Abstract
Most functional magnetic resonance imaging (fMRI) studies in animals are conducted under anesthesia to minimize motion artifacts. However, methods and techniques have been developed recently for imaging fully conscious rats. Functional MRI studies on conscious animals report enhanced BOLD signal changes as compared to the anesthetized condition. In this study, rats were exposed to different concentrations of carbon dioxide (CO2) while conscious and anesthetized to test whether cerebrovascular reactivity may be contributing to these enhanced BOLD signal changes. Hypercapnia produced significantly greater increases in MRI signal intensity in fully conscious animals (6.7–13.3% changes) as when anesthetized with 1% isoflurane (3.2–4.9% changes). In addition, the response to hypercapnia was more immediate in the conscious condition (< 30s) with signal risetimes twice as fast as in the anesthetized state (60s). Both cortical and subcortical brain regions showed a robust, dose- dependent increase in MRI signal intensity with hypercapnic challenge while the animals were conscious but little or no change when anesthetized. Baseline variations in MRI signal were higher while animals were conscious but this was off set by greater signal intensity changes leading to a greater contrast-to-noise ratio, 13.1 in conscious animals, as compared to 8.0 in the anesthetized condition. In summary, cerebral vasculature appears to be more sensitive to hypercapnic challenge in the conscious condition resulting in enhanced T2* MRI signal intensity and the potential for better BOLD signal changes during functional imaging.
Keywords: Functional magnetic resonance imaging, Vascular reactivity, Bold signal intensity, Regional cerebral blood flow
1. Introduction
Head movement in the field of view or disturbances in magnetic field homogeneity caused by respiration, swallowing and muscle contractions in the face and neck are the major sources of motion artifact in fMRI [1–3]. While motion artifact is problematic in human imaging studies, it is a major limitation in animal imaging. Animals, unlike humans, must be physically restrained to minimize motion artifacts during an imaging session. Consequently, a majority of animal imaging studies have been done under anesthetized conditions. Unfortunately, anesthesia precludes the investigation of cognitive processes that are critical to the study of the brain and, in addition, anesthesia alters both the cellular and cardiovascular components contributing to the fMRI signal.
Functional MRI is a technique sensitive to the oxygenation status of hemoglobin and therefore produces images that reflect changes in cerebral blood flow and volume. The general anesthetics commonly used in animal fMRI studies depress CNS metabolic activity causing a reduction in basal cerebral blood flow [4,5] and BOLD signal intensity [6]. Even an anesthetic known to preserve neuronal function, α-chloralose, reduces blood flow and metabolism in certain brain regions [7].
Recently, technologies and methods have been developed for imaging conscious rats [6–10] and monkeys [12–16]. Studies comparing conscious vs. anesthetized conditions in the same animal during a single imaging session report a greater BOLD signal change in the conscious condition as compared to the anesthetized state [8,17]. The enhanced BOLD signal change in conscious animals is most likely due to elevation in cerebral blood flow to areas of activation. Data obtained from laser-Doppler studies show large increases in cerebral blood flow in conscious animals as compared to the anesthetized condition [5]. Enhanced cerebral blood flow in the conscious condition may reflect an increase in cerebrovascular reactivity that is normally depressed with anesthetics [18]. The effect of anesthesia on cerebrovascular reactivity and cerebral blood flow can be studied with MRI and hypercapnic challenge.
Cerebral arterial smooth muscle is very sensitive to the partial pressure of CO2 in the blood. In the presence of carbonic anhydrase, elevated CO2 is rapidly hydrated to form carbonic acid and its dissociation products, bicarbonate and hydrogen ions. The local acidic environment enhances the vasodilatory effects of adenosine [19] and increases potassium ion conductance across vascular smooth muscle [20] resulting in a passive dilation of blood vessels, decreased resistance and increased blood flow [21]. Indeed, the BOLD effect is mediated in part by metabolic changes in pH affecting cerebrovascular reactivity. Since hypercapnic challenge does not alter metabolic oxygen consumption [22] the change in T2* MRI signal caused by enhanced cerebral blood flow is directly related to the change in PaCO2 [23]. Mapping changes in T2* MRI signal in response to hypercapnic challenge is a simple, robust method for assessing cerebrovascular reactivity in functional imaging studies [24].
In the present study, animals were challenged with gas mixtures of 5% and 10% CO2 while conscious and anesthetized during a single imaging session. Cerebrovascular reactivity was much greater in the conscious condition as measured by dose-dependent changes in MRI signal to cortical and subcortical brain regions. While baseline signal-to-noise was higher in the conscious condition, the contrast-to-noise ratio of the hypercapnic challenge was equal to and greater than that of the anesthetized state. These findings indicate the enhanced T2* signal observed in conscious animals during hypercapnic challenge is due to increased cereborvascular reactivity.
2. Methods
2.1. Animal preparation
Male Sprague-Dawley rats weighing 200–300 gm were obtained from Charles River Laboratories (Charles River, MA). Animals were housed in pairs, maintained on 12:12 light:dark cycle (lights on at 9:00 h) and provided food and water ad libitum. All animals were acquired and cared for in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications No. 85-23, Revised 1985).
On the day of imaging, animals (n = 6) were anesthetized with an IM injection of medetomidine, (Domitor®, 1 mg/kg; Pfizer) and ketamine (10 mg/kg) and the femoral artery catheterized. The incision was sutured and treated with 2% lidocaine gel, a topical anesthetic. Following surgery animals were placed into a restrainer consisting of a multiconcentric, acrylic head and body holder with built-in dual coil radiofrequency electronics fitted with a ventilation tube for gas inhalation (Insight Neuroimaging Systems, LLC, Worcester MA). When secured in the restrainer, the anesthesia was reversed with an IM injection of atipamezole (Antisedan®, 5 mg/kg; Pfizer). The duration of metedomidine anesthesia was approximately 30 min. Animals were monitored for pulse oximetry, capnography and respiration for the duration of the experiment. Heart rate was measured with a pulse oximeter. Blood samples (0.1 mL) were drawn prior to and at the end of each imaging trial and analyzed for PaO2, PaCO2 and pH using an I-Stat PCA system (I-Stat Corp). Respiratory rate and the relative force of respiration were collected with a piezo electric force transducer mounted on the animal's thorax that output to an amplified voltage waveform. A mean peak to peak time calculation for baseline and hypercapnic conditions produced the respiratory rate. The respiratory force was calculated as the mean baseline to peak signal change (in volts) and normalized to arbitrary units in order to calculate the percent change between baseline and hypercapnic conditions.
2.2. Imaging
Experiments were conducted in a Bruker Biospec 4.7T animal imager with a 20G/cm gradient set. Anatomic, fast spin echo images were acquired once for each animal (TR = 3 s, TE = 48 msec, echo train length = 8,8 averages, 256 × 256 matrix, 2.7 × 2.7 cm FOV, 2 slices, 1.5 mm slice thickness). BOLD fMRI images were acquired with a two-segment, gradient-echo planar image (TR = 1 s, TE = 25 msec, 128 × 128 matrix, 2.7 × 2.7 cm FOV, 2 slices, 1.5 mm slice thickness). The functional EPI sequence was repeated 100 times with an acquisition time of 2 sec for each repeat. Each of these 100 repetitions took 200 s and constituted an imaging trail. Functional images were collected using the same geometry as the anatomic scan. For the first 50 repetitions, rats inhaled ambient air. For the last 50 repetitions, the air was premixed with 5 or 10% CO2 (Air-gas). The mole fraction of O2 in these gas mixtures was maintained at 21% by reducing the fraction of N2. The presentation of different CO2 mixtures was counter balanced. At the end of an imaging trial, the CO2 was turned off and the animals were given 10 min to recover. After 2–3 trials in the conscious condition, animals were anesthetized with 2% isoflurane. Anesthesia was confirmed by a reduction in the breathing rate and the absence of a withdrawal reflex in response to tail pinch. After 2–3 imaging trials, the anesthesia was discontinued. When animals regained consciousness (ca. 5–10 min) 2–3 more trials were conducted followed by a final set of 2–3 trials during anesthesia.
2.3. Data analysis
All imaging trials were viewed and assessed for motion artifact. Two of the twenty- three imaging trials during the conscious condition were excluded for animal movement. The first three repetitions were eliminated from each trial because the acquisition was not at steady state. Using Stimulate [25], Two regions of interest (ROIs) were defined for each animal (Fig. 1). One ROI covered all cortical regions above the corpus callosum and outside of the external capsule, while the second encompassed subcortical brain regions below the corpus callosum and inside the external capsule. Mean pixel intensity was calculated using every pixel within the ROI and tabulated for each repetition. Eight of 2100 total repetitions were eliminated from the conscious condition for being more then 6 standard deviations from neighboring repetitions. These single point errors were the result of motion related phase artifacts.
Fig. 1.
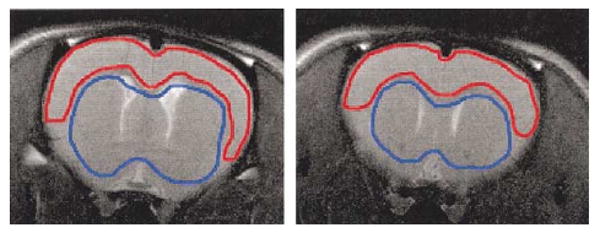
Cortical (red) and subcortical (blue) regions of interest outlined on an anatomic scan.
Percent change, risetime, and contrast-to-noise ratios were calculated for each imaging trial. The baseline mean and standard deviation was calculated for the period from repetitions 3–49. The risetime was calculated as the time from repetition 50 until the data reached one standard deviation of the last 10 points in the trial. The hypercapniainduced changes in MRI signal were calculated as the mean of the signal from the end of the risetime until the end of the imaging trial. Data were tabulated for each of the four groups; conscious inhaling 5% CO2, conscious inhaling 10% CO2, anesthetized inhaling 5% CO2, and anesthetized inhaling 10% CO2. For each time course, the pixel intensities were normalized by dividing each value by the mean of the baseline. The time courses for all the trials from each condition were averaged. Physiological data were tabulated at time points just before each imaging trial, during the baseline period, and during hypercapnia.
3. Results
3.1. Physiological measures
Table 1 summarizes the physiological data collected prior to and during the imaging trials for each of the four conditions. There was a significant decrease in blood pH for both conscious and anesthetized conditions during inhalation of CO2 corresponding to a concentration-dependent increase in PaCO2. There was also a significant concentration- dependent, linear increase in the rate of respiration in the anesthetized condition but not the conscious conditions in response to hypercapnia. However, there was a significant increase in the force of respiration in the conscious condition suggesting an increase in tidal volume. This increase in the force of respiration was not observed in the anesthetized condition. The enhanced rate and depth of respiration in the anesthetized and conscious conditions, respectively, in response to hypercapnic challenge was accompanied by an increase in PaO2. There was also a significant bradycardia in response to hypercapnia for both conscious and anesthetized conditions.
Table 1.
Physiologic changes under conscious and anesthetized conditions before and during hypercapnia. Respiratory force for 5 and 10% CO2 was calculated as a percent change from baseline (Before). Shown are the mean ± standard deviation (parenthesis) for each physiological measure
| Before | 5% CO2 | 10% CO2 | |
|---|---|---|---|
| Conscious | |||
| Arterial pH | 7.246 (.05) | 7.133 (.089)* | 6.975 (.088)** |
| Arterial PO2 (mmHg) | 96 (15) | 129 (16)** | 131 (6)** |
| Arterial PCO2 (mmHg) | 38 (6) | 47 (9)* | 88 (12)** |
| Respiratory rate (BPM) | 97 (2) | 103 (14) | 96 (9) |
| % Change in Respiratory Force | 93 (61) | 157 (127) | |
| Heart rate (BPM) | 332 (29) | 283 (31)* | 243 (29)** |
| Anesthetized | |||
| Arterial pH | 7.161 (.063) | 7.102 (.070)** | 7.018 (.039)** |
| Arterial PO2 (mmHg) | 105 (25) | 126 (33) | 141 (36)* |
| Arterial PCO2 (mmHg) | 49 (13) | 62 (18) | 87 (8.7)** |
| Respiratory rate (BPM) | 30 (2) | 47 (6)** | 62 (6)** |
| % Change in Respiratory Force | 12 (23) | 13 (33) | |
| Heart rate (BPM) | 233 (10) | 215 (6)** | 208 (10)* |
P < .05,
< .01005.
3.2. MRI data
All conditions showed a significant increase in MRI signal in response to hypercapnia as presented in Fig. 2. However, there was an ostensible difference in cerebrovascular reactivity between conscious and anesthetized conditions. There was a marked and rapid concentration-dependent increase in MRI signal in the conscious condition that was suppressed with anesthesia. MRI signal increased by an average of 13.3% in response to 10% CO2 in the conscious condition as compared to an average of 4.9% during anesthesia.
Fig. 2.
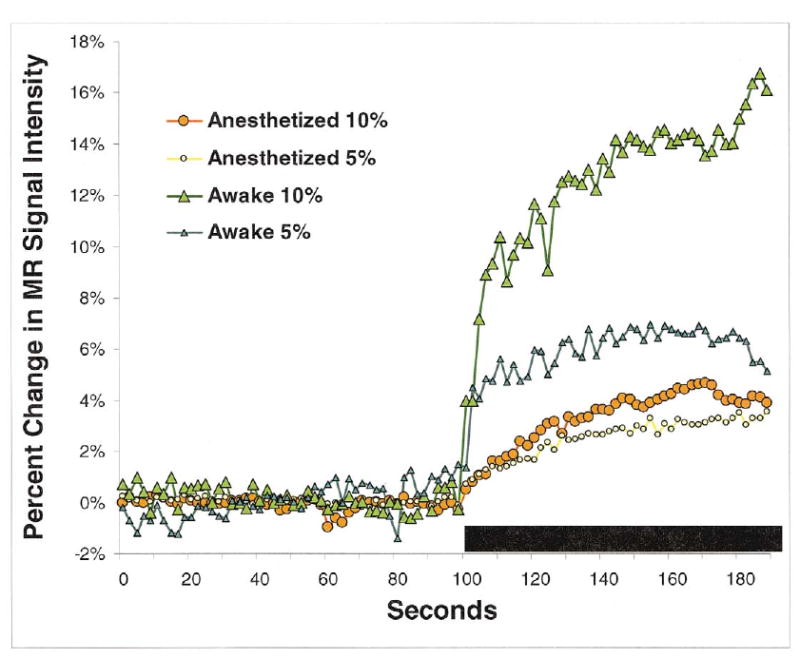
Time course of CO2 inhalation under anesthetized and conscious conditions. Raw cortical signal intensities averaged for all animals are shown for 100 repetitions over 200 s. The bar indicates the time of exposure to CO2.
The average risetime in MRI signal was more than twice as fast under conscious conditions as compared to anesthetized (Table 2). The contrast-to-noise ratio in the conscious condition was greater with a 10% CO2 challenge compared to anesthesia. The contrast-to-noise was similar between conscious and anesthetized conditions at 5% CO2.
Table 2.
Summary of MRI signal changes under conscious and anesthetized conditions. The rate of increase (risetime), and magnitude of T2* MRI signal change (% change) and contrast-to-noise (CNR) is significantly higher in conscious animals for both CO2 concentrations. Data are presented as mean ± standard deviation (parenthesis).
| 5% CO2 | 10% CO2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Cortical | Subcortical | Cortical | Subcortical | |||||
| Conscious | Anesth. | Conscious | Anesth. | Conscious | Anesth. | Conscious | Anesth. | |
| Risetime(s) | 18.8 (13.6) | 60 (28)** | 28 (13.6) | 64 (30)** | ||||
| % change | 6.7 (2.1) | 3.2 (2)** | 4.2 (2.2) | 2.7 (1.4)* | 13.3 (5.4) | 4.9 (3.5)** | 9.1 (2) | 2.4 (2)** |
| CNR | 6.3 (4.8) | 7.3 (4) | 3 (2.8) | 6.4 (3.6)* | 13.1 (6.2) | 8.0 (4.9)* | 10.8 (6.9) | 5.3 (4.1)* |
P < .05,
< .005.
Fig. 3 shows the percent change in MRI signal in cortical regions under conscious and anesthetized conditions for different concentration of CO2. The MRI signal changes were twice as much in the conscious condition as the anesthetized condition during inhalation of 5% CO2. When animals were exposed to 10% CO2, this difference increased to nearly threefold. Subcortical regions showed similar response patterns to the different hypercapnic challenges under anesthetized conditions (Fig. 4).
Fig. 3.
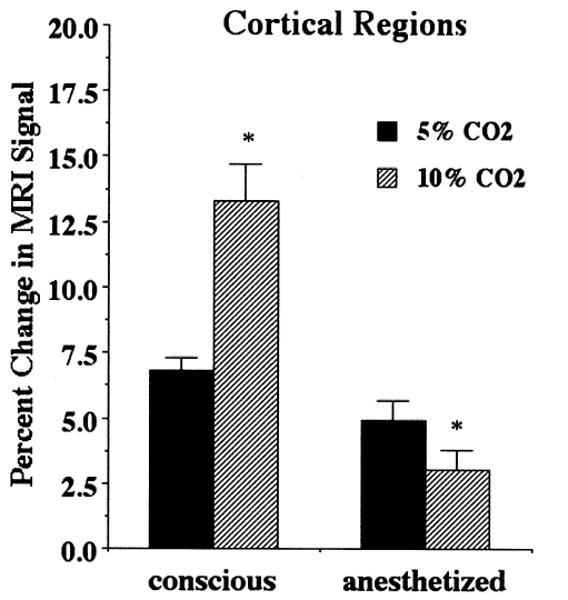
Percent change in MRI signal intensity in cortical regions under conscious and anesthetized conditions during hypercapnic challenge. There was a significant (p < 0.001) dose-dependent change in the conscious and anesthetized condition.
Fig. 4.
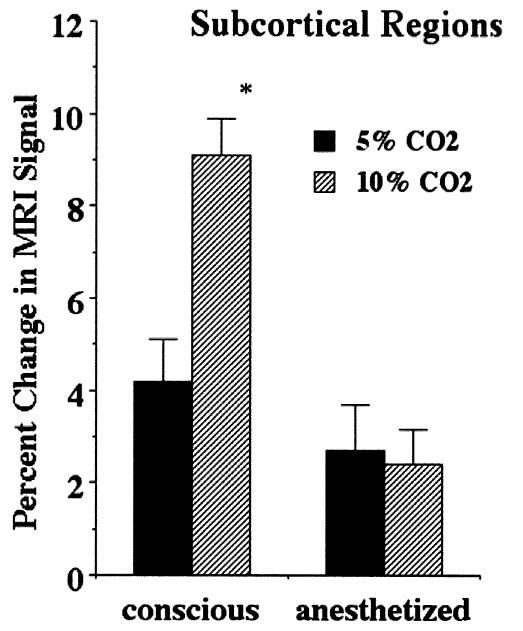
Percent change in subcortical regions under anesthetized and conscious conditions during hypercapnic challenge. There was a significant (p < 0.001) dose-dependent change in the conscious but not in the anesthetized condition.
4. Discussion
The present study clearly demonstrates a difference in cerebrovascular reactivity to hypercapnic challenge in conscious and isoflurane anesthetized rats. The enhanced T2* MRI signal in the conscious condition was concentration-dependent, robust and rapid in onset. While there was a significant increase in T2* MRI signal response to hypercapnia in the anesthetized condition, there was no clear dose-dependence to this response. The depressed responsiveness of cerebral vascular smooth muscle to hypercapnia during anesthesia is well documented [18,26,27]. However, this is the first study known to date to characterize and quantify changes in MRI signal intensity as a reflection of cerebrovascular reactivity in conscious and anesthetized conditions.
As expected, increasing the partial pressure of CO2 in blood by inhalation of 5% and 10% CO2 gas mixtures caused a concentration-dependent decreased in pH under conscious and anesthetized conditions. As a volatile acid, CO2 reduces blood pH under hypercapnic conditions. What was unexpected was the low baseline pH of 7.25 for the conscious condition as compared to normal pH of around 7.4 for a rat [28]. In the anesthetized condition, the blood gases and respiratory rate suggest hypoventilation and respiratory acidosis as contributing to the low baseline pH of 7.13. However, the normal PaCO2 value and respiratory rate exclude hypoventilation as the cause of the low pH in the conscious condition. Most likely, the low pH is caused from the medetomidine-ketamine anesthetic. While studies in dogs [29-31], and rabbits [32] have shown similar decreases in blood pH following administration of medetomidine or medetomidine/ketamine, the precise mechanism for this anesthetic induced low pH is unknown. While bicarbonate was not measured in the present study, any decrease in this ion could contribute to a metabolic acidosis. Indeed, dogs given a threshold dose of 40 μg/kg of medetomidine show a rapid reduction in arterial blood bicarbonate with a modest metabolic acidosis [31].
The baseline rate of respiration was markedly different between the conscious and anesthetized conditions. In general, anesthetics like isoflurane reduce minute volume by reducing the rate and depth of respiration [33] as confirmed in this study. In the conscious condition, the respiratory rate of 96 breaths/min is on the high end of the normal range and is probably associated with the stress of the imaging protocol. In addition, the modest medetomidine-induced metabolic acidosis in the presence of a PCO2 of 38 mmHg would be expected to enhance the rate and depth of respiration as predicted from PCO2/ventilation response studies [34]. Hypercapnic challenged affected respiration differently in the conscious and anesthetized conditions. In the conscious condition there was an increase in force of respiration while in the anesthetized condition there was an increase in respiratory frequency. These different responses to hypercapnic challenge probably reflect the most efficient way of increasing alveolar ventilation for each condition. For example, increasing tidal volume as suggested by the increased force of respiration is the more efficient way of increasing alveolar ventilation for the conscious condition given the high baseline respiratory frequency. Conversely, in the anesthetized condition increasing the frequency of respiration in the presence of a very low baseline rate is the more efficient way of increasing alveolar ventilation.
Hypercapnia caused a decrease in heart rate in both the conscious and anesthetized conditions. This reactive bradycardia is reported in both clinical and preclinical studies [35–38]. Presumably, hypercapnic stimulation of the peripheral chemoreceptors, carotid and aortic bodies leads to an increase in sympathetic tone and peripheral resistance followed by a baroreceptor mediated increase in vagal activity and decrease in heart rate [37,39].
4.1. Conscious vs. anesthetized-implications in functional imaging
The enhanced cerebrovascular reactivity demonstrated in this study in the conscious condition would predict greater BOLD signal changes during functional imaging as compared to animals studied under anesthesia. This prediction is consistent with data reported in the few other functional imaging studies of conscious animals. Specifically, Lahti [6] and coworkers examined changes in BOLD signal intensity in response to foot shock in conscious and propofol anesthetized rats. The greatest increases in BOLD signal in the somatosensory cortex occurred in the conscious condition. Indeed, the BOLD signal changes were 4–5 times greater in the conscious versus the propofol anesthetized condition. Furthermore, the areas of activation in the contralateral and ipsilateral somatosensory cortices were greater in the conscious condition suggesting a more complex response pattern. These findings related to foot-shock induced cortical activation were corroborated by Peeters [17] et al. in conscious curarized and α-chloralose anesthetized rats.
While there is a definite advantage to imaging conscious animals both from the standpoint of broader experimental applications and enhanced BOLD signal there are still the concerns of higher noise levels and complications with stress that are minimized or eliminated with anesthesia. While noise levels are a major concern with fMRI studies, signal change and contrast-to-noise are the final determinates of whether a significant change is detected during activation.
Functional MRI relies on the detection of BOLD changes in regions in order to imply activation within that region. Anesthesia is commonly utilized to reduce the baseline noise in order to better detect functional changes on the order of a several percent. In these studies, as expected, base line noise was higher in the conscious condition (2–3%) as compared to anesthesia (1–2%), making signal changes of a few percent undetectable. Despite the presence of higher physiological noise, mostly likely caused by muscle movement around the head and neck, there was a higher contrast-to-noise ratio in the conscious condition particularly with 10% CO2 challenge. This would suggest that the enhanced BOLD signal accompanying robust increases in brain activity that occur with electric shock and intense emotion like fear and sexual arousal would easily exceed baseline noise of 2–3%. Indeed, conscious rats exposed to stimulation via foot shock [6] or fear from exposure to the odors of a predator [10] show increases of BOLD signal from 10– 15%. Conscious marmoset monkeys imaged during sexual arousal induced by odors of novel receptive females show BOLD signals changes of 7–8% [13]. Whether these robust changes in BOLD signal would occur in more subtle experimental paradigms like simple cognitive tasks is unknown.
5. Conclusion
These data confirm the feasibility of fMRI studies in fully conscious animals. The increased responsiveness to carbon dioxide seen in conscious animals suggest that the cerebrovasculature of these animals is more sensitive to the factors that contribute to BOLD signal during neuronal activity. The benefits of studying conscious animals; increased BOLD signal, increased contrast to noise, higher orders of sensory input and cognition etc, seem to encourage the further use of conscious animals in fMRI studies.
References
- 1.Hajnal JV, Myers R, Oatridge A, Schwieso JE, Young IR, Bydder GM. Artifacts due to stimulus correlated motion in functional imaging of the brain. Magn Reson Med. 1994;31(3):283–91. doi: 10.1002/mrm.1910310307. [DOI] [PubMed] [Google Scholar]
- 2.Yetkin FZ, Haughton VM, Cox RW, Hyde J, Bim RM, Wong EC, Prost R. Effect of motion outside the field of view on functional MR. AJNR Am J Neuroradiol. 1996;17(6):1005–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Bim RM, Bandettini PA, Cox RW, Jesmanowicz A, Shaker R. Magnetic field changes in the human brain due to swallowing or speaking. Magn Reson Med. 1998;40(1):55–60. doi: 10.1002/mrm.1910400108. [DOI] [PubMed] [Google Scholar]
- 4.Ueki M, Mies G, Hossmann KA. Effect of α-chloralose, halothane, pentobarbital and nitrous oxide anesthesia on metabolic coupling in somatosensory cortex of rat. Acta Anaesthesiol Scand. 1992;36(4):318–22. doi: 10.1111/j.1399-6576.1992.tb03474.x. [DOI] [PubMed] [Google Scholar]
- 5.Bonvento G, Seylaz J, Lacombe P. Widespread attenuation of the cerebrovascular reactivity to hypercapnia following inhibition of nitric oxide synthase in the conscious rat. J Cereb Blood Flow Metab. 1994 doi: 10.1038/jcbfm.1994.90. [DOI] [PubMed] [Google Scholar]
- 6.Lahti KM, Ferris CF, Li F, Sotak CH, King JA. Comparison of evoked cortical activity in conscious and propofol-anesthetized rats using functional MRI. Magn Reson Med. 1999;41(2):412–6. doi: 10.1002/(sici)1522-2594(199902)41:2<412::aid-mrm28>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Nakao Y, Itoh Y, Kuang T, Cook M, Jehle J, Sokoloff L. Effects of anesthesia on functional activation of cerebral blood flow and metabolism. PNAS. 2001;98(13):7593–98. doi: 10.1073/pnas.121179898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahti KM, Ferris CF, Li F, Sotak CH, King JA. Imaging brain activity in conscious animals using functional MRI. J Neurosci Methods. 1998;82:75–82. doi: 10.1016/s0165-0270(98)00037-5. [DOI] [PubMed] [Google Scholar]
- 9.Brevard ME, Ferris CF, Mattingly M, Olson D, Lee RMKW, Zhu G, King JA. Magnetic Resonance Imaging of Spontaneous Hemorrhagic Stroke in Conscious Rats. Proc Intl Soc Mag Reson Med, 9th Meeting. 2001:676. [Google Scholar]
- 10.King JA, Ferris CF, Duong TQ, Does MD, Ludwig R, Sullivan JM. Functional Imaging of Brain Activity in Conscious Animals: The Smell of Fear. Proc ISMRM 10th Meeting. 2002:1352. [Google Scholar]
- 11.Wyrwicz AM, Chen N, Li L, Weiss C, Disterhoft JF. fMRI of visual system activation in the conscious rabbit. Magn Reson Med. 2000;44(3):474–8. doi: 10.1002/1522-2594(200009)44:3<474::aid-mrm19>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Logothetis NK, Guggenberger H, Peled S, Pauls J. Functional imaging of the monkey brain. Nat Neurosci. 1999;2(6):555–62. doi: 10.1038/9210. [DOI] [PubMed] [Google Scholar]
- 13.Ferris C, Snowdon CT, King J, Duong TQ, et al. Functional imaging of Brain Activity in Conscious Monkeys Responding to Sexually Arousing Cues. NeuroReport. 2001;12:2231–235. doi: 10.1097/00001756-200107200-00037. [DOI] [PubMed] [Google Scholar]
- 14.Vanduffel W, Fize D, Mandeville JB, Nelissen K, Van Hecke P, Rosen BR, Tootell RB, Orban GA. Visual motion processing investigated using contrast agent-enhanced fMRI in awake-behaving monkeys. Neuron. 2001;32(4):565–77. doi: 10.1016/s0896-6273(01)00502-5. [DOI] [PubMed] [Google Scholar]
- 15.Dubowitz DJ, Bernheim KA, Chen DY, Bradley WG, Andersen RA. Enhancing fMRI contrast in awake-behaving primates using intravascular magnetite dextran nanopartieles. Neuroreport. 2001;12(11):2335–40. doi: 10.1097/00001756-200108080-00011. [DOI] [PubMed] [Google Scholar]
- 16.Andersen A, Zhang Z, Barber T, Rayens W, Zhang J, Grondin R, Hardy P, Gerhardt G, Gash D. Functional MRI studies in conscious rhesus monkeys: methodological and analytical strategies. J Neurosci Methods. 2002;118(2):141. doi: 10.1016/s0165-0270(02)00123-1. [DOI] [PubMed] [Google Scholar]
- 17.Peeters RR, Tindemans I, De Schutter E, Van der Linden A. Comparing BOLD fMRI signal changes in the awake and anesthetized rat during electrical forepaw stimulation. Magn Reson Imaging. 2001;19(6):821–6. doi: 10.1016/s0730-725x(01)00391-5. [DOI] [PubMed] [Google Scholar]
- 18.Levasseur JE, Kontos HA. Effects of anesthesia on cerebral arteriolar responses to hypercapnia. Am J Physiol. 1989;257(1 Pt 2):H85–8. doi: 10.1152/ajpheart.1989.257.1.H85. [DOI] [PubMed] [Google Scholar]
- 19.Fenton RA, Rubio R, Berne RM. Adenosine and the acid-base state of vascular smooth muscle. J Appl Physiol. 1981;51(1):179–84. doi: 10.1152/jappl.1981.51.1.179. [DOI] [PubMed] [Google Scholar]
- 20.Karaki H, Weiss GB. Effect of transmembrane pH gradient changes on potassium- induced relaxation in vascular smooth muscle. Blood Vessels. 1981;18(1-2):36–44. doi: 10.1159/000158336. [DOI] [PubMed] [Google Scholar]
- 21.Grubb RL, Raichle ME, Eichling JO, Ter-Pogossian MM. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke. 1974;5(5):630–9. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- 22.Vorstrup S, Henriksen L, Paulson OB. Effect of acetazolamide on cerebral blood flow and cerebral metabolic rate for oxygen. J Clin Invest. 1984;74(5):1634–9. doi: 10.1172/JCI111579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SG, Rostrup E, Larsson HB, Ogawa S, Paulson OB. Determination of relative CMRO2 from CBF and BOLD changes: significant increase of oxygen consumption rate during visual stimulation. Magn Reson Med. 1999;41(6):1152–61. doi: 10.1002/(sici)1522-2594(199906)41:6<1152::aid-mrm11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 24.Rostrup E, Larsson HB, Toft PB, Garde K, Thomsen C, Ring P, Sondergaard L, Henriksen O. Functional MRI of CO2 induced increase in cerebral perfusion. NMR Biomed. 1994;7(1-2):29–34. doi: 10.1002/nbm.1940070106. [DOI] [PubMed] [Google Scholar]
- 25.Strupp JP. Stimulate: A GUI based fMRI analysis software package. NeuroImage. 1996;3:S607. [Google Scholar]
- 26.Schieve JF, Wilson WP. The influence of age, anesthesia and cerebral arteriosclerosis on cerebral vascular activity to CO2. Am J Med. 1953;15:171–74. doi: 10.1016/0002-9343(53)90067-9. [DOI] [PubMed] [Google Scholar]
- 27.Fujishima M, Scheinberg P, Busto R, Reinmuth OM. The relation between cerebral oxygen consumption and cerebral vascular reactivity to carbon dioxide. Stroke. 1971;2(3):251–7. doi: 10.1161/01.str.2.3.251. [DOI] [PubMed] [Google Scholar]
- 28.Flecknell PA, Waynsorth HB. Experimental and surgical techniques in rats. San Diego: Academic Press; 1992. [Google Scholar]
- 29.Jalanka H, Skutnabb K, Damsten Y. Preliminary results on the use of medetomidine- ketamine combinations in the dog. Acta Vet Scand Suppl. 1989;85:125–7. [PubMed] [Google Scholar]
- 30.Venugopalan CS, Holmes EP, Fucci V, Keefe TJ, Crawford MP. Cardiopulmonary effects of medetomidine in heartworm-infected and noninfected dogs. Am J Vet Res. 1994;55(8):1148–52. [PubMed] [Google Scholar]
- 31.Kuusela E, Raekallio M, Vaisanen M, Mykkanen K, Ropponen H, Vainio O. Comparison of medetomidine and dexmedetomidine as premedicants in dogs undergoing propofol- isoflurane anesthesia. Am J Vet Res. 2001;62(7):1073–80. doi: 10.2460/ajvr.2001.62.1073. [DOI] [PubMed] [Google Scholar]
- 32.Mero M, Vainionpaa S, Vasenius J, Vihtonen K, Rokkanen P. Medetomidine-ketamine- diazepam anesthesia in the rabbit. Acta Vet Scand Suppl. 1989;85:135–7. [PubMed] [Google Scholar]
- 33.Eger El. Isoflurane: a review. Anesthesiology. 1981;55(5):559–76. doi: 10.1097/00000542-198111000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Lumb AB. In: Nunn's Applied Respiratory Physiology. Fifth. Lumb A, editor. Butterworth-Heinemann Reed Educational and Professional Publishing; 2000. pp. 82–106. [Google Scholar]
- 35.Weiss HR, Cohen JA, McPherson LA. Blood flow and relative tissue PO2 of brain and muscle: effect of various gas mixtures. Am J Physiol. 1976;230(3):839–44. doi: 10.1152/ajplegacy.1976.230.3.839. [DOI] [PubMed] [Google Scholar]
- 36.Walker BR, Brizzee BL. Cardiovascular responses to hypoxia and hypercapnia in barodenervated rats. J Appl Physiol. 1990;68(2):678–86. doi: 10.1152/jappl.1990.68.2.678. [DOI] [PubMed] [Google Scholar]
- 37.Gootman PM, Gandhi MR, Steele AM, Hundley BW, Cohen HL, Eberle LP, Sica AL. Respiratory modulation of sympathetic activity in neonatal swine. Am J Physiol. 1991;261(5 Pt 2):R1147–54. doi: 10.1152/ajpregu.1991.261.5.R1147. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Boujedaini N, Cazin L, Mallet E, Clabaut M. Developmental changes in cardio- respiratory responses to hypoxia and hypercapnia in anesthetized low-birth-weight rats. Respir Physiol. 2000;123(3):189–99. doi: 10.1016/s0034-5687(00)00176-6. [DOI] [PubMed] [Google Scholar]
- 39.Daly MD, Angell-James JE, Elsner R. Role of carotid-body chemoreceptors and their reflex interactions in bradycardia and cardiac arrest. Lancet. 1979;1(8119):764–7. doi: 10.1016/s0140-6736(79)91218-2. [DOI] [PubMed] [Google Scholar]


