Abstract
The etiology of multiple sclerosis (MS) is considered to involve genetic, environmental, infective, and immunological factors which affect the integrity of a normally assembled myelin sheath, either directly or indirectly resulting in demyelination. In a correlative study involving protein chemical, mass spectrometric, and electron microscopic techniques we have determined that myelin obtained from victims of MS is arrested at the level of the first growth spurt (within the first 6 yr of life) and is therefore developmentally immature. The data supporting this conclusion include (a) the pattern of microheterogeneity of myelin basic protein (MBP); (b) the NH2-terminal acylation of the least cationic component of MBP ("C-8"); (c) the phase transition temperature (Tc) of myelin isolated from victims of MS correlated with the increased proportion of the least cationic component of MBP; and (d) immunogold electron microscopy using an antibody specific for "C-8" showed that the distribution of gold particles in a 2-yr-old infant was similar to the distribution found in a victim of MS. We postulate that this developmentally immature myelin is more susceptible to degradation by one or a combination of factors mentioned above, providing the initial antigenic material to the immune system.
Full text
PDF

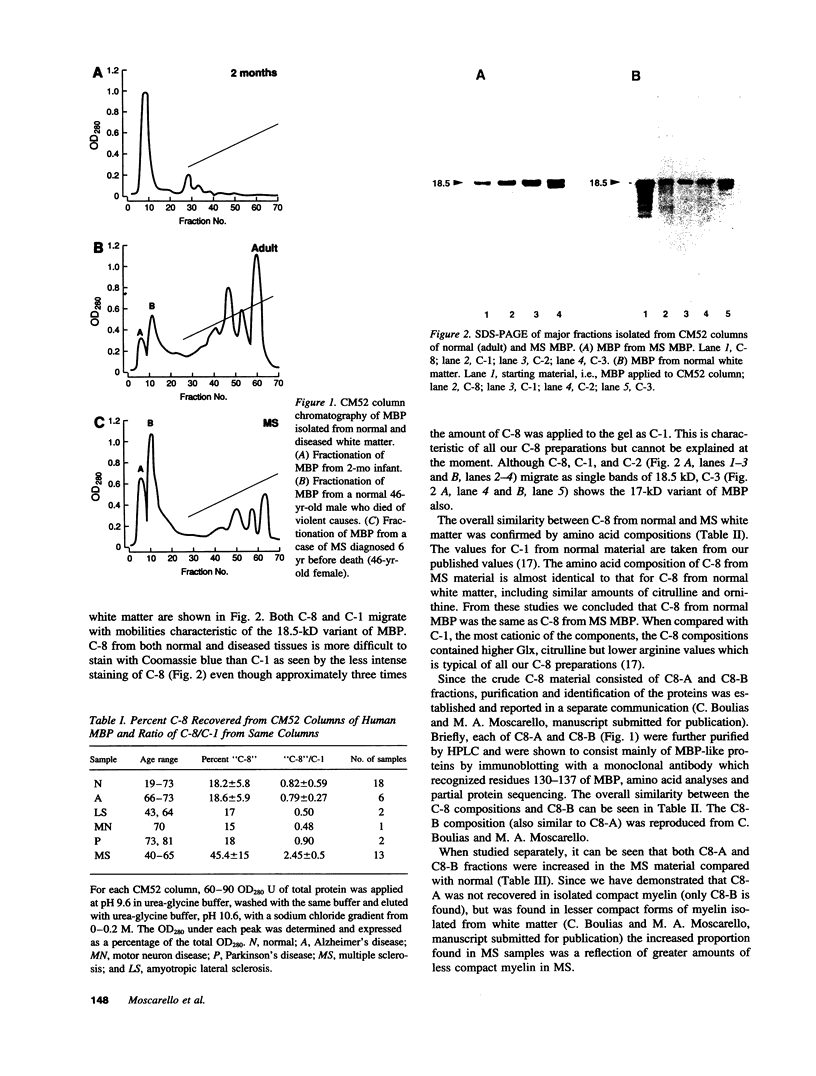




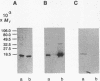
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady G. W., Fein D. B., Wood D. D., Moscarello M. A. The role of charge microheterogeneity of human myelin basic protein in the formation of phosphatidylglycerol multilayers. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1161–1165. doi: 10.1016/0006-291x(85)90307-9. [DOI] [PubMed] [Google Scholar]
- Campagnoni A. T., Pribyl T. M., Campagnoni C. W., Kampf K., Amur-Umarjee S., Landry C. F., Handley V. W., Newman S. L., Garbay B., Kitamura K. Structure and developmental regulation of Golli-mbp, a 105-kilobase gene that encompasses the myelin basic protein gene and is expressed in cells in the oligodendrocyte lineage in the brain. J Biol Chem. 1993 Mar 5;268(7):4930–4938. [PubMed] [Google Scholar]
- Carnegie P. R. N-terminal sequence of an encephalitogenic protein form human myelin. Biochem J. 1969 Jan;111(2):240–242. doi: 10.1042/bj1110240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheifetz S., Moscarello M. A., Deber C. M. NMR investigation of the charge isomers of bovine myelin basic protein. Arch Biochem Biophys. 1984 Aug 15;233(1):151–160. doi: 10.1016/0003-9861(84)90611-8. [DOI] [PubMed] [Google Scholar]
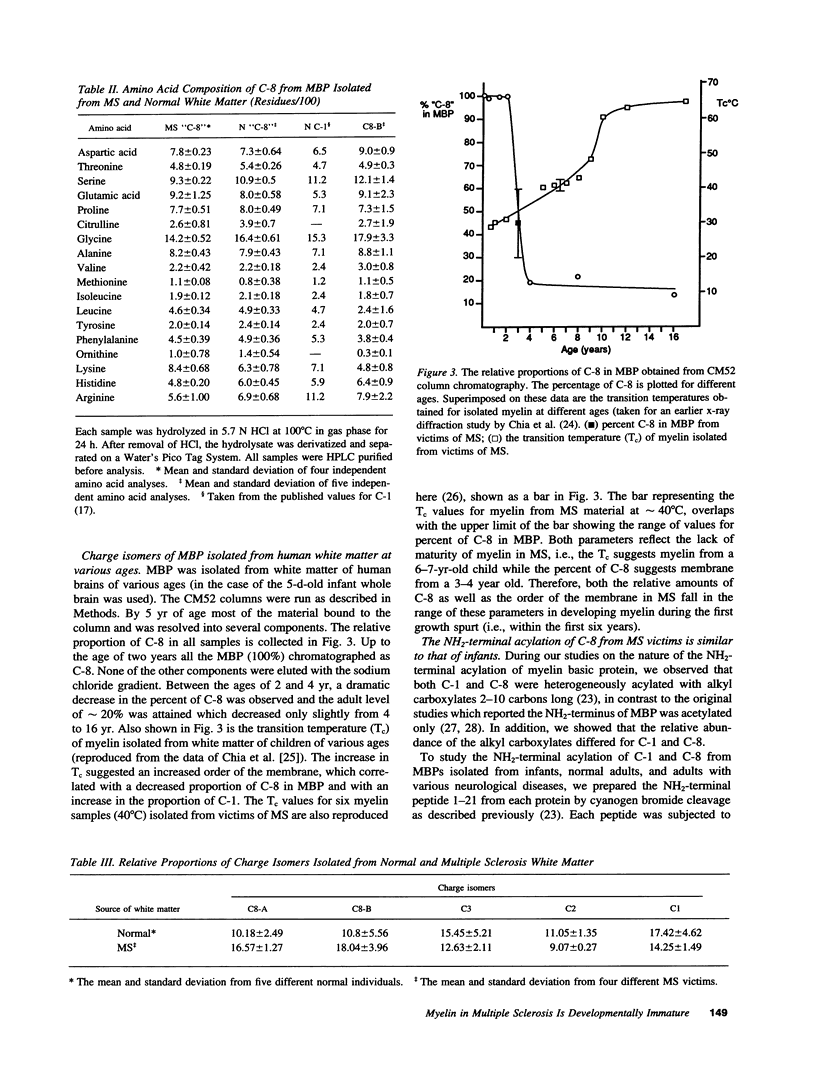
- Chia L. S., Thompson J. E., Moscarello M. A. Alteration of lipid-phase behavior in multiple sclerosis myelin revealed by wide-angle x-ray diffraction. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1871–1874. doi: 10.1073/pnas.81.6.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia L. S., Thompson J. E., Moscarello M. A. Changes in lipid phase behaviour in human myelin during maturation and aging. Involvement of lipid peroxidation. FEBS Lett. 1983 Jun 27;157(1):155–158. doi: 10.1016/0014-5793(83)81136-3. [DOI] [PubMed] [Google Scholar]
- Chou F. C., Chou C. H., Shapira R., Kibler R. F. Basis of microheterogeneity of myelin basic protein. J Biol Chem. 1976 May 10;251(9):2671–2679. [PubMed] [Google Scholar]
- Chou F. C., Chou C. H., Shapira R., Kibler R. F. Modifications of myelin basic protein which occur during its isolation. J Neurochem. 1977 May;28(5):1051–1059. doi: 10.1111/j.1471-4159.1977.tb10668.x. [DOI] [PubMed] [Google Scholar]
- Deibler G. E., Krutzsch H. C., Kies M. W. A new form of myelin basic protein found in human brain. J Neurochem. 1986 Oct;47(4):1219–1225. doi: 10.1111/j.1471-4159.1986.tb00743.x. [DOI] [PubMed] [Google Scholar]
- Deibler G. E., Martenson R. E., Kramer A. J., Kies M. W. The contribution of phosphorylation and loss of COOH-terminal arginine to the microheterogeneity of myelin basic protein. J Biol Chem. 1975 Oct 10;250(19):7931–7938. [PubMed] [Google Scholar]
- Elias S. B. Oligodendrocyte development and the natural history of multiple sclerosis. A new hypothesis for the pathogenesis of the disease. Arch Neurol. 1987 Dec;44(12):1294–1299. doi: 10.1001/archneur.1987.00520240066016. [DOI] [PubMed] [Google Scholar]
- Ellison G. W. Multiple sclerosis: why? Biomed Pharmacother. 1989;43(5):327–333. doi: 10.1016/0753-3322(89)90058-9. [DOI] [PubMed] [Google Scholar]
- Fannon A. M., Moscarello M. A. Characterization of myelin basic protein charge isomers from adult mouse brain. Neuroreport. 1991 Mar;2(3):135–138. doi: 10.1097/00001756-199103000-00006. [DOI] [PubMed] [Google Scholar]
- Fannon A. M., Moscarello M. A. Myelin basic protein is affected by reduced synthesis of myelin proteolipid protein in the jimpy mouse. Biochem J. 1990 May 15;268(1):105–110. doi: 10.1042/bj2680105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas S., Gordon J., Khalili K. A developmentally regulated DNA-binding protein from mouse brain stimulates myelin basic protein gene expression. Mol Cell Biol. 1993 May;13(5):3103–3112. doi: 10.1128/mcb.13.5.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashim G. A., Eylar E. H. The structure of the terminal regions of the encephalitogenic basic protein from bovine myelin. Arch Biochem Biophys. 1969 Dec;135(1):324–333. doi: 10.1016/0003-9861(69)90546-3. [DOI] [PubMed] [Google Scholar]
- Julien J., Ferrer X. Multiple sclerosis: an overview. Biomed Pharmacother. 1989;43(5):335–346. doi: 10.1016/0753-3322(89)90059-0. [DOI] [PubMed] [Google Scholar]
- Kamholz J., de Ferra F., Puckett C., Lazzarini R. Identification of three forms of human myelin basic protein by cDNA cloning. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4962–4966. doi: 10.1073/pnas.83.13.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlero De Rosbo N., Carnegie P. R., Bernard C. C., Linthicum D. S. Detection of various forms of brain myelin basic protein in vertebrates by electroimmunoblotting. Neurochem Res. 1984 Oct;9(10):1359–1369. doi: 10.1007/BF00964663. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamensa J. W., Moscarello M. A. Deimination of human myelin basic protein by a peptidylarginine deiminase from bovine brain. J Neurochem. 1993 Sep;61(3):987–996. doi: 10.1111/j.1471-4159.1993.tb03612.x. [DOI] [PubMed] [Google Scholar]
- Lowden J. A., Moscarello M. A., Morecki R. The isolation and characterization of an acid-soluble protein from myelin. Can J Biochem. 1966 May;44(5):567–577. doi: 10.1139/o66-068. [DOI] [PubMed] [Google Scholar]
- Martenson R. E., Gaitonde M. K. Electrophoretic analysis of the highly basic proteins of the rat brain fraction which induces experimental allergic encephalomyelitis. J Neurochem. 1969 Mar;16(3):333–347. doi: 10.1111/j.1471-4159.1969.tb10372.x. [DOI] [PubMed] [Google Scholar]
- McDonald W. I. The mystery of the origin of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1986 Feb;49(2):113–123. doi: 10.1136/jnnp.49.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
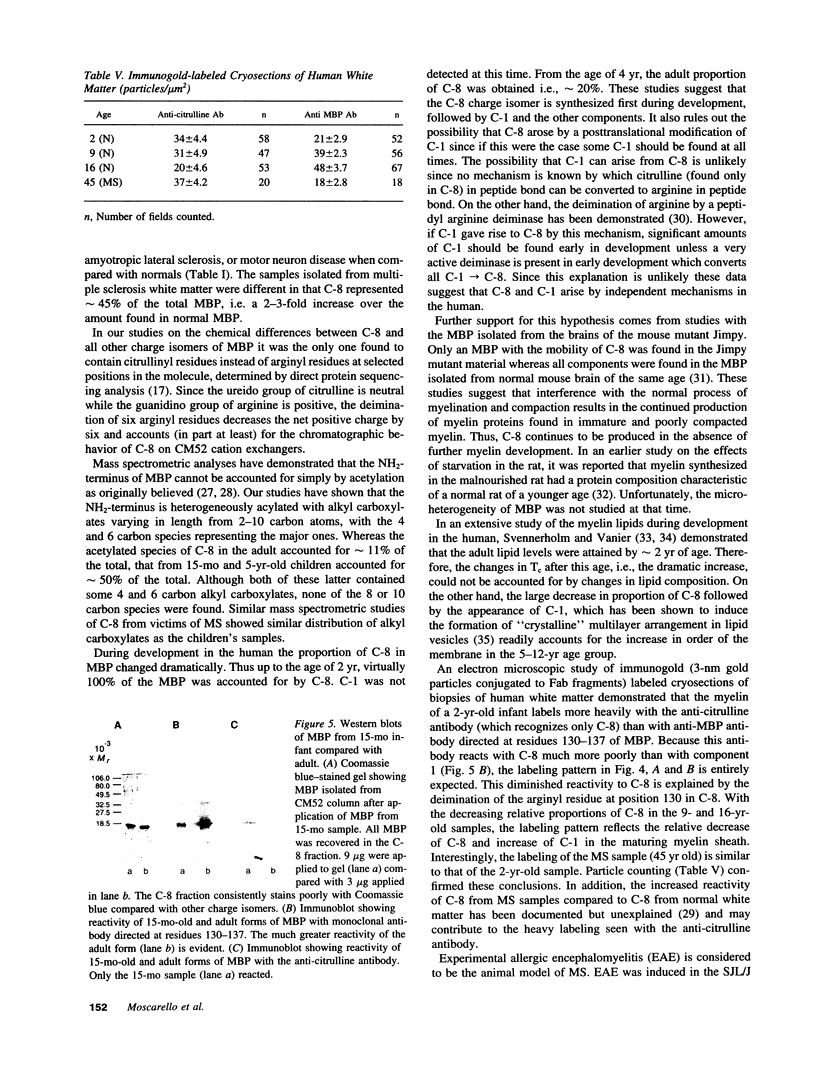
- McLaurin J., Ackerley C. A., Moscarello M. A. Localization of basic proteins in human myelin. J Neurosci Res. 1993 Aug 15;35(6):618–628. doi: 10.1002/jnr.490350605. [DOI] [PubMed] [Google Scholar]
- McLaurin J., Hashim G., Moscarello M. A. An antibody specific for component 8 of myelin basic protein from normal brain reacts strongly with component 8 from multiple sclerosis brain. J Neurochem. 1992 Oct;59(4):1414–1420. doi: 10.1111/j.1471-4159.1992.tb08455.x. [DOI] [PubMed] [Google Scholar]
- Moscarello M. A., Brady G. W., Fein D. B., Wood D. D., Cruz T. F. The role of charge microheterogeneity of basic protein in the formation and maintenance of the multilayered structure of myelin: a possible role in multiple sclerosis. J Neurosci Res. 1986;15(1):87–99. doi: 10.1002/jnr.490150109. [DOI] [PubMed] [Google Scholar]
- Moscarello M. A., Pang H., Pace-Asciak C. R., Wood D. D. The N terminus of human myelin basic protein consists of C2, C4, C6, and C8 alkyl carboxylic acids. J Biol Chem. 1992 May 15;267(14):9779–9782. [PubMed] [Google Scholar]
- Newcombe J., Glynn P., Cuzner M. L. The immunological identification of brain proteins on cellulose nitrate in human demyelinating disease. J Neurochem. 1982 Jan;38(1):267–274. doi: 10.1111/j.1471-4159.1982.tb10880.x. [DOI] [PubMed] [Google Scholar]
- Poser C. M. Multiple sclerosis. Observations and reflections--a personal memoir. J Neurol Sci. 1992 Feb;107(2):127–140. doi: 10.1016/0022-510x(92)90280-x. [DOI] [PubMed] [Google Scholar]
- Roth H. J., Kronquist K. E., Kerlero de Rosbo N., Crandall B. F., Campagnoni A. T. Evidence for the expression of four myelin basic protein variants in the developing human spinal cord through cDNA cloning. J Neurosci Res. 1987;17(4):321–328. doi: 10.1002/jnr.490170402. [DOI] [PubMed] [Google Scholar]
- Roth H. J., Kronquist K., Pretorius P. J., Crandall B. F., Campagnoni A. T. Isolation and characterization of a cDNA coding for a novel human 17.3K myelin basic protein (MBP) variant. J Neurosci Res. 1986;16(1):227–238. doi: 10.1002/jnr.490160120. [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Vanier M. T. The distribution of lipids in the human nervous system. 3. Fatty acid composition of phosphoglycerides of human foetal and infant brain. Brain Res. 1973 Feb 28;50(2):341–351. doi: 10.1016/0006-8993(73)90735-x. [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Vanier M. T. The distribution of lipids in the human nervous system. IV. Fatty acid composition of major sphingolipids of human infant brain. Brain Res. 1973 Jun 15;55(2):413–423. doi: 10.1016/0006-8993(73)90306-5. [DOI] [PubMed] [Google Scholar]
- Wiggins R. C., Fuller G. N. Early postnatal starvation causes lasting brain hypomyelination. J Neurochem. 1978 Jun;30(6):1231–1237. doi: 10.1111/j.1471-4159.1978.tb10450.x. [DOI] [PubMed] [Google Scholar]
- Wood D. D., Moscarello M. A. The isolation, characterization, and lipid-aggregating properties of a citrulline containing myelin basic protein. J Biol Chem. 1989 Mar 25;264(9):5121–5127. [PubMed] [Google Scholar]
- Young P. R., Snyder W. R., Vacante D. A., Waickus C. M., Zygas A. P., Grynspan F., Karunatilake C., Wilson D. H. The acid instability of myelin. A model for myelin degeneration in multiple sclerosis. Med Hypotheses. 1988 May;26(1):31–37. doi: 10.1016/0306-9877(88)90110-7. [DOI] [PubMed] [Google Scholar]