Abstract
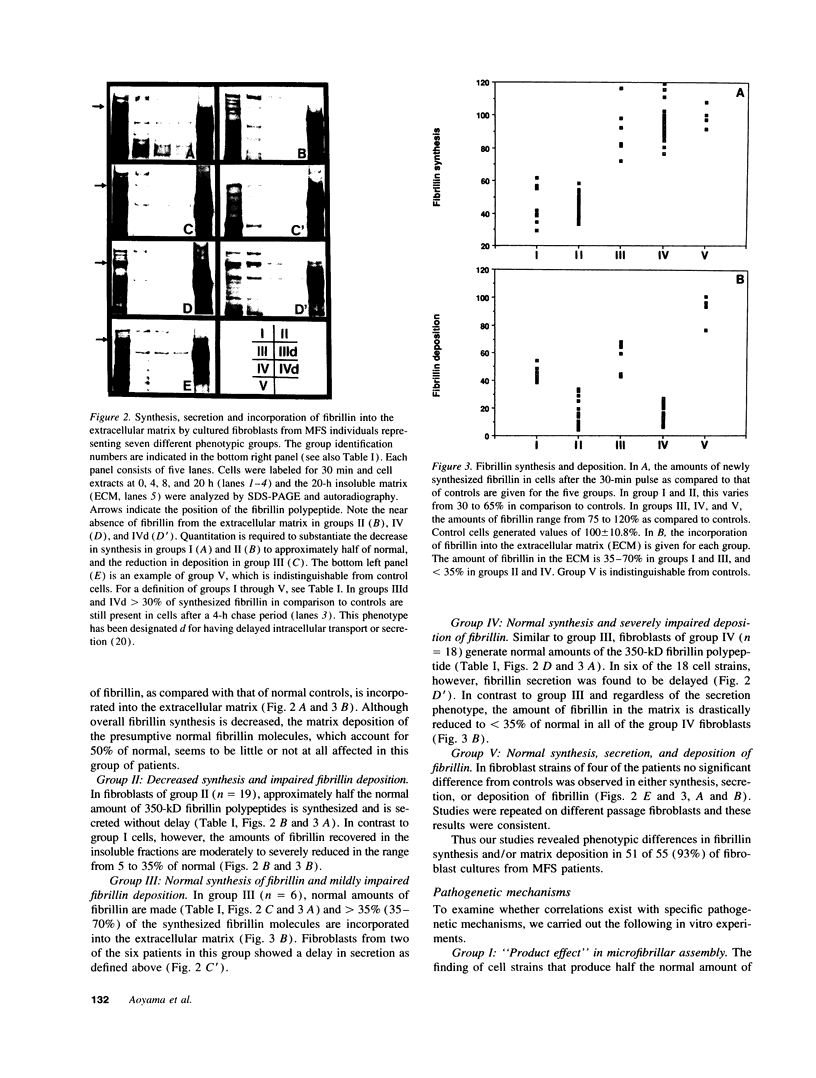
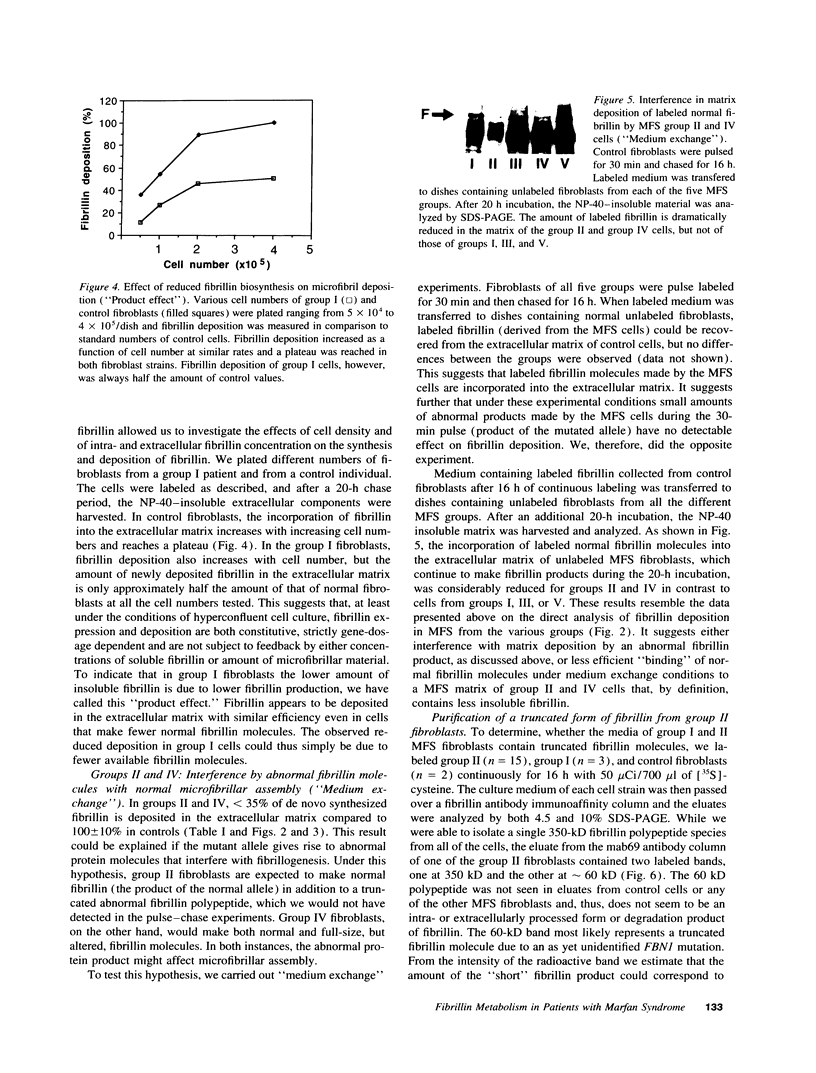
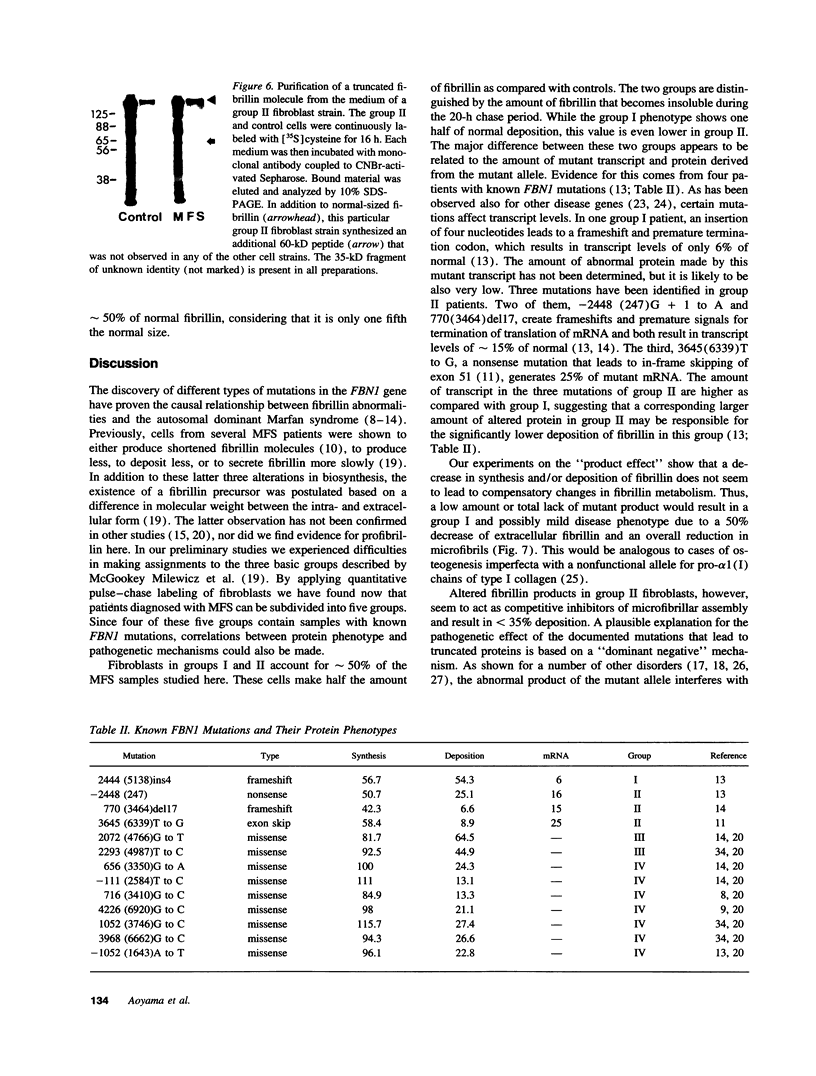
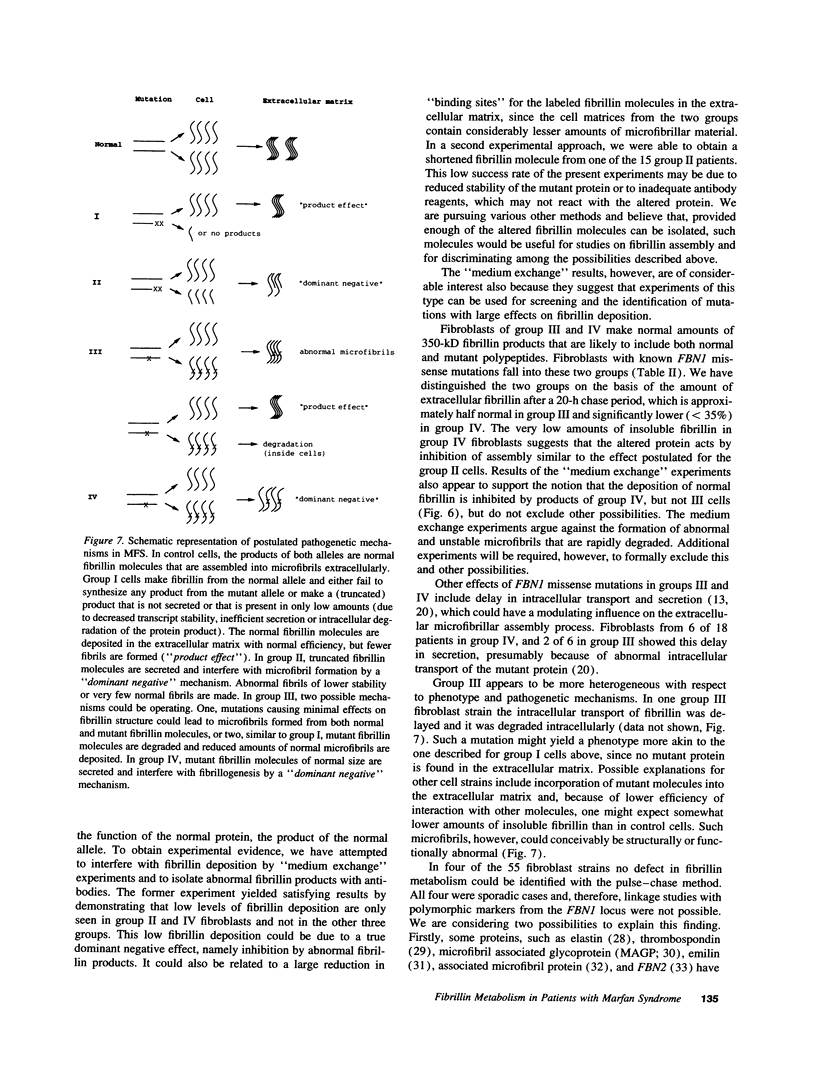
Pulse-chase studies of [35S]cysteine-labeled fibrillin were performed on fibroblast strains from 55 patients with Marfan syndrome (MFS), including 13 with identified mutations in the fibrillin-1 gene and 10 controls. Quantitation of the soluble intracellular and insoluble extracellular fibrillin allowed discrimination of five groups. Groups I (n = 8) and II (n = 19) synthesize reduced amounts of normal-sized fibrillin, while synthesis is normal in groups III (n = 6), IV (n = 18), and V (n = 4). When extracellular fibrillin deposition is measured, groups I and III deposit between 35 and 70% of control values, groups II and IV < 35%, and group V > 70%. A deletion mutant with a low transcript level from the mutant allele and seven additional patients have the group I protein phenotype. Disease in these patients is caused by a reduction in microfibrils associated with either a null allele, an unstable transcript, or an altered fibrillin product synthesized in low amounts. In 68% of the MFS individuals (groups II and IV), a dominant negative effect is invoked as the main pathogenetic mechanism. Products made by the mutant allele in these fibroblasts are proposed to interfere with microfibril formation. Insertion, deletion, and exon skipping mutations, resulting in smaller fibrillin products, exhibit the group II phenotype. A truncated form of fibrillin of 60 kD was identified with specific fibrillin antibodies in one of the group II cell culture media. Seven of the nine known missense mutations, giving rise to abnormal, but normal-sized fibrillin molecules, are in group IV.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama T., Tynan K., Dietz H. C., Francke U., Furthmayr H. Missense mutations impair intracellular processing of fibrillin and microfibril assembly in Marfan syndrome. Hum Mol Genet. 1993 Dec;2(12):2135–2140. doi: 10.1093/hmg/2.12.2135. [DOI] [PubMed] [Google Scholar]
- Arbeille B. B., Fauvel-Lafeve F. M., Lemesle M. B., Tenza D., Legrand Y. J. Thrombospondin: a component of microfibrils in various tissues. J Histochem Cytochem. 1991 Oct;39(10):1367–1375. doi: 10.1177/39.10.1940308. [DOI] [PubMed] [Google Scholar]
- Barsh G. S., David K. E., Byers P. H. Type I osteogenesis imperfecta: a nonfunctional allele for pro alpha 1 (I) chains of type I procollagen. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3838–3842. doi: 10.1073/pnas.79.12.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beighton P., de Paepe A., Danks D., Finidori G., Gedde-Dahl T., Goodman R., Hall J. G., Hollister D. W., Horton W., McKusick V. A. International Nosology of Heritable Disorders of Connective Tissue, Berlin, 1986. Am J Med Genet. 1988 Mar;29(3):581–594. doi: 10.1002/ajmg.1320290316. [DOI] [PubMed] [Google Scholar]
- Bressan G. M., Daga-Gordini D., Colombatti A., Castellani I., Marigo V., Volpin D. Emilin, a component of elastic fibers preferentially located at the elastin-microfibrils interface. J Cell Biol. 1993 Apr;121(1):201–212. doi: 10.1083/jcb.121.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz H. C., Cutting G. R., Pyeritz R. E., Maslen C. L., Sakai L. Y., Corson G. M., Puffenberger E. G., Hamosh A., Nanthakumar E. J., Curristin S. M. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991 Jul 25;352(6333):337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., McIntosh I., Sakai L. Y., Corson G. M., Chalberg S. C., Pyeritz R. E., Francomano C. A. Four novel FBN1 mutations: significance for mutant transcript level and EGF-like domain calcium binding in the pathogenesis of Marfan syndrome. Genomics. 1993 Aug;17(2):468–475. doi: 10.1006/geno.1993.1349. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., Pyeritz R. E., Puffenberger E. G., Kendzior R. J., Jr, Corson G. M., Maslen C. L., Sakai L. Y., Francomano C. A., Cutting G. R. Marfan phenotype variability in a family segregating a missense mutation in the epidermal growth factor-like motif of the fibrillin gene. J Clin Invest. 1992 May;89(5):1674–1680. doi: 10.1172/JCI115766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz H. C., Saraiva J. M., Pyeritz R. E., Cutting G. R., Francomano C. A. Clustering of fibrillin (FBN1) missense mutations in Marfan syndrome patients at cysteine residues in EGF-like domains. Hum Mutat. 1992;1(5):366–374. doi: 10.1002/humu.1380010504. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., Valle D., Francomano C. A., Kendzior R. J., Jr, Pyeritz R. E., Cutting G. R. The skipping of constitutive exons in vivo induced by nonsense mutations. Science. 1993 Jan 29;259(5095):680–683. doi: 10.1126/science.8430317. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Contard P., Schwartz E., MacDonald E. D., 2nd, Jacobs L., 2nd, Sakai L. Y. Elastin-associated microfibrils (10 nm) in a three-dimensional fibroblast culture. J Invest Dermatol. 1991 Oct;97(4):638–643. doi: 10.1111/1523-1747.ep12483132. [DOI] [PubMed] [Google Scholar]
- Gibson M. A., Cleary E. G. The immunohistochemical localisation of microfibril-associated glycoprotein (MAGP) in elastic and non-elastic tissues. Immunol Cell Biol. 1987 Aug;65(Pt 4):345–356. doi: 10.1038/icb.1987.39. [DOI] [PubMed] [Google Scholar]
- Gibson M. A., Sandberg L. B., Grosso L. E., Cleary E. G. Complementary DNA cloning establishes microfibril-associated glycoprotein (MAGP) to be a discrete component of the elastin-associated microfibrils. J Biol Chem. 1991 Apr 25;266(12):7596–7601. [PubMed] [Google Scholar]
- Hamosh A., Rosenstein B. J., Cutting G. R. CFTR nonsense mutations G542X and W1282X associated with severe reduction of CFTR mRNA in nasal epithelial cells. Hum Mol Genet. 1992 Oct;1(7):542–544. doi: 10.1093/hmg/1.7.542. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Hewett D. R., Lynch J. R., Smith R., Sykes B. C. A novel fibrillin mutation in the Marfan syndrome which could disrupt calcium binding of the epidermal growth factor-like module. Hum Mol Genet. 1993 Apr;2(4):475–477. doi: 10.1093/hmg/2.4.475. [DOI] [PubMed] [Google Scholar]
- Hollister D. W., Godfrey M., Sakai L. Y., Pyeritz R. E. Immunohistologic abnormalities of the microfibrillar-fiber system in the Marfan syndrome. N Engl J Med. 1990 Jul 19;323(3):152–159. doi: 10.1056/NEJM199007193230303. [DOI] [PubMed] [Google Scholar]
- Horrigan S. K., Rich C. B., Streeten B. W., Li Z. Y., Foster J. A. Characterization of an associated microfibril protein through recombinant DNA techniques. J Biol Chem. 1992 May 15;267(14):10087–10095. [PubMed] [Google Scholar]
- Kadowaki T., Kadowaki H., Rechler M. M., Serrano-Rios M., Roth J., Gorden P., Taylor S. I. Five mutant alleles of the insulin receptor gene in patients with genetic forms of insulin resistance. J Clin Invest. 1990 Jul;86(1):254–264. doi: 10.1172/JCI114693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainulainen K., Pulkkinen L., Savolainen A., Kaitila I., Peltonen L. Location on chromosome 15 of the gene defect causing Marfan syndrome. N Engl J Med. 1990 Oct 4;323(14):935–939. doi: 10.1056/NEJM199010043231402. [DOI] [PubMed] [Google Scholar]
- Kainulainen K., Sakai L. Y., Child A., Pope F. M., Puhakka L., Ryhänen L., Palotie A., Kaitila I., Peltonen L. Two mutations in Marfan syndrome resulting in truncated fibrillin polypeptides. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5917–5921. doi: 10.1073/pnas.89.13.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Godfrey M., Vitale E., Hori H., Mattei M. G., Sarfarazi M., Tsipouras P., Ramirez F., Hollister D. W. Linkage of Marfan syndrome and a phenotypically related disorder to two different fibrillin genes. Nature. 1991 Jul 25;352(6333):330–334. doi: 10.1038/352330a0. [DOI] [PubMed] [Google Scholar]
- Magenis R. E., Maslen C. L., Smith L., Allen L., Sakai L. Y. Localization of the fibrillin (FBN) gene to chromosome 15, band q21.1. Genomics. 1991 Oct;11(2):346–351. doi: 10.1016/0888-7543(91)90142-2. [DOI] [PubMed] [Google Scholar]
- Maslen C. L., Corson G. M., Maddox B. K., Glanville R. W., Sakai L. Y. Partial sequence of a candidate gene for the Marfan syndrome. Nature. 1991 Jul 25;352(6333):334–337. doi: 10.1038/352334a0. [DOI] [PubMed] [Google Scholar]
- Milewicz D. M., Pyeritz R. E., Crawford E. S., Byers P. H. Marfan syndrome: defective synthesis, secretion, and extracellular matrix formation of fibrillin by cultured dermal fibroblasts. J Clin Invest. 1992 Jan;89(1):79–86. doi: 10.1172/JCI115589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyeritz R. E., McKusick V. A. The Marfan syndrome: diagnosis and management. N Engl J Med. 1979 Apr 5;300(14):772–777. doi: 10.1056/NEJM197904053001406. [DOI] [PubMed] [Google Scholar]
- Sakai L. Y., Keene D. R., Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986 Dec;103(6 Pt 1):2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai L. Y., Keene D. R., Glanville R. W., Bächinger H. P. Purification and partial characterization of fibrillin, a cysteine-rich structural component of connective tissue microfibrils. J Biol Chem. 1991 Aug 5;266(22):14763–14770. [PubMed] [Google Scholar]
- Tsipouras P., Del Mastro R., Sarfarazi M., Lee B., Vitale E., Child A. H., Godfrey M., Devereux R. B., Hewett D., Steinmann B. Genetic linkage of the Marfan syndrome, ectopia lentis, and congenital contractural arachnodactyly to the fibrillin genes on chromosomes 15 and 5. The International Marfan Syndrome Collaborative Study. N Engl J Med. 1992 Apr 2;326(14):905–909. doi: 10.1056/NEJM199204023261401. [DOI] [PubMed] [Google Scholar]
- Tynan K., Comeau K., Pearson M., Wilgenbus P., Levitt D., Gasner C., Berg M. A., Miller D. C., Francke U. Mutation screening of complete fibrillin-1 coding sequence: report of five new mutations, including two in 8-cysteine domains. Hum Mol Genet. 1993 Nov;2(11):1813–1821. doi: 10.1093/hmg/2.11.1813. [DOI] [PubMed] [Google Scholar]
- Vogel B. E., Minor R. R., Freund M., Prockop D. J. A point mutation in a type I procollagen gene converts glycine 748 of the alpha 1 chain to cysteine and destabilizes the triple helix in a lethal variant of osteogenesis imperfecta. J Biol Chem. 1987 Oct 25;262(30):14737–14744. [PubMed] [Google Scholar]
- Williams C. J., Prockop D. J. Synthesis and processing of a type I procollagen containing shortened pro-alpha 1(I) chains by fibroblasts from a patient with osteogenesis imperfecta. J Biol Chem. 1983 May 10;258(9):5915–5921. [PubMed] [Google Scholar]
- Willing M. C., Cohn D. H., Starman B., Holbrook K. A., Greenberg C. R., Byers P. H. Heterozygosity for a large deletion in the alpha 2(I) collagen gene has a dramatic effect on type I collagen secretion and produces perinatal lethal osteogenesis imperfecta. J Biol Chem. 1988 Jun 15;263(17):8398–8404. [PubMed] [Google Scholar]