Abstract
Hypothesis
When cochlear implant (CI) users are allowed to self-select the “most intelligible” frequency-to-electrode table, some of them choose one that differs from the default frequency table that is normally used in clinical practice.
Background
Cochlear implants (CI) reproduce the tonotopicity of normal cochleas using frequency-to-electrode tables that assign stimulation of more basal electrodes to higher frequencies and more apical electrodes to lower frequency sounds. Current audiological practice uses a default frequency-to-electrode table for most patients. However, individual differences in cochlear size, neural survival and electrode positioning may result in different tables sounding most intelligible to different patients. No clinical tools currently exist to facilitate this fitting.
Methods
A software tool was designed that enables CI users to self-select a “most intelligible” frequency table. Users explore a two dimensional space that represents a range of different frequency tables. Unlike existing tools, this software enables users to interactively audition speech processed by different frequency tables, and quickly identify a preferred one. Pilot testing was performed in eleven long-term, postlingually deaf CI users.
Results
The software tool was designed, developed, tested and debugged. Patients successfully utilized the tool to sample frequency tables and to self-select tables deemed most intelligible, which for approximately half of the users differed from the clinical default.
Conclusion
A software tool allowing CI users to self-select frequency-to-electrode tables may help in fitting postlingually deaf users. This novel approach may transform current methods of CI fitting.
INTRODUCTION
Although cochlear implants (CI) provide substantial benefit to many users, there remains significant variability in patient outcomes1. This widespread variability is notable given that many aspects of patient care with CI users is done with a “one-size-fits-all” paradigm. For example, current surgical manufacturer manuals promote a uniform insertion depth of the electrode array for all patients. Markers along the electrode array are designed to be placed at the cochleostomy site, an aid to assist surgeons in a uniform insertion depth, measured in millimeters beyond the cochleostomy2–3. While the desire for uniformity in surgical procedure is generally desirable, it is entirely possible that the procedure recommended to achieve a uniform insertion depth may actually yield differing insertion angles for a ‘fully-inserted’ electrode. Such variations may occur because anatomical studies have shown the size of the cochlea to vary by as much as 40%4. A more recent study of cochlear duct length reported an average duct length of 33.5mm with a standard deviation of 2.28mm and a difference of 13.78mm between the shortest and longest5. Given that variations in insertion angle have been correlated with word-recognition scores in postlingually-deafened CI users6, it is possible that this “one-size-fits-all” approach may unintentionally contribute to the poorer speech perception performance seen in some CI users.
One side effect of differing electrode insertion depths is the possibility that in postlingually-deafened CI users, the degree of frequency mismatch can also vary. Frequency mismatches can occur in CI users when frequency of the input signals differ from the characteristic frequency of the neurons being stimulated by the implant’s electrodes. Cochlear implants attempt to reproduce the tonotopicity of normal cochleas by mapping input signal frequencies to electrode locations in the implanted cochlea. A frequency-to-electrode table is used to assign stimulation of more basal electrodes to high frequency inputs and stimulation of more apical electrodes to lower frequency inputs. In current clinical practice, most CI users are given a standard, or default frequency-to-electrode allocation table. If the insertion angle varies between patients because of differing cochlear sizes, however, then it is likely that the same electrode number may stimulate neural elements tuned to different frequencies in different patients.
The assumption underlying the use of a standard frequency table for all cochlear implant patients is that the human brain is sufficiently plastic to adapt to any frequency mismatch inadvertently imposed by their frequency table and electrode insertion depth. However, it is possible that a subset of patients will be unable to fully adapt to a frequency mismatch, raising the possibility that their speech-perception could be somewhat hindered. It is not practical to deal with the issue of frequency mismatch via re-insertion of the electrode array, although such an approach has been successfully used in a few extreme cases7–8. Rather, a better approach may be to identify patients who have not completely adapted to the frequency mismatch imposed by their device, and to modify the frequency allocation table so as to reduce the degree of frequency mismatch that patient experiences. Appropriate modifications of the frequency table have already been shown to aid speech understanding in CI users9–10. However, there are several issues with implementing modifications to the frequency table clinically. For example, what procedure would enable audiologists to identify patients who may have unadapted frequency mismatches? How would a frequency table that could potentially ameliorate any negative effects of frequency mismatch on speech understanding be obtained? Finally, how might both of these goals be achieved in a time-efficient manner?
One possible solution to these issues would be to allow patients to self-select a frequency table that maximizes perceived speech intelligibility. If patient-driven self-selections could be obtained in a timely fashion, this approach could address the issues denoted above provided we assume the following: If an experienced CI user selects a frequency table that deviates from the standard clinical table, then this patient may have a frequency mismatch to which they have not fully adapted. Unfortunately, current clinical tools that are used for fitting speech processors do not have the kind of flexibility that, for example, an optometrist has when fitting a patient for prescription eyeglasses. For example, in the fitting software for Advanced Bionics CIs (SoundWave Professional Suite) the frequency boundaries for the 14 intermediate electrodes (out of 16 total electrodes) are completely fixed. Current versions of the fitting software for Cochlear Corporation’s CIs (Custom Sound 3.0) do provide more flexibility for choosing frequency boundaries, but the audiologist’s manuals provide little guidance to clinicians as to which frequency allocations to select. As a result, the majority of CI users are fit with the standard frequency table recommended by the fitting software. Furthermore, even the more flexible versions of CI fitting software do not allow quick and convenient comparisons across a large number of frequency tables. These comparisons could potentially be used in the clinic to guide the selection of frequency tables, following the optometry model.
Given this situation, the goal of the present research study was to develop a new software tool which could address these issues, and may allow for customized fitting of frequency allocation tables in postlingually deafened CI users. The study also aimed to bench test the functionality of the newly developed tool to ensure that the software and hardware worked to specification and, finally, conduct feasibility tests with actual CI users.
MATERIALS AND METHODS
A software tool which we have named Gridstream was designed and created to allow CI users to self-select frequency allocation tables using a novel interface. With the new software tool, a sample sound file is provided offline as input to the program, which then processes the sound using a variety of frequency allocation tables, and prepares outputs for presentation to the end listener. Outputs can be processed for presentation as either direct electrical stimulation to a CI user, or represented in an acoustic model format for testing of normal hearing listeners.
Programming for this tool was completed using MATLAB (The MathWorks, Inc.) and the Nucleus Implant Communicator (NIC) v2 software interface with associated Nucleus MATLAB Toolbox v4.20 (Cochlear). The NIC software provides a programming interface to communicate with the cochlear implant device, both at the speech processing strategy level or at a lower level to directly create sequences of electrode stimulation instructions, and the ability to programmatically stream these instructions directly to a device.
Filterbank processing
Sound input is processed by frequency analysis filterbanks to create unique stimuli, each representing a different frequency to electrode table. Filterbanks for the acoustic model were based on those previously used11–12. The first filterbank to be created was identical to the standard frequency table for the Nucleus Freedom device (Cochlear Corp.).
A range of filterbanks was then created that differed from this standard analysis filter by varying both the “span” of the filter and the “midpoint.” The “span” of the filters represents the difference between the minimum and maximum frequencies of the filterbank. If the minimum and maximum frequencies are considered as points along the basilar membrane, estimated as distances from the cochlear apex by Greenwood’s equation13, then the “span” of a filter bank can also be represented by a distance along the cochlea. So, filter banks with larger“spans” would cover a broader range of frequencies, or a greater distance along the basilar membrane.
In addition to changing the span, filterbanks were created that varied in their midpoint. According to the frequency place map in an average sized human basilar membrane, the default analysis filter would have a midpoint correlating to 16.9mm from the cochlear apex. Moving the midpoint higher or lower would therefore result in shifting the frequency table higher or lower in frequency. For any given “span”, several filterbanks were created that varied by their midpoint, with both basalward and apical shifts (higher and lower in frequency), corresponding to cochlear distances between 10.7mm and 26.7mm.The combination of varying spans and midpoints resulted in the creation of 288 different filterbanks, each essentially representing a different frequency to electrode table.
Filterbank processing was then applied to production of both acoustic and electrical stimulation patterns. Integrated into the NIC interface, sound samples were passed through each individual filterbank and the resulting electrode stimulation patterns written to disk in the NIC interface’s specified XML format. For acoustic presentation, the temporal envelope was extracted for each filterbank and used to modulate eight noise bands representing the CI electrodes, providing an acoustic simulation of each filterbanks’ output, saved as wav files. Each input wave file had 200 ms of silence in the beginning in order to ensure that the CI would be powered up by the time actual speech stimulation starts. For electrical stimulation, the default stimulation rate is 7200 Hz, although other rates can be utilized. Threshold and comfort levels are obtained for each electrode from the patients clinical map, and are incorporated into the signal processing.
Graphical User Interface
The graphical user interface begins with a setup window for entering patient and tester information, selecting acoustic or electrical output, and choosing a particular sound file sample for testing. The software then presents a two dimensional grid of buttons to the user, each button representing the sample sound file processed by a different frequency table. The buttons are systematically laid out, with movements in the X axis corresponding to differences in frequency span (e.g., overall frequency range) and movements in the Y axis corresponding to differences in the “midpoint” of the filterbank (higher or lower in frequency for a given range). The program can randomize the directionality of these changes in filterbank after a frequency table selection has been finalized, to ensure that the listener cannot rely on the absolute position within the grid if he is asked to repeat the selection process. By clicking on any given button in the grid, the subject is presented with the acoustic or electrical stimulation representing the sound processed by that particular frequency to electrode table. In this way, users can quickly audition and explore a wide range of frequency tables. A visual depiction of the graphical user interface employed here is provided in Figure 1.
Figure 1.
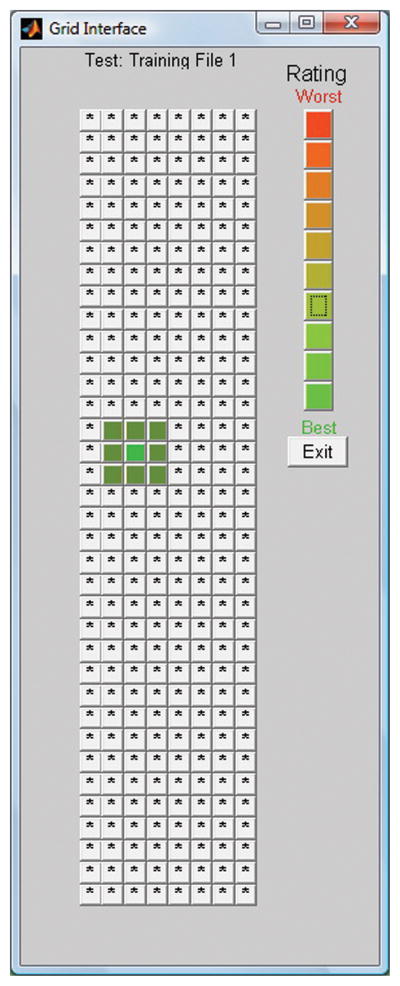
A depiction of the graphical user interface that the CI patient sees when using Gridstream. Each square within the grid represents the same sentence processed with a different frequency table. When the frequency tables are not randomized, as the patient moves from left to right within the grid, the overall bandwidth (span) increases. Conversely, as one moves from top to bottom, the frequency range within a given span becomes higher in frequency. Listeners can rate the intelligibility of a given table by right-clicking on a given rating within the rating scale shown on the upper right of the grid.
In addition to the frequency-table selections, this tool also offers listeners the opportunity to provide a subjective rating of the quality of speech heard with a given frequency table. In the upper right hand corner of the GUI, a set of ten colored buttons varying in hue from red (top) to green (bottom) are available. Upon selecting a given frequency table, listeners can then ‘color in’ that point in the grid with their subjective evaluation of that particular frequency table. Subjects can interactively search the grid, comparing different frequency tables and rating them (green for best, red for worst sounding), to find a frequency table, or a range of frequency tables, which maximizes speech intelligibility for them.
Equipment
Over several months of testing, Gridstream was designed, developed and underwent rounds of debugging. Testing was initially performed utilizing a clinical programming system capture device (Cochlear), which accepts the radiofrequency signals from a CI transmitting coil and represents individual electrode stimuli in a panel of blinking LEDs. This provided a gross depiction of electrical output stimulation, which is appropriate to test that the frequency content of steady state input signals is adequately represented. More refined testing was completed using a Nucleus-24 implant-in–a-box which is a CI receiver that is connected to a bank of resistors instead of being implanted in a real patient. Electrode outputs were connected to an oscilloscope. Gridstream runs on a PC computer, attached to a Cochlear Programming pod (Nucleus 24), which is the equipment that serves as the interface between the computer and the patient’s speech processor. An experimental L34 processor provided by the manufacturer was used for all testing, allowing for theoretically unlimited streaming of speech data.
Listeners
A total of eleven post-lingually deafened CI users have been tested to date with Gridstream. Table 1 shows the unaided pure tone average of the unimplanted ear, duration of CI use, age at testing, and duration of hearing aid use. With the exception of S5, who had used his CI for only 5 months, all the other patients had reached asymptotic performance on tests of speech understanding prior to participating in this experiment (as measured by speech perception tests in their last two clinical evaluations). Given the long-term device use and stable level of performance exhibited by these patients, we hypothesize that self-selection of a frequency table different from the standard represents some form of frequency distortion or mismatch to which the patient has not yet adapted.
Table 1.
Patient Characteristics
| Subject | PTA of better ear (dB) | Duration of CI use | Age at testing | Duration of Hearing Aid use (years) |
|---|---|---|---|---|
| S1 | 63 | 13 months | 73 | 18 |
| S2 | >100 | 7 years | 63 | 52 |
| S2 | 80 | 7 years | 35 | 7 |
| S4 | >100 | 12 years | 80 | 11 |
| S5 | >100 | 9 years | 68 | 26 |
| S6 | >100 | 18 months | 54 | 48 |
| S7 | 73 | 5 months | 51 | 36 |
| S8 | >100 | 23 months | 74 | 33 |
| S9 | 90 | 3 years | 28 | 20 |
| S10 | n/a | 5 years | 88 | 40 |
| S11 | >100 | 9 years | 42 | 42 |
PTA, pure-tone average; CI, cochlear implant
RESULTS
Gridstream was developed and successfully tested. The graphical user interface functionality worked as expected and electronic logging of user input and test results was accurately recorded. Electrical stimulation of the clinical programming device revealed appropriate timing and delivery of streaming speech. Output was then monitored using using the implant-in-a-box, and voltages were measured with an oscilloscope, showing that speech was being successfully streamed. Further testing revealed that the initial elements of a streamed speech file were not being delivered to the CI. As a result, the software was adjusted to provide 200ms of silence as a buffer before each speech sequence after which no further clipping of speech information was found.
Our first goal was to ensure that the GridStream system was able to stimulate patients at an appropriate level of loudness without truncation of the speech signal or other processing errors. Regarding the stimulation process itself, all patients were able to navigate the grid interface with a comfortable loudness level without any incidences of overstimulation. Either no instances, or very few instances of clipping of speech samples were noted by these patients during testing. However, a brief delay of approximately one second was noted between the user input (mouse click) to presentation of speech samples to CI users.
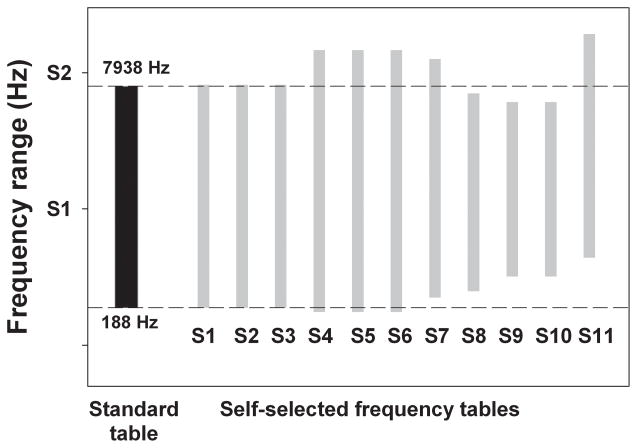
Our second goal was to observe whether patients could successfully utilize the Gridstream system to obtain a self-selected frequency table. Here, all 11 patients successfully explored the two dimensional space to obtain a self-selected frequency to electrode table that they believed maximized speech intelligibility. Some patients explored the two-dimensional space independent of investigator control, while others directed the self-selection with verbal comments as the investigator controlled the presentation of different frequency tables. The first self-selection took between 10–15 minutes for most listeners, which included initial instructions concerning the nature of their task. The second self-selection, done with a different sentence, took between 4–8 minutes. A representation of the frequency to electrode tables that the 11 patients chose is depicted in Figure 2. The dotted horizontal lines bracket the frequency range for the standard clinical frequency table for the Nucleus Freedom device (188–7938 Hz; shaded rectangle), while the grey rectangles represent self-selected frequency tables for each of the 11 patients tested here.
Figure 2.
Frequency range of the standard clinical table for the Nucleus Freedom device (shaded left column), and the self-selected frequency tables for the 11 patients tested to date (thin gray rectangles). Dashed lines indicate the edges of the standard clinical table.
There are several points of interest in this figure. First, three of eleven patients selected the exact same frequency table which they were wearing in their speech processor. Second, an additional three individuals selected a table with a low-frequency edge very similar to that of the standard table, while the high-frequency edge is elevated relative to the standard table. Taken together, 6 of 11 patients appeared to prefer a table that is quite similar to the standard table which they are using on a daily basis, suggesting that these patients have adapted completely, or almost completely, to their clinical frequency table and therefore have no residual frequency mismatch. More notable, however, are the self-selected tables from the other 5 patients, which are all shifted higher in frequency than the standard, and some of these tables are also compressed in frequency range as well. These differences suggest that these patients may possess some uncompensated frequency mismatch.
DISCUSSION
In the current experiment, our goal was to develop a tool which could be used to obtain custom-fit frequency tables in individual patients in a time-efficient manner. The present data reflect a significant engineering effort, and both bench tests and preliminary data obtained thus far suggest that our initial version of the Gridstream system is safe for patient use. We speculate that this may be one such tool which can successfully identify patients who have a frequency mismatch to which they have not fully adapted.
The issue of the ability of individuals to adapt to a frequency mismatch has been widely explored, yet the implications of this issue have yet to be fully resolved for postlingually deafened CI users. On one hand, there is clear evidence that patients can adapt to frequency distortions at least partially. Evidence for such adaptation to frequency mismatch has been observed with a number of different experimental paradigms, including 1) longitudinal examination and mathematical modeling of vowel identification and electrode discrimination after initial stimulation14, 2) an analysis of the first and second formant values that CI patients report correspond to a given vowel such analyses have been conducted in both experienced15 and newly-implanted patients16. 3) changes over time in the perceived pitch elicited by electrical stimulation17–18, 4) comparison of vowel perception with different frequency tables before and after training19, and 5) long term studies of speech perception after imposition of a large frequency shift via an experimental frequency table20.
On the other hand, there is also evidence indicating that not all patients can adapt to a frequency mismatch, particularly if that mismatch is large. Clear support for this concept stems from 1) the inability of three experienced CI users to fully adapt to an experimental frequency table with a severe frequency shift20–21, 2) the poorer speech-understanding abilities in patients with short insertion depths, presumably because this induces a larger frequency mismatch when a standard frequency table is used6, 3) the improved speech perception scores observed when frequency tables were reprogrammed to ideally accommodate from a frequency shift imposed by overly deep electrode insertions7,10, and 4) the observation that while in many CI users, the pitch percepts elicited by electrical stimulation match what would be predicted from their standard frequency table, approximately half of individuals have pitch percepts that differ from their frequency table17–18, 22–23.
Given our hypothesis that an experienced CI user may have a frequency mismatch to which they have not fully adapted if they select a frequency table that deviates from the standard clinical table, then the present data suggest that as many as 50% of postlingually deafened CI patients may have some form of uncompensated mismatch. Such an estimate is consistent with the pitch-matching data obtained in experienced CI users who have sufficient residual hearing to make reliable pitch matches, as approximately 50% of those patients have pitch-matched values that differ from what would be predicted from their standard frequency table17–18, 22–23. It is unclear at this time whether, in these patients, speech-understanding abilities can be improved using a frequency table that reduces a frequency mismatch. Until now there have been no clinical fitting tools that allow the presentation of speech samples using different frequency tables in a quick and efficient manner, which is a prerequisite for optimizing frequency-table selection. The Gridstream tool described in this manuscript has shown its feasibility as a new instrument that has the potential to do so, and may therefore allow for improved customization of CI fitting to individual patients.
Acknowledgments
This research was supported by AAO-HNS Core Resident Research Grant (awarded to D.J.), and by NIH/NIDCD (R01 DC003937 awarded to M.A.S., and K99 DC009459 awarded to M.B.F). The corresponding author is the author of a patent for a system to help select frequency-to-electrode tables in cochlear implants, but has not received any royalties and has pledged to NYU’s committee of conflicts of interest that he will donate to charity any potential future royalties. Cochlear Americas generously provided us with the NIC research system, the L34 processor, and with other valuable technical resources and assistance. We also acknowledge the invaluable assistance of NYU’s Cochlear Implant Center, its Co-Directors J. Thomas Roland, MD and Susan B. Waltzman, PhD, and its chief audiologist, William Shapiro, AuD.
References
- 1.Firszt JB, Holden LK, et al. Recognition of speech presented at soft to loud levels by adult cochlear implant recipients of three cochlear implant systems. Ear and Hearing. 2004;25(4):375–87. doi: 10.1097/01.aud.0000134552.22205.ee. [DOI] [PubMed] [Google Scholar]
- 2.Cochear: Nucleus CI512 cochlear implant with Contour Advance electrode Surgeons Guide. Cochlear Americas; [Google Scholar]
- 3.HiRes 90K Surgeon’s Manual for the HiFocus Helix and HiFocus 1j Electrodes C2005; Advanced Bionics Corp.
- 4.Hardy M. The length of the organ of Corti in man. American Journal of Anatomy. 1938;62:291–311. [Google Scholar]
- 5.Miller JD. Sex differences in the length of the organ of Corti in humans. J Acoust Soc Am. 2007;121(4):EL151–5. doi: 10.1121/1.2710746. [DOI] [PubMed] [Google Scholar]
- 6.Skinner MW, Ketten DR, et al. CT-derived estimation of cochlear morphology and electrode array position in relation to word recognition in Nucleus-22 recipients. J Assoc Res Otolaryngol. 2002;3(3):332–50. doi: 10.1007/s101620020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kos M-I, et al. Partial withdrawal of deeply inserted cochlear electrodes: observations of two patients. Eur Arch Otorhinolaryngol. 2007;264(11):1369–72. doi: 10.1007/s00405-007-0354-5. [DOI] [PubMed] [Google Scholar]
- 8.Roland JT, et al. Large speech perception improvement after reimplantation of a cochlear implant user with extremely deep electrode insertion. Presented at the House Working Group Conference.2007. [Google Scholar]
- 9.Skinner MW, Holden LK, Holden TA. Effect of frequency boundary on speech recognition with the SPEAK speech-coding strategy. Ann Otolo Rhinol Laryngol Suppl. 1995;166:307–311. [PubMed] [Google Scholar]
- 10.Gani M, Valentini G, Sigrist A, Kos M-I, Boex C. Implications of deep electrode insertion on cochlear implant fitting. J Assoc Res Otolaryngol. 2007;8(1):69–83. doi: 10.1007/s10162-006-0065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzgerald MB, Morbiwala TA, Svirsky MA. Customized selection of frequency maps in an acoustic cochlear implant simulation. Proceedings of the IEEE 2006 International Conference of the Engineering in Medicine and Biology Society; New York, NY, USA. Aug 30–Sept 3, 2006; 2006. pp. 3596–3599. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald MB, Tan C-T, Morbiwala TA, Svirsky MA. Listener-selected frequency maps and modeling of vowel identification in an acoustic simulation of a cochlear implant. Ear Hear (submitted) [Google Scholar]
- 13.Greenwood DD. A cochlear frequency-position function for several species--29 years later. Journal of the Acoustical Society of America. 1990;87(6):2592–605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- 14.Svirsky MA, Silveira A, et al. Auditory learning and adaptation after cochlear implantation: a preliminary study of discrimination and labeling of vowel sounds by cochlear implant users. Acta Otolaryngol. 2001;121(2):262–5. doi: 10.1080/000164801300043767. [DOI] [PubMed] [Google Scholar]
- 15.Harnsberger JD, Svirsky MA, et al. Perceptual “vowel spaces” of cochlear implant users: implications for the study of auditory adaptation to spectral shift. Journal of the Acoustical Society of America. 2001;109(5 Pt 1):2135–45. doi: 10.1121/1.1350403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svirsky MA, Silveira A, et al. Long-term auditory adaptation to a modified peripheral frequency map. Acta Otolaryngol. 2004;124(4):381–6. [PubMed] [Google Scholar]
- 17.Reiss LAJ, Turner CW, Erenberg SR, Gantz BJ. Changes in pitch with a cochlear implant over time. JARO. 2007;8(2):241–257. doi: 10.1007/s10162-007-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiss LAJ, Gantz BJ, Turner CW. Cochlear implant speech processor frequency allocations may influence pitch perception. Otology & Neurotology. 2008;29(2):160–167. doi: 10.1097/mao.0b013e31815aedf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu Q-J, Galvin JJ. Perceptual learning and auditory training in cochlear implant recipients. Trends in Amplification. 2007;11:193–205. doi: 10.1177/1084713807301379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu QJ, Shannon RV, Galvin JJ. Perceptual learning following changes in the frequency-to-electrode assignment with the Nucleus-22 cochlear implant. Journal of the Acoustical Society of America. 2002;112(4):1664–1674. doi: 10.1121/1.1502901. [DOI] [PubMed] [Google Scholar]
- 21.Sagi E, Fu QJ, Galvin JJ, III, Svirsky MA. A model of incomplete adaptation to a severely shifted frequency-to-electrode mapping by cochlear implant users. JARO; 2009. Published online ahead of print: 23 September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francart T, Brokx J, Wouters J. Sensitivity to interaural level difference and loudness growth with bilateral bimodal stimulation. Audiology and Neurotology. 2008;13:309–319. doi: 10.1159/000124279. [DOI] [PubMed] [Google Scholar]
- 23.McDermott H, Sucher C, Simpson A. Electro-acoustic stimulation acoustic and electric pitch comparisons. Audiology and Neurotology. 2009;14(suppl 1):2–7. doi: 10.1159/000206489. [DOI] [PubMed] [Google Scholar]