Abstract
Neuroblastoma is the most common malignant tumour in infancy; the reversion-inducing cysteine-rich protein with Kazal motifs gene (RECK) is a tumour suppressor gene. Previous studies show that RECK inhibits tumour invasion and metastasis through negative regulation of the matrix metalloproteinase (MMP)-2, MMP-9 and MMP-14. Therefore, we wanted to detect the expression of RECK and MMP-14 in neuroblastomas to assess the correlation between the expression levels of these proteins, and to investigate the roles in the metastasis and development of the tumour. PV-6000 immunohistochemistry method was used to detect the expression levels of RECK and MMP-14 in 36 samples of neuroblastoma tissue. Samples from paraffin wax-embedded specimens and the complete clinicopathological data of 36 neuroblastoma and 10 ganglioneuroma patients were collected. The rate of expression of the RECK protein in the neuroblastoma was low (16.7%). Furthermore, it reduced with the increase in the invasive depth and distant metastasis (P=0.015; P<0.05). The rate of expression of the MMP-14 protein in the neuroblastoma was high (58.3%) and increased with the increase in the extent of invasive depth and distant metastasis (P=0.002; P<0.05). The expression of the RECK protein correlated negatively with that of MMP-14 (r =−0.418; P<0.05). Low levels of the RECK protein are expressed in the neuroblastoma, while the MMP-14 protein is expressed at high levels. The RECK and MMP-14 proteins may serve as markers in the estimation of the extent of metastasis and dissemination of the neuroblastoma.
Keywords: cysteine, metastasis, neuroblastoma, tumour
Background
A neuroblastoma (NB) is an embryonal tumour of the sympathetic nervous system seen commonly in childhood and the most common malignant tumour in infancy and therefore NB is prone to early metastasis; in 60–70% of NB cases, systemic organ or bone marrow metastasis is detected at presentation and is the leading cause of death in these patients. It is of great significance to explore the metastatic mechanisms of NB, particularly the mechanisms underlying its systemic dissemination. The reversion-inducing cysteine-rich protein with Kazal motifs gene (RECK) which was discovered in 1998 is a tumour suppressor gene. Studies have shown that RECK inhibits tumour invasion and metastasis through negative regulation of the matrix metalloproteinase (MMP)-2, MMP-9 and membrane-type 1-matrix metalloproteinase (MT1-MMP/MMP-14)(Takahashi et al. 1998; Eisenberg et al. 2002; Masui et al. 2003). In our study, the expression of the RECK and MMP-14 proteins in the neuroblastoma metastasis and non-metastasis groups was assessed by immunohistochemistry to clarify further the molecular mechanisms of the RECK and MMP-14 proteins in the occurrence, development, invasion and metastasis of neuroblastoma. The insights gained provide a theoretical basis for the prevention, diagnosis and treatment of neuroblastoma.
Materials and methods
Materials
Samples were obtained from paraffin wax-embedded specimens of surgically removed and pathologically confirmed neuroblastoma and ganglioneuroma. These included 36 samples of neuroblastoma and 10 of ganglioneuroma. These specimens, with complete clinicopathological data from January 1995 to April 2008, were selected from the paediatric surgery department of our hospital. Of the 46 patients included, 35 were male and 11 were female, with age ranging from 1 to 8 years (average age: 4.40 years). The tumours were classified using the International Neuroblastoma Staging System (INSS): of the 36 cases, 7 were in stage I; 8 in stage II; 11 in stage III (including 8 cases with huge tumours across the midline and 3 cases with bilateral lymph node metastases); and 10 in stage IV (including 2 cases with distant lymph node metastasis and 8 cases with distant organs metastasis such as liver, lung, testis, or bone marrow metastasis). Condensed rabbit anti-human RECK monoclonal antibodies (mAb) were purchased from Santa Cruz, Biotechnology Inc. (Santa Cruz, CA, USA). The MMP-14 rabbit anti-human mAbs were purchased from Wuhan Boster Biological Technology Co., Ltd. (Wuhan, China). The PV-6000 immunohistochemistry kit and diaminobenzidine (DAB) chromogenic kit were purchased from Beijing Zhongshan Goldenbridge Biotechnology Co., Ltd. (Beijing, China). The conventional reagents required in immunohistochemical staining were citrate buffer (0.01 mmol/L, pH 6.0), disodium hydrogen phosphate, sodium dihydrogen phosphate, sodium chloride, xylene, alcohol, hydrogen peroxide and haematoxylin, all of which were chemical or analytical pure reagents. The equipment and instruments, including a low-temperature refrigerator, freezing microtome, drying machine, high-pressure sterilizing pots, incubator, microscopes, microphotographic apparatus and wet boxes, were provided by the pathology department.
Methods
Based on the presence or absence of metastasis, the cases with neuroblastoma were divided into groups A and B as follows: group A which has no metastasis included 13 cases in stages I and IIA, group B which has local or distant metastasis comprised 23 cases in stages IIB, III and IV. Group C comprised 10 cases of ganglioneuroma. Slices of the wax block were stained with haematoxylin and eosine (H&E), and the extent of tissue differentiation was determined. PV-6000 immunohistochemistry method was used to evaluate the expression of the proteins RECK and MMP-14 in the neuroblastoma and ganglioneuroma specimens. The working concentration of the primary antibody for both the proteins was 1:100. The negative control was prepared in the above-mentioned manner, but by using phosphate-buffered saline (PBS) instead of the primary antibody. The paraffin-embedded tissue sections (3 μm) were de-waxed and hydrated according to standard protocols and incubated in deionized water (with 3% hydrogen peroxide) for 10 min. The sections were pretreated using microwave oven processing as per the requirements of reaction with primary antibodies; the sections were then stained with the primary antibody and maintained at 37 °C for 1 h. Thereafter, the stained samples were treated with the universal IgG antibody, maintained at 37 °C for 20 min and the colour was developed by adding the DAB solution.
Statistical analysis
The RECK and MMP-14 positive signals were observed to originate from a brown granular substance, located mainly in the cytoplasm. The cells were observed under a high-power microscope, and 5–10 visual fields containing not less than 200 cells in each field were randomly selected. The results were determined based on the percentage of positive cells and the density of staining as follows (Xu & Yang 1996): (1) Cells in sections were scored according to the density of staining: score 0 = no colour; score 1 = light yellow; score 2 = yellow-brown; score 3 = brown (2) according to the percentage of positive cells among the same cells, score 1 for positive cells at ≤30%; score 2, 30%–70%; and score 3, >70%. The product obtained by multiplying the score of (1) and (2) was the total score, where a total score of 0–1 was considered a negative score (-); 2–3, a weak positive score (+), and ≥4, a positive score (++). The (+) and (++) values were combined to form the positive group to facilitate the analysis. The Statistical Package for the Social Sciences (spss) 15.0 software was used in the statistical process (spss Inc, Chicago, IL, USA). The exact fourfold table chi-square test was performed to establish the difference among the indicators. The results of the inter-group correlation analysis were confirmed by the Spearman correlation analysis used for inter-group comparison. P<0.05 was considered as representing significant differences.
Results
Expression of RECK and MMP-14 in Neuroblastoma groups
Table 1 indicates that the RECK-positive rate in group B was 4.3% (1/23), while that in group A was 38.5% (5/13). There were statistically significant differences between the 2 groups (P= 0.015). The positive rate of MMP-14 expression in group A was 23.1% (3/13), while that in group B was 78.3% (18/23). There were statistically significant differences between the 2 groups (P= 0.002) (Figure 1).
Table 1.
RECK and MMP-14 expression in the metastasized and non-metastasized neuroblastoma groups (case number, %)
| RECK |
MMP-14 |
||||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Total | |
| group A | 5(38.5) | 8(61.5) | 3(23.1) | 10(76.9) | 13(100) |
| group B | 1(4.3) | 22(95.7) | 18(78.3) | 5(21.7) | 23(100) |
(P< 0.05)
RECK, reversion-inducing cysteine-rich protein with Kazal motifs; MMP, matrix metalloproteinase.
Figure 1.
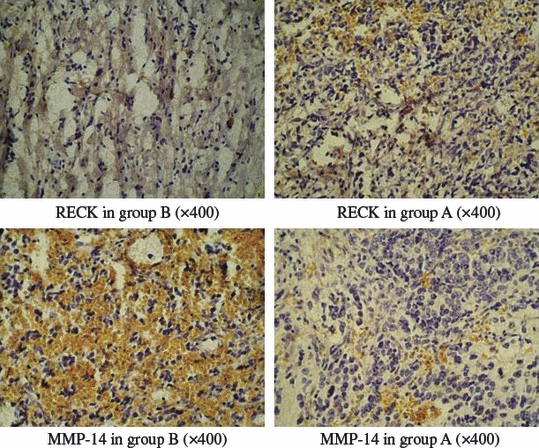
RECK and MMP-14 protein expression in the neuroblastoma (group A and B).
Expression of RECK and MMP-14 in the ganglioneuroma and neuroblastoma groups
Table 2 indicates that the positive rate of RECK expression in the ganglioneuroma group was 80.0% (8/10), which was significantly higher than the 16.7% (6/36) in the neuroblastoma group (the metastasized and non-metastasized neuroblastoma groups were combined) and the difference between the 2 groups was statistically significant (P= 0.000). The positive rate of MMP-14 expression in the ganglioneuroma group was 10.0% (1/10), which was significantly lower than the 58.3% (21/36) in the neuroblastoma group, and the difference between the 2 groups was statistically significant (P= 0.007) (Figure 2).
Table 2.
RECK and MMP-14 expression in the ganglioneuroma and neuroblastoma groups (case number, %)
| RECK |
MMP-14 |
||||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Total | |
| group A and B | 6(16.7) | 30(83.3) | 21(58.3) | 15(41.7) | 36(100) |
| group C | 8(80.0) | 2(20.0) | 1(10.0) | 9(90.0) | 10(100) |
(P< 0.05)
RECK, reversion-inducing cysteine-rich protein with Kazal motifs; MMP, matrix metalloproteinase.
Figure 2.
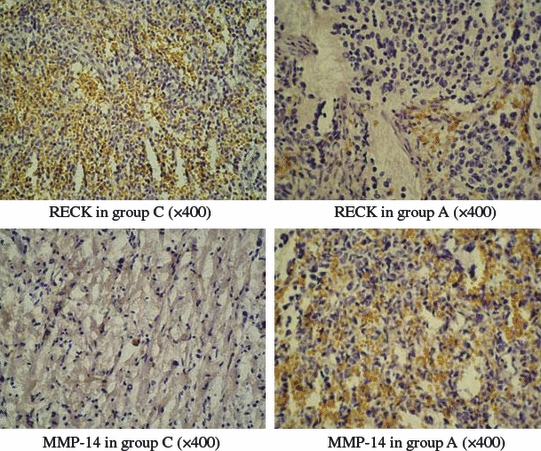
RECK and MMP-14 protein expression in the neuroblastoma (group A/B) and ganglioneuroma (group C).
Correlation analysis between RECK and MMP-14
The expressions of the RECK and MMP-14 proteins in the neuroblastoma and ganglioneuroma tissue were negatively correlated [(P< 0.05; r = −0.418) and (P< 0.005; r = −0.821) respectively].
Discussion
Neuroblastoma is the most common malignant tumour in infancy, its high degree of malignancy and early metastasis are critical factors that affect the cure rate of NB patients. It is important to investigate the mechanism of metastasis and factors influencing the metastasis of the neuroblastoma to develop improved treatment strategies for this disease as well as to improve its survival rates. In our study, RECK and MMP-14 expressions in neuroblastoma tissues were investigated, and the groups in this study were designed to focus on the relationship between the 2 types of protein and tumour metastasis, with the limited data.
The basal membrane and extracellular matrix (ECM) are the main barriers that prevent tumour invasion and metastasis. Tumour invasion and metastasis are influenced by the capacity to degrade the ECM and basal membrane; these degradation processes involve huge amounts of proteases and their inhibitors. The MMP family constitutes enzymes that are essential for the degradation of the ECM. These enzymes can degrade almost all the components of the ECM and the collagen in the basal membrane and thus promote the invasion of the tumour cells into the surrounding connective tissues and the entry of these cells into the circulation leading to metastasis of the tumour to other tissues and organs. The MMPs, comprising more than 20 zinc-dependent endopeptidases, are a class of highly conserved enzymes. They are widely distributed in plants, vertebrates and invertebrates; occur in various molecular sizes; and exhibit a certain degree of substrate specificity. They are the key enzymes that influence tumour invasion and metastasis. They can be broadly divided according to their substrates into the following categories: (1) collagenases, (2) gelatinases, (3), matrilysin, (4) membrane-type MMPs (MT-MMPs), including MT1-MMP/MMP-14 and (5) others. Studies have shown that overexpression of MMPs is closely associated with the degree of malignancy of the tumour (Cheng et al. 2003).
The main role of the MMPs includes the following: (1) Degradation of the ECM: MMPs can break down the matrix constituents of healthy tissues and thus facilitate the dissemination of tumour cells, along the defects and gaps in the basal membrane, and the subsequent tumour proliferation; (2) Precipitating the tumour invasion and metastasis: MMP causes structure relaxation of the tissue, and the autolysis of tissues contributes to the release of MMP, which causes proliferation of the tumour; (3) Promoting angiogenesis: By causing the degradation of the ECM, the MMP provides space for the growth of new blood vessels. (4) Regulating cell adhesion: The adhesion between tumour cells and between tumour cells and host cells plays an important role in tumour invasion and metastasis; (5) Activating protein with potential activity: Studies have shown that MMP can activate the potential activity of 2 types of protein, plasma fibrinogen and laminin-5, both of which play an important role in attracting inflammatory cells and spontaneously stimulating the migration of tumour cells. RECK is a novel gene discovered by Takahashi in the NIH3T3 cells transfected with v-Ki-ras in 1998, and is located on chromosome 9p12-p13, with a length of about 87 kb, containing 21 exons and 20 introns; the RECK gene is highly expressed in normal tissues and is expressed in low amounts in cell lines originating from a variety of tumours, and cells transfected with the Ras oncogene (Masui et al. 2003; Takeuchi et al. 2004). Gene knockout studies revealed that the RECK gene is also closely associated with angiogenesis; a moderate expression of the RECK gene can inhibit angiogenesis(Oh et al. 2001; Sasahara et al. 2002; Welm et al. 2002).
Recent studies show that the RECK gene can inhibit at least 3 MMPs namely, MMP-2, MMP-9 and MMP-14, post transcriptionally and thereby inhibit angiogenesis and metastasis of the tumour. Specifically, the membrane-anchored RECK gene inhibits the secretion of MMP-9 precursors, while the anchored RECK protein and soluble RECK protein can inhibit the activity of MMP-2, MMP-9 and MMP-14. RECK regulates MMP-9 in 2 ways: (1) Inhibition of the secretion of MMP-9 from cells by directly inhibiting its activity. Studies have shown that while the expression of the RECK gene inhibits the invasion and metastasis of tumour cells and angiogenesis, it is accompanied by a reduction in the MMP-9 secretion. Downregulation of this gene by tumourigenic signals contributes to tumour invasion and metastasis. (2) The RECK protein can inhibit the activation process of the MMP-2 precursor protein. By inhibiting the activity of the MT1-MMP, the RECK protein can inhibit the transformation of the MMP-2 precursor protein into the MMP-2 protein and likewise, the RECK protein can inhibit the final step of the MMP-2 precursor protein activation during which its intermediate forms are activated by autolysis. It was found in our study that the expression of the RECK protein in the neuroblastoma tissues (16.7%) was significantly lower than in the ganglioneuroma tissues (80.0%); furthermore, the expression of the RECK protein in the group B neuroblastoma group (4.3%) was significantly lower than in group A (38.5%). That is, the RECK protein had a low expression in neuroblastoma tissues and its expression was reduced with the increase in the depth of tumour invasion and distant metastasis, which is consistent with the findings of other studies (Span et al. 2003; Xu et al. 2005; Li et al. 2006, 2007; Song et al. 2006; Oshima et al. 2008). It is indicated that the termination of the expression of the RECK gene is closely related to the incidence, development, invasion and metastasis of the neuroblastoma and may provide the basis for determining the degree of malignancy of the neuroblastoma. The expression of the MMP-14 in neuroblastoma tissues (58.3%) was significantly higher than in the ganglioneuroma group (10.0%). The expression of MMP-14 in the neuroblastoma group B (78.3%) was significantly higher than in group A (23.1%),suggesting that MMP-14 had a high expression in neuroblastoma tissues, and its expression levels were elevated with the increase in the depth of tumour invasion of the tumour and distant metastasis.
The experimental results further show that the expressions of RECK and MMP-14 in neuroblastoma and ganglioneuroma tissues were negatively correlated. The expression of MMP-14 was enhanced with the decline in expression of the RECK protein, indicating that the termination of RECK expression in neuroblastoma tissues led to the decreased inhibition of MMP-14 and its resultant high expression, thereby promoting the invasion and metastasis of the tumour cells.
Conclusion
In summary, the expressions of RECK and MMP-14 may play an important role in the occurrence, development and infiltrating metastasis of the neuroblastoma and probably represent an important indicator for the evaluation of the biological behaviour of the neuroblastoma. However, the specific mechanisms and channels are not yet clear. More in-depth studies are needed to be carried out to identify the relationship between the channels and mechanisms. This article focused on the relationship between the RECK and MMP-14 protein expression and tumour metastasis. The prognosis in children with neuroblastoma depends on age, primary tumour location, pathological stage and other factors. We intend to study further the relationship between the RECK and MMP-14 proteins and the prognosis of children with neuroblastoma.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation and the Ministry of Education of China and grants from Qingdao city. (no. 30471800, 30872702).
References
- Cheng Y, Dong Q, Jiang BX, Sun LR. The expression and significance of MMP-2,-9,TIMP-2,-1 in neuroblastoma. Chin. J. Pediatr. Surg. 2003;24(5):452–454. [Google Scholar]
- Eisenberg I, Hochner H, Sadeh M, Argov Z, Mitrani-Rosenbaum S. Establishment of the genomic structure and identification of thirteen single-nucleotide polymorphisms in the human RECK gene. Cytogenet. Genome Res. 2002;97(1-2):58–61. doi: 10.1159/000064042. [DOI] [PubMed] [Google Scholar]
- Li Z, Sun T, Wei LZ. RECK expression in gliomas and its clinical significance. Chin. J. Neurosurg. 2006;22(9):550–552. [Google Scholar]
- Li SL, Zhao QM, Liu ZW. Correlation between the protein expression of reversion-inducing cysteine-rich protein with Kazal motifs and matrix matalloproteinase-9 in esophageal squamous cell carcinoma and its clinical pathological significance. World Chinese J. Digestol. 2007;15(10):1082–1086. [Google Scholar]
- Masui T, Doi R, Koshiba T, et al. RECK expression in pancreatic cancer: its correlation with lower invasiveness and better prognosis. Clin. Cancer Res. 2003;9(5):1779–1784. [PubMed] [Google Scholar]
- Oh J, Takahashi R, Kondo S, et al. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell. 2001;107(6):789–800. doi: 10.1016/s0092-8674(01)00597-9. [DOI] [PubMed] [Google Scholar]
- Oshima T, Kunisaki C, Yoshihara K, et al. Clinicopathological significance of the gene expression of matrix metalloproteinases and reversion-inducing cysteine-rich protein with Kazal motifs in patients with colorectal cancer: MMP-2 gene expression is a useful predictor of liver metastasis from colorectal cancer. Oncol. Rep. 2008;19(5):1285–1291. [PubMed] [Google Scholar]
- Sasahara RM, Brochado SM, Takahashi C, et al. Transcriptional control of the RECK metastasis/angiogenesis suppressor gene. Cancer Detect. Prev. 2002;26(6):435–443. doi: 10.1016/s0361-090x(02)00123-x. [DOI] [PubMed] [Google Scholar]
- Song SY, Son HJ, Nam E, Rhee JC, Park C. Expression of reversion-inducing- cysteine-rich protein with Kazal motifs (RECK) as a prognostic indicator in gastric cancer. Eur. J. Cancer. 2006;42(1):101–108. doi: 10.1016/j.ejca.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Span PN, Sweep CG, Manders P, Beex LV, Leppert D, Lindberg RL. Matrix metalloproteinase inhibitor reversion-inducing cysteine -rich protein with Kazal motifs: a prognostic marker for good clinical outcome in human breast carcinoma. Cancer. 2003;97(11):2710–2715. doi: 10.1002/cncr.11395. [DOI] [PubMed] [Google Scholar]
- Takahashi C, Sheng Z, Horan TP, et al. Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane anchored glycoprotein RECK. Proc. Natl. Acad. Sci. USA. 1998;95(22):13221–13226. doi: 10.1073/pnas.95.22.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Hisanaga M, Nagao M, et al. The membrane anchored matrix metalloproteinase (MMP) RECK in combination with MMP-9 serves as an informative prognostic indicator for colorectal cancer. Clin. Cancer Res. 2004;10(16):5572–5579. doi: 10.1158/1078-0432.CCR-03-0656. [DOI] [PubMed] [Google Scholar]
- Welm B, Mott J, Werb Z. Developmental biology: vasculogenesis is a wreck without RECK. Curr. Biol. 2002;12(6):R209–R211. doi: 10.1016/s0960-9822(02)00752-2. [DOI] [PubMed] [Google Scholar]
- Xu LZ, Yang WT. The criteria of immunohistochemical reaction results. China Oncol. 1996;6:229–231. [Google Scholar]
- Xu ZY, Gao JP, Zhang ZY. Expression and significance of the matrix metalloproteinase inhibitor RECK gene in prostate cell strains. Nat. J. Androl. 2005;11(10):727–730. [PubMed] [Google Scholar]


