Abstract
Introduction
Semantic memory and language deficits are associated with schizophrenia. Understanding how these systems operate in this disorder will likely require a multi-factorial model that explains their linkages with cognition and modulation by dopamine. A biological factor that may provide causal convergence for these connections is cell membrane composition and dynamics.
Methods
N400 is an electrophysiological measure of semantic memory and language that is sensitive to deficits in schizophrenia. Relationships among N400, cognition, dopamine, and cell membrane polyunsaturated fatty acids (PUFAs) were examined for patients tested under medicated (haloperidol only) and unmedicated (placebo) conditions. Relationships between these factors and clinical symptoms were also evaluated. The sample included 37 male schizophrenia inpatients and 34 male normal controls. The N400 priming effect was measured from visual event-related potentials recorded during a semantic priming-lexical decision task, in which semantic association (related versus unrelated words) and presentation rate (Stimulus Onset Asynchrony/SOAs: 350 and 950 ms) were varied.
Results
N400 was associated with cognition (speed, visuoperception, attention) in patients and controls. These relationships were influenced by SOA in both groups, and by pharmacological condition in patients. Levels of total PUFAs and arachidonic acid were associated with N400 in unmedicated patients. Clinical symptoms (paranoia, thought disturbance) were associated with N400, but not with cognition or PUFAs.
Conclusions
Results suggest cell membrane fatty acids are associated with semantic memory and language in schizophrenia. Findings also suggest a series of linkages that are modulated by dopamine: cell membrane fatty acids are associated with N400 semantic priming; N400 semantic priming is associated with clinical symptoms.
Keywords: N400, Arachidonic acid, Dopamine, Paranoia, Thought disturbance
1. Introduction
Memory impairment is an associated feature of schizophrenia (American Psychiatric Association, 2000) with deficits observed across a wide range of memory systems and processes, including semantic memory (reviews: Aleman et al., 1999; Condray, 2005; Minzenberg et al., 2002). Semantic memory represents factual knowledge about the world (Schacter et al., 2000) and provides a dynamic record of an individual’s learning and experience. Language is one medium for contact between the environment, learning, and semantic memory, and is regarded by some theorists as a key cognitive input system (Fodor, 1983). Expressive language disturbance, manifested in disorganized speech (derailment, incoherence), was among the first clinical features recorded for schizophrenia (Bleuler, 1911/1950; Kraepelin, 1919/1971), and currently serves as a characteristic diagnostic symptom (APA, 2000). Its receptive language counterparts, listening and reading impairments, may predate the onset of schizophrenia (Cannon et al., 2002; Crow et al., 1995), and are observed in patients across clinical states and medication regimens, and in their healthy family members (reviews: Condray, 2005; DeLisi, 2001; Kremen et al., 1994; Minzenberg et al., 2002). Linguistic codes for semantic memory are therefore an important focus for schizophrenia.
The pattern of findings to date is suggestive of receptive language as a mediating vulnerability trait for schizophrenia, as ‘vulnerability’ was earlier conceptualized by J. Zubin and colleagues (Zubin and Spring, 1977; Zubin and Steinhauer, 1981) and ‘mediating vulnerability’ was conceived by K. Nuechterlein, M. Dawson, et al. (1992; also ‘endophenotype’: Braff et al., 2007). Mediating vulnerability traits involve stable, enduring decrements from normal responding. Although this type of liability can be influenced by fluctuations in internal state, produced by pharmacological agents and exacerbations or stabilizations of clinical symptoms, it never fully resolves to normal performance. As developed by Neuchterlein et al., the term ‘mediating’ refers to characteristics that are genetically influenced and which serve as links in causal chains that are close to the formation of psychotic symptoms.
1.1. Semantic memory and N400
Much of the electrophysiological evidence concerning semantic memory in schizophrenia is based on findings for the N400 component of the scalp-recorded event-related brain potential (ERP). The N400 is a negative-going deflection in amplitude that typically occurs approximately 250–500 ms post-stimulus onset and prior to an overt behavioral response. A commonly used strategy is the semantic priming paradigm in which stimulus pairs are varied along linguistic (legal words versus nonword letter strings) and semantic (meaningfully and semantically related versus unrelated) dimensions. Healthy individuals show increased N400 amplitude to semantically unrelated word pairs (moon vote) compared to semantically related pairs (lion tiger) (‘N400 priming effect’). Currently, the N400 is considered to provide an electrophysiological index of on-line semantic activation that reflects both access of long-term semantic memory and late-stage semantic integration processes that are influenced by working memory (for analysis of the N400 literature, see Kutas and Federmeier, 2000). Examination of the patterns of relationships between performance on neuropsychological tests and N400, elicited across psycholinguistic conditions and tasks, can provide insight about the cognitive functions reflected in N400. For example, Salisbury (2004) reported evidence for an association between healthy individuals’ performance on a neuropsychological test presumed to involve working memory and their N400 response elicited during a sentence comprehension task. Most priming studies have shown schizophrenia patients, as a group, to be characterized by reduced N400 priming. It is important to emphasize, however, that heterogeneity of N400 response (abnormal and normal N400) has been noted for individual patients within groups showing group decrements in N400 (Condray, 2005; Grillon et al., 1991). Understanding this heterogeneity is an important task for the field.
1.2. Model of semantic memory in schizophrenia
Activation of linguistic codes in memory likely involves a dynamic interplay among perceptual processes and executive or attention functions. The extent to which either domain is prominent in its influence on semantic processes may be determined by presentation rate (stimulus onset asynchrony or SOA: time interval between onsets of successive stimuli) (Neely, 1991). Dopamine is assumed to modulate further the influences of cognition (Cohen et al., 2002; Goldman-Rakic et al., 2000) and timing. A strong version of this hypothesis involves timing as fundamental to the operational links between semantic memory and cognition. Timing-deficit hypotheses have been advanced for both schizophrenia (Andreasen et al., 1998; 1999) and developmental language disorders, including dyslexia (Llinás, 1993), and disturbance to semantic memory in schizophrenia patients has been documented for a wide range of SOAs (Condray, 2005; Minzenberg et al., 2002). However, the influence of timing-based processing in shaping the hypothesized dopamine-mediated connectivity among semantic memory, cognition, and clinical symptoms has not been determined.
1.3. Cell membrane phospholipids and semantic memory in schizophrenia
Phospholipids account for a large proportion of the makeup of all cell membranes, including brain tissue, and were early recognized as potentially important to the pathophysiology of schizophrenia (Feldberg, 1976; Horrobin, 1977). Polyunsaturated fatty acids (PUFAs) play important roles in cell membrane dynamics and in normal brain development, and defects have been observed for schizophrenia patients across medication regimens and at different points in illness course (reviewed in Mahadik and Yao, 2006). The pattern of results reported is suggestive of PUFA dysregulation for schizophrenia. Of relevance for considerations regarding receptive language in schizophrenia are the findings of PUFA abnormalities in dyslexic individuals using 31P MRS (MacDonnell et al., 2000; Richardson et al., 1997). It is therefore timely to explore the potential relationships among language codes for semantic memory, cognition, and cell membrane constituents and dynamics in schizophrenia.
Peripheral tissue measures, such as erythrocyte (Red Blood Cell) fatty acid concentrations, provide a method for examining relationships among cognition and membrane fatty acids in clinical populations. Moreover, linkages have been demonstrated among peripheral erythrocyte fatty acid concentrations, central fatty acid composition and metabolism, and the brain regions implicated as sources for semantic memory function reflected in the N400 component of the visual ERP. Firstly, correspondence among frontal cortex, plasma, and erythrocyte levels of polyunsaturated fatty acids has been demonstrated for non-human primates (Connor et al., 1990). Secondly, erythrocyte fatty acid concentrations in healthy human adults were correlated with cerebral phospholipid breakdown (phosphodiester), assessed by in vivo cerebral 31-Phosphorous Magnetic Resonance Spectroscopy (31P MRS) (Richardson et al., 2001). Correlation between peripheral (RBC) polyunsaturated fatty acids and central (31P MRS) phospholipid metabolism has also been documented for first-episode neuroleptic naïve schizophrenia patients (Yao et al., 2002). This latter finding was selective for bilateral prefrontal cortex regions. With respect to concentrations of fatty acids in central loci, recent work based on postmortem cortical tissue also implicates abnormal fatty acid composition in frontal cortex in schizophrenia patients (McNamera et al., 2007). Finally, the brain regions implicated by the 31P MRS and postmortem studies have also been tied to the N400 of the event-related brain potential (ERP) elicited during semantic processing (Frishkoff et al., 2004; Halgren et al., 2002), as well as to visual attention (Nobre et al., 1999).
In the present paper, data are reported to address the hypothesis that understanding how semantic memory operates in schizophrenia will likely require a multi-factorial model that explains its connections with processing speed, cognition (perception, attention) and dopamine. Data are also presented which address the hypothesis that cell membrane fatty acids may be associated with language and memory function in schizophrenia.
2. Methods
2.1. Subjects
Study participants (37 male schizophrenia inpatients; 34 male normal controls) were tested in the Pittsburgh Biometrics Research Program. Complete details regarding characteristics of the full sample and clinical profile of patients are provided in Condray et al. (1999). All individuals were evaluated with the Structured Clinical Interview for DSM-III-R (SCID: Spitzer et al., 1989) and consensus diagnoses were assigned during case conferences. Exclusion criteria included current substance use disorder and neurological conditions, including any history of loss of consciousness ≥ 10 min. The schizophrenia patient group was comprised of 37 physically healthy (medical and neurological examination) male veterans (38.5±8.7 (mean/SD) years of age; 13.2±1.8 years education; 91.7±8.3 WAIS-R Full-scale IQ) who were voluntarily admitted to the Schizophrenia Research Unit at Highland Drive VA Pittsburgh Healthcare System. Of these 37 patients, artifact-free event-related potential (ERP) data were recorded for 30 patients during medication maintenance therapy and for 21 patients during placebo replacement. Patients were tested during their participation in a separate, double-blind medication (haloperidol only) maintenance and placebo replacement protocol (protocol details in van Kammen et al., 1996). At the time of ERP testing, medicated patients were receiving only haloperidol (mean/SD=8.9±3.9 mg/daily; median=8; range: 3–20). Seven (7) of the 30 medicated patients had received an adjunct anticholinergic medication (benztropine: mean/SD=2.71±0.95 mg daily; median=2; range: 2–4), but this agent was discontinued two weeks before testing. After completion of the study, patients were returned to their pre-study clinical treatment regimens, which followed the standard of clinical care (van Kammen et al., 1996). Study participation for patients involved treatment; patients were therefore not paid.
Data are reported regarding the relationship between N400 and neuropsychological test performance for a subset of the control group, which was composed of 34 physically healthy (self-report) males (36.1±7.9 years; 15.2±2.3 years education; 109.06±10.05 WAIS-R Full-scale IQ) who were group-matched to the patient group for age and premorbid intelligence. Controls were diagnosed as having had no lifetime psychiatric disorder (SCID) and were paid $80 for study participation.
All protocols were approved by the Institutional Review Boards of the VA Pittsburgh Healthcare System and University of Pittsburgh. After explanation of study procedures and before enrollment and testing in study protocols, subjects provided written, informed consent to participate using VA Human Studies Committee and University of Pittsburgh Institutional Review Board approved consent forms. This research was conducted in accordance with the World Medical Association (Declaration of Helsinki) Code of Ethics.
2.2. Clinical assessment
At the time of ERP testing, patients were clinically stable as determined from ratings conducted by trained clinicians and nursing staff who were blind to medication condition (van Kammen et al., 1996). For this report, the following clusters were used from the Brief Psychiatric Rating Scale (Overall and Gorham, 1962) administered during the week of ERP testing: paranoia (items 10, 11, 14), thought disturbance (items 4, 12, 15), and psychosis (items 4, 7, 8, 10, 11, 12, 14, 15). For medicated patients (n=29), mean (SD) ratings for these symptom clusters during the week of ERP testing were: paranoia: 4.55 (1.1), thinking disturbance: 4.79 (2.2); psychosis: 11.9 (3.3). For unmedicated patients (n=20), mean (SD) ratings during the week of ERP testing were: paranoia: 5.80 (3.1), thought disturbance: 6.05 (3.5); psychosis: 14.6 (6.8).
2.3. Electrophysiology and cognitive assessment
2.3.1. Semantic memory: N400
Visual event-related potentials (ERPs) were recorded (midline electrodes) while subjects performed a semantic priming-lexical decision (word/nonword discrimination) task in which word primes were paired with associated, unassociated, and nonword distractor targets. Presentation rate was varied using two stimulus onset asynchronies (SOAs: 350 ms and 950 ms): prime word duration of 100 ms followed by a blank-screen (fixation box only) interstimulus interval (ISI) of either 250 ms or 850 ms; target stimuli followed the ISIs and remained on the screen for 1200 ms until participants completed behavior discrimination. Semantic expectancy was also varied, but is not a focus in this report. Intertrial interval=2 s.
The ERP measures of interest for this report are N400 amplitude to target words for correct behavioral response trials. The mean area integration of N400 amplitude was computed with signed deviations from baseline to produce the mean amplitudes at each electrode over the 270 to 550 ms window post-target onset. Results for amplitudes of N400 mean area integration and N400 peak (most negative peak between 300 and 500 ms post-target onset) were highly similar, and, for brevity, analyses reported are based on the amplitude of N400 mean area integration.
Complete details for methodology and results are reported in Condray et al. (1999, 2003).
2.3.2. Processing speed, visuoperceptual ability, and executive function
The Wide Range Achievement Test–Revised (WRAT-R) reading subtest (Jastak and Wilkinson, 1984) and Wechsler Adult Intelligence Scale–Revised (WAIS-R; Wechsler, 1981) were administered at the time of study enrollment. In addition to measuring word-level reading ability, the WRAT-R reading subtest is considered a measure of premorbid intelligence (Johnstone et al., 1996; Kareken et al., 1995). Performance on WAIS-R Digit Symbol Substitution was used to estimate attention and mental flexibility (Ryan et al., 1987) and processing speed (Joy et al., 2003). WAIS-R Picture Completion estimated visuoperceptual ability (Ryan et al., 1987), while WAIS-R Vocabulary was used to estimate stored semantic knowledge. Scaled scores from the selected WAIS-R subtests were used in analyses.
2.4. Biochemical measurement
Procedures for blood sample collection and methods used to determine levels of RBC membrane fatty acids were essentially the same as described by Yao et al. (1994), Yao and Reeddy, (2000). Briefly, freshly drawn blood with anticoagulant citrate dextrose (ACD) was centrifuged at 750×g for 7 min to remove platelet-rich plasma and leukocytes. Hemoglobin-free RBC ghost membranes were prepared by the method of Dodge et al. (1963). Lipid extraction of RBC ghost membranes was performed according to the procedure of Rose and Oklander (1965). Fatty acid methyl esters (FAME) were prepared using methanolic KOH reagent as described by Ichihara et al. (1996). Diheptadecanoyl lecithin (Matraya, Inc.) was used as an internal standard. All FAME were analyzed on a Hewlett-Packard capillary gas chromatograph, Model 5890 Series II, equipped with a hydrogen flame ionization detector. A 30-meter, fused silica SP-2380 column, with an inner diameter of 0.25 mm and a 0.20 μm film thickness (Suppelco, Inc.) was used. Each sample was run under a splitless injection mode with hydrogen as the carrier gas (30 mL/min) and with an inlet pressure of 6.5 psi. Oven temperature was programmed under three stages: Stage 1 – From 50 to 150 °C at a rate of 25 °C/min; Stage 2 – From 150 to 190 °C at a rate of 4 °C/min; Stage 3 – from 190 to 250 °C at a rate of 6 °C/min, with a final time of 3 min at 250 °C. Peaks on the chromatograms were identified by comparing the retention times with those of standard mixtures (Supelco, Inc.), and calculated by an Agilent ChemStation, Rev. A.09.03, using an internal standard mode.
2.5. Data analysis
Associations among semantic memory, cognitive functions of theoretical interest, clinical symptoms, and red blood cell (RBC) membrane fatty acids were tested using first-order bivariate correlation (Pearson r, Spearman’s rho) and simple linear regression (unstandardized betas; Pedhazur, 1997). Data were first assessed for normality (Shapiro–Wilk W test), and most variables either conformed to or were able to be transformed to normality using natural log (ln) transformation. A non-parametric statistic (Spearman’s rho) was used for those cases in which transformation did not result in normality. Partial correlation was used to test the association between N400 and cognitive function while controlling for premorbid intelligence, as measured by WRAT-R reading. The following measures were entered into analyses: semantic memory – mean area integration of N400 amplitude for correct response trials; cognitive processes –WRAT-R reading score and scaled scores for the selected WAIS-R subtests; BPRS ratings – paranoia, thinking disturbance, psychosis clusters; total levels of RBC polyunsaturated fatty acids and arachidonic acid. Control of Type I error for these multiple comparisons was accomplished by adjusting the standard level of alpha (p=.05) for logically-derived sets of comparisons using a modified Bonferroni strategy (Keppel, 1991), which has the advantage of balancing the risk for both Type I and Type II errors. A Type I familywise error rate (.20) was adopted for logically-derived sets of comparisons, and alpha levels were adjusted based on the number of comparisons for each set (i.e., familywise error/number in each set of comparisons). These adjusted alphas were then used to evaluate the significance of all of the planned comparisons within a given set of comparisons.
3. Results
Results are organized to emphasize the patterns of relationship observed across pharmacology conditions, which have implications for the status of semantic memory function as a mediating vulnerability trait and for the influence of dopaminergic transmission.
Variations in degrees of freedom across comparisons reflect missing data for some participants.
Characteristics of the full sample and clinical profile of patients are provided in Condray et al. (1999). Patients and controls included in the comparisons for this report did not differ for age or performance on the measure of word reading ability and premorbid intelligence: (1) age –medicated patients (mean/SD/n=37.8 years±9.0/29) versus normal controls (36.5 years±7.9/30) (p=.54), and unmedicated patients (37.1 years±7.8/20) versus normal controls (p=.78); WRAT-R Reading – medicated patients (73.4±9.0/30) versus normal controls (77.1±9.8/33) (p=.13), and unmedicated patients (76.3±7.3/21) versus normal controls (p=.74).
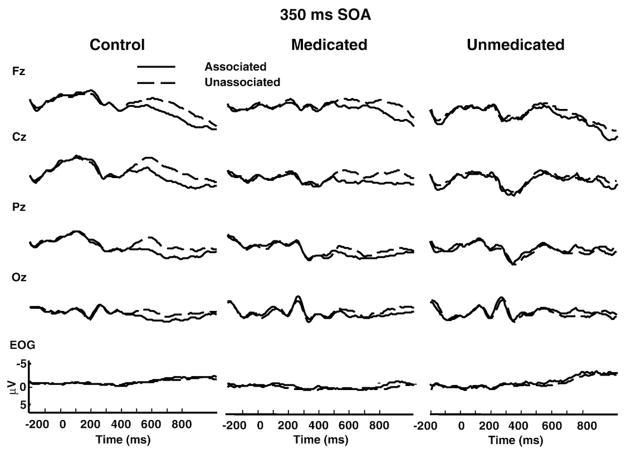
For ease of reference and interpretation, Fig. 1 presents the grand mean event-related potentials elicited by the 350 ms SOA for normal controls and schizophrenia patients (data adapted from Condray et al., 2003); waveforms for all conditions are provided in previous reports (Condray et al., 1999, 2003). Correlation coefficients are provided in tables; results of simple linear regression analyses are reported below. For interpretative purposes, it is important to recall that N400 is a negative-going deflection in amplitude and the N400 priming effect refers to the difference between N400 to unassociated and associated word pairs: N400-Unrelated minus N400-Related. Typical or normal N400 priming is reflected in increased amplitude negativity. Direction of correlation between N400 and other variables reflect this fact.
Fig. 1.
Grand mean event-related potentials elicited by the 350 ms SOA (stimulus onset asynchrony) for normal controls (n=19), and medicated (n=17) and unmedicated (n=9) schizophrenia patients tested under low expectancy (31% associated word pairs) condition. Waveforms represent responses for semantically associated (solid line) and unassociated (dotted line) target words in prime-target pairs. Positivity is downward. The target word was presented at time zero and remained on for the duration of the recording epoch. EOG=electrooculogram. Data adapted from Condray et al., 2003.
Red blood cell (RBC) membrane fatty acid data were available for a subset of the patient sample (63% medicated and 67% unmedicated patients). Patients for whom RBC membrane fatty acid data were available did not differ from patients for whom these data were not available (all p-values=n.s.) with respect to demographic factors (age and education), reading ability and premorbid intelligence, or clinical symptoms (BPRS paranoia, thinking disturbance, psychosis). Levels of RBC membrane total polyunsaturated fatty acids (PUFAs) and arachidonic acid (AA) concentrations are the focus of primary interest, which is reflected in analyses.
3.1. Unmedicated condition (placebo replacement)
3.1.1. N400 and neuropsychological tests
Table 1 presents the correlation coefficients obtained for N400 and performance on neuropsychological tests that tap word-level reading, processing speed, visuo-perceptual ability, and attention and executive function. Results from simple linear regression analyses are provided in the following. Figs. 2 and 3 show those relationships between unmedicated patients’ N400 and cognitive performance that remained statistically significant after controlling for premorbid intelligence (WRAT-R Reading).
Table 1.
Association between semantic memory and cognitive processes for schizophrenia patients and normal controls
| Unmedicated patients (n=21) | ||||
|---|---|---|---|---|
| N400 priming effect (μV) (Unassociated minus Associated) |
||||
| 350 s SOA |
950 s SOA |
|||
| Fz | Pz | Fz | Pz | |
| WRAT-R Reading (n=21) | −.008 | .277 | −.611* | −.380 |
| WAIS-R (n=20) | ||||
| Vocabulary | .281 | .631** | −.241 | −.142 |
| Picture Completion | .294 | −.002 | −.503 | −.246 |
| Digit Symbol | −.220 | −.183 | −.566** | −.459 |
| Medicated patients (n=30) | ||||
| N400 priming effect (μV) (Unassociated minus Associated) |
||||
| 350 s SOA |
950 s SOA |
|||
| Fz | Pz | Fz | Pz | |
| WRAT-R Reading (n=30) | −.006 | −.234 | .250 | .199 |
| WAIS-R (n=29) | ||||
| Vocabulary | .302 | .245 | .256 | .107 |
| Picture Completion | .060 | .108 | .463** | .119 |
| Digit Symbol | −.025 | −.006 | .269 | .276 |
| Normal controls (n=34) | ||||
| N400 priming effect (μV) (Unassociated minus Associated) |
||||
| 350 s SOA |
950 s SOA |
|||
| Fz | Pz | Fz | Pz | |
| WRAT-R Reading (n=33) | −.292 | −.357 | −.150 | −.031 |
| WAIS-R | ||||
| Vocabulary (n=16) | −.138 | −.202 | .197 | .195 |
| Picture Completion (n=17) | −.364 | −.354 | .175 | .081 |
| Digit Symbol (n=17) | −.332 | −.631* | .137 | −.200 |
Adjusted alpha (2-tailed): p≤.0125.
Remained statistically significant after controlling for premorbid IQ.
Fig. 2.
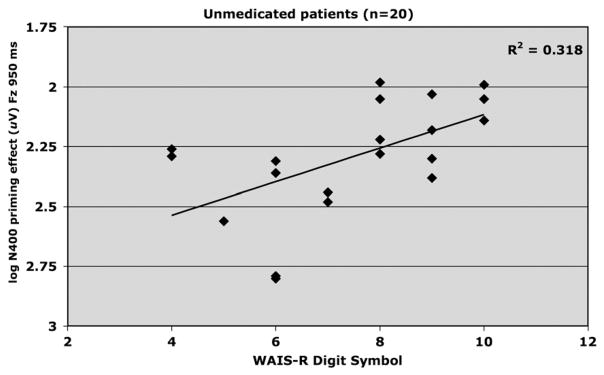
Relationship between WAIS-R Digit Symbol scaled score and log amplitude of N400 priming effect elicited at FZ by the 950 ms SOA in unmedicated (placebo) schizophrenia patients (n=20).
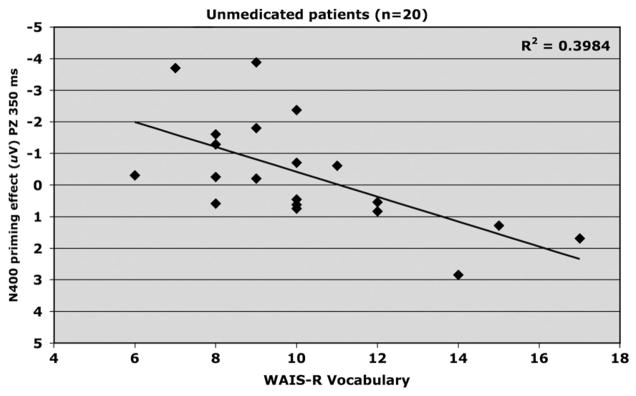
Fig. 3.
Relationship between WRAT-R Vocabulary scaled score and amplitude of N400 priming effect elicited at PZ by the 350 ms SOA in unmedicated (placebo) schizophrenia patients (n=20).
3.1.1.1. WRAT-R Reading
N400 and word reading ability were correlated only for unmedicated patients. Reading score and amplitude of the N400 priming effect (Fz; 950 ms SOA) were negatively associated (R2 = .373, adjusted R2 = .34, F1,20 = 11.30, p < .01, beta=−.019) with the direction indicating increases in patients’ reading score were associated with increases in the magnitude of their N400 priming.
3.1.1.2. Digit Symbol
Significant associations were observed between N400 and Digit Symbol performance for both unmedicated patients and controls. Fig. 2 shows that Digit Symbol score and N400 priming (Fz; 950 ms SOA) were negatively correlated (R2 =.321, adjusted R2 =.283, F1,19 =8.50, p <.01, beta= −.070) for unmedicated patients, and this relationship remained significant after controlling for premorbid intelligence (p <.05). For controls, Digit Symbol score and magnitude of N400 priming (Pz; 350 ms SOA) were also negatively correlated (R2 =.398, adjusted R2 = .357, F1,16 =9.90, p <.01, beta= −.311). The direction of the relationship was the same for both groups; increases in Digit Symbol score were associated with increases in the magnitude of N400 priming.
3.1.1.3. Picture Completion
N400 and Picture Completion score were not significantly associated for unmedicated patients or controls.
3.1.1.4. Vocabulary
N400 (Pz; 350 ms SOA) and Vocabulary were significantly and positively correlated for unmedicated patients (R2 =.398, adjusted R2 =.365, F1,19 =11.92, p <.01, beta=.394) with the direction showing more accurate vocabulary performance was associated with decreased magnitude of N400 priming (Fig. 3). This relationship remained significant after controlling for premorbid intelligence (p <.05).
3.1.2. N400 and clinical symptoms
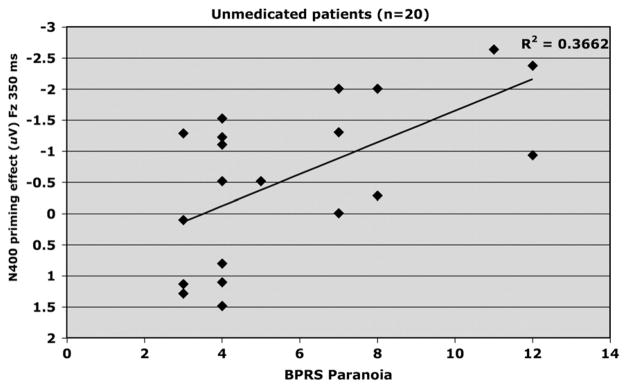
Table 2 presents the correlation coefficients for N400 and clinical symptoms. Fig. 4 shows symptoms of paranoia and the N400 priming effect (Fz; 350 ms SOA) were negatively correlated (R2 = .366, adjusted R2 = .331, F1,19 =10.40, p <.01, beta= −.255) indicating increases in unmedicated patients’ symptoms of paranoia were associated with increases in the magnitude of their N400 priming response.
Table 2.
Association between semantic memory and clinical symptoms for medicated and unmedicated schizophrenia patients
| Unmedicated patients (n=20) | ||||
|---|---|---|---|---|
| BPRS | N400 priming effect (μV) (Unassociated minus Associated) |
|||
| 350 ms SOA | 950 ms SOA | |||
| Fz | Pz | Fz | Pz | |
| Paranoia | −.577* | .107 | −.222 | −.217 |
| Thought disturbance | −.317 | .227 | −.200 | −.089 |
| Psychosis | −.505 | .197 | −.172 | −.122 |
| Medicated patients (n=29) | ||||
| N400 priming effect (μV) (Unassociated minus Associated) |
||||
| 350 ms SOA | 950 ms SOA | |||
| BPRS | Fz | Pz | Fz | Pz |
| Paranoia | −.283 | .066 | .285 | .247 |
| Thought disturbance | .150 | .327 | .513* | .460* |
| Psychosis | .191 | .259 | .186 | .280 |
Adjusted alpha (2-tailed): p≤.0166.
Fig. 4.
Relationship between BPRS Paranoia and amplitude of N400 priming effect elicited at FZ by the 350 ms SOA in unmedicated (placebo) schizophrenia patients (n=20).
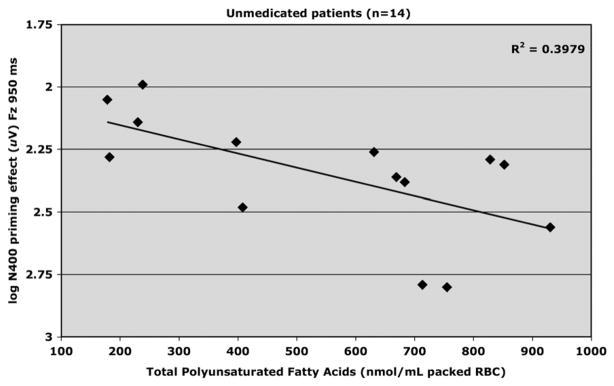
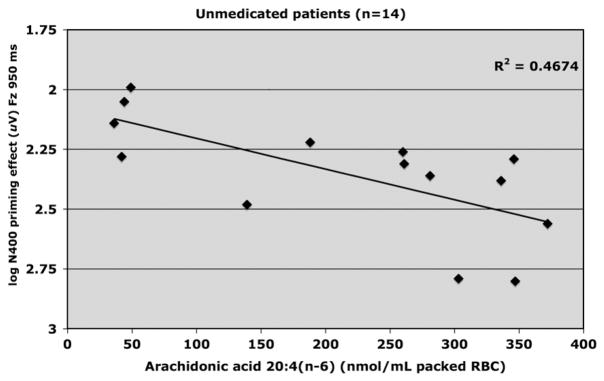
3.1.3. N400 and RBC membrane polyunsaturated fatty acids
Levels of fatty acids in red blood cell membranes of patients are provided in Table 3. Table 4 presents correlations for N400 and cell membrane fatty acids. Fig. 5 shows that for unmedicated patients concentration of total polyunsaturated fatty acids (PUFAs) and N400 priming (Fz; 950 ms SOA) were positively correlated (R2 = .401, adjusted R2 = .351, F1,13 = 8.03, p <.02, beta=.001), which indicates that as level of total PUFAs increased, magnitude of N400 priming decreased. A similar effect was observed for the relationship between level of arachidonic acid (AA) and N400 priming (Fz; 950 ms SOA) (R2 = .469, adjusted R2 = .425, F1,13 =10.60, p <.01, beta=.001) (Fig. 6).
Table 3.
Fatty acids in red blood cell membranes of medicated and unmedicated schizophrenia patients
| Fatty acidsa | Unmedicated patients |
Medicated patients |
|---|---|---|
| (n=14) |
(n=19) |
|
| Mean (SD) | Mean (SD) | |
| Saturated | 1212.14 (308.82) | 1142.74 (216.32) |
| Monounsaturated | 518.14 (144.16) | 524.42 (114.53) |
| Polyunsaturatedb | 549.57 (268.47) | 634.63 (243.65) |
| Total fatty acids | 2282.36 (628.72) | 2301.47 (546.03) |
| Arachidonic acid, 20:4(n-6) | 214.57 (128.72) | 259.95 (112.82) |
Mean (SD).
nmol/mL packed red blood cells.
Sum of fatty acids with more than one double bond.
Table 4.
Association between semantic memory and fatty acids for medicated and unmedicated schizophrenia patients
| Unmedicated patients (n=14) | ||||
|---|---|---|---|---|
| N400 priming effect (μV) (Unassociated minus Associated) |
||||
| 350 ms SOA | 950 ms SOA | |||
| Fatty acids | Fz | Pz | Fz | Pz |
| Polyunsaturated | −.044 | −.157 | .633* | .357 |
| Arachidonic acid, 20:4(n-6) | −.198 | −.138 | .749* | .292 |
| Medicated patients (n=19) | ||||
| N400 priming effect (μV) (Unassociated minus Associated) |
||||
| 350 ms SOA | 950 ms SOA | |||
| Fatty acids | Fz | Pz | Fz | Pz |
| Polyunsaturated | .238 | −.131 | .096 | −.197 |
| Arachidonic acid, 20:4(n-6) | .223 | −.088 | .108 | −.085 |
Adjusted alpha (2-tailed): p≤.025.
Fig. 5.
Relationship between total polyunsaturated fatty acids (PUFAs: nmol/mL packed red blood cell) and log amplitude of N400 priming effect elicited at FZ by the 950 ms SOA in unmedicated (placebo) schizophrenia patients (n=14).
Fig. 6.
Relationship between Arachidonic acid (nmol/mL packed red blood cell) and log amplitude of N400 priming effect elicited at FZ by the 950 ms SOA in unmedicated (placebo) schizophrenia patients (n=14).
3.2. Medicated condition (haloperidol only)
Daily dose of haloperidol was not significantly correlated with N400 semantic priming elicited by either of the two SOAs (all p-values>.10), which is consistent with findings reported in the literature for this dosage range and cognitive performance (meta-analysis by Woodward et al., 2007).
3.2.1. N400 and neuropsychological tests
Table 1 presents correlation coefficients for N400 and performance on neuropsychological tests for medicated patients. Effects from simple linear regression analyses are provided in the following, and Fig. 7 shows the relationship between medicated patients’ N400 and cognitive performance that remained statistically significant after controlling for premorbid intelligence.
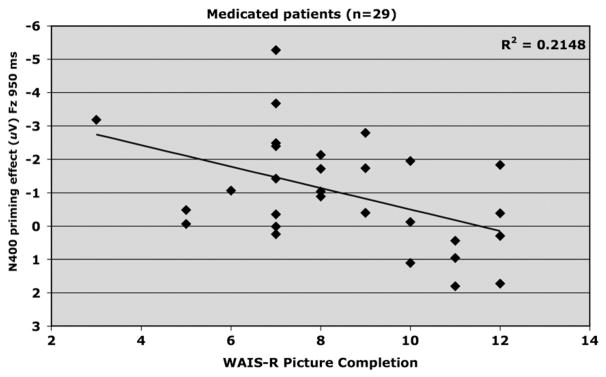
Fig. 7.
Relationship between WAIS-R Picture Completion scaled score and amplitude of N400 priming effect elicited at FZ by the 950 ms SOA in medicated (haloperidol only) schizophrenia patients (n=29).
3.2.1.1. WRAT-R Reading
N400 and word reading ability were not correlated for medicated patients.
3.2.1.2. Digit Symbol
N400 and Digit Symbol score were not correlated for medicated patients.
3.2.1.3. Picture Completion
Picture Completion score and N400 priming effect (Fz; 950 ms SOA) were positively correlated (R2 = .215, adjusted R2 = .186, F1,28 = 7.38, p = .011, beta = .321), which indicated increases in Picture Completion performance to be associated with decreases in magnitude of N400 priming (Fig. 7). This relationship remained significant after controlling for premorbid intelligence (p <.05).
3.2.1.4. Vocabulary
N400 response was not associated with Vocabulary score in medicated patients.
3.2.2. N400 and clinical symptoms
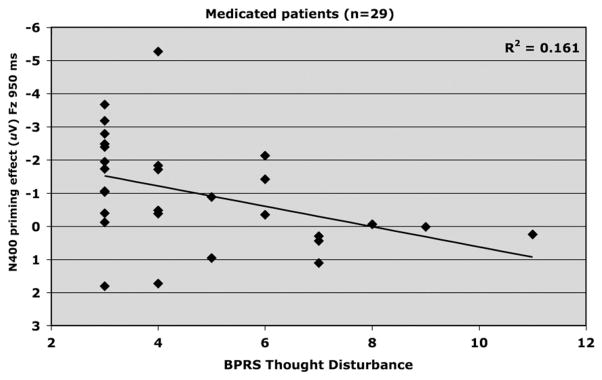
Table 2 presents correlation coefficients for N400 and clinical symptoms. Fig. 8 shows thought disturbance and N400 priming effect (Fz; 950 ms SOA) were positively correlated (R2 =.161, adjusted R2 =.13, F1,28 =5.18, p <.05, beta =.307) and indicates that increases in patients’ thought disturbance were associated with decreases in magnitude of their N400 priming.
Fig. 8.
Relationship between BPRS Thought Disturbance and amplitude of N400 priming effect elicited at FZ by the 950 ms SOA in medicated schizophrenia patients (n=29).
3.2.3. N400 and RBC membrane polyunsaturated fatty acids
N400 response was not correlated with cell membrane PUFAs in medicated patients.
3.3. Clinical symptoms, neuropsychological performance, and RBC membrane fatty acids
Clinical symptoms were not associated with patients’ neuropsychological test performance under either pharmacological condition. Clinical symptoms and concentrations of membrane fatty acids were also not associated during either medicated or unmedicated phase.
3.4. Pharmacological condition as a repeated measure
Artifact-free ERP data recorded for the same individuals under both pharmacological conditions were available for subset of patients (n=14). This subgroup therefore provided a complete crossing of pharmacological condition, and analyses were reapplied to the data from these patients for the eight comparisons determined significant in the main analyses. Results were evaluated using an adjusted-alpha =.025, and were generally consistent with those based on the full sample of patients. For unmedicated patients, associations remained statistically significant between N400 and single-word reading, digit symbol, paranoia, and cell membrane fatty acids. For medicated patients, the relationship between N400 and picture completion (p=.027) narrowly missed statistical significance.
4. Discussion
Semantic memory function, reflected in N400, was associated with cognitive processes (speed, visuoperception, attention) in patients and controls. These relationships were influenced by presentation rate (SOA) in both groups, and by pharmacological condition in patients. An hypothesized ‘upstream’ biological cause, cell membrane polyunsaturated fatty acids, was associated with N400 semantic priming in patients. Symptoms relevant to the clinical profile of schizophrenia (paranoia, thinking disturbance) were associated with N400, but were not associated with the targeted cognitive processes or cell membrane fatty acids under either pharmacological condition. Results suggest a series of linkages: cell membrane fatty acids influence N400 semantic priming; N400 priming influences clinical symptoms. These hypothesized linkages can be tested using more refined strategies (e.g., path analysis) given sufficient sample sizes. If reliable, this pattern is suggestive of semantic memory as a vulnerability trait or endophenotype for schizophrenia that serves as a link in a causal chain that is close to the formation of psychotic symptoms.
Several points regarding these findings merit discussion.
Dopamine and cognitive coordination
Patterns of association between semantic memory and cognition differed across pharmacological condition and cognitive domains, which is suggestive of a modulatory effect of dopamine (DA) on cognitive coordination. Dopamine as a primary influence on cognition has strong precedent in the literature (Castner et al., 2000; Cohen et al., 2002; Goldman-Rakic et al., 2000; Servan-Schreiber et al., 1990). Moreover, hypotheses regarding possible mechanisms for disturbance to neural synchrony and coordinated cognition in schizophrenia have favored N-methyl D-aspertate (NMDA) receptor function (Ford et al., 2007; Phillips and Silverstein, 2003), which may be modulated by DA activity (reviewed in Thompson et al., 2004). Importantly, the direction of associations observed in the present study varied across pharmacological condition and cognitive domain thereby raising the possibility of an interaction of the two factors. For medicated patients, more accurate visuoperceptual (Picture Completion) performance was associated with reduced semantic memory (N400 priming). Patients with above average scores on the former showed the greatest reduction and abnormality in N400 priming response. In contrast, typical or enhanced N400 priming was associated with greater attention, mental flexibility, and processing speed (Digit Symbol) in both unmedicated patients and controls. However, the inverse relationship observed for unmedicated patients’ vocabulary performance and semantic memory function indicates the relationship between dopamine and cognitive coordination is far from straightforward. In summary, there are at least two important implications of the overall pattern of relationships observed. Firstly, inverse relationships suggest interference to cognitive integration rather than reduced or absent cognitive coordination. Non-significant correlations indicating random associations would be expected for the latter circumstance. It is therefore possible that altered DA transmission produced a failure of cognitive coordination that was manifested as functional interference. Secondly, the occurrence of inverse relationships under both pharmacological conditions and for multiple cognitive functions suggests an interaction of DA and cognitive domain that influences cognitive coordination.
Support for the hypothesized role of dopamine (DA) in semantic processing is provided by studies in which the effects of DA agonists on behavioral semantic priming have been examined in healthy volunteers. A question of interest for those studies has concerned whether DA narrows the spread of activation in the neurocognitive semantic network by reducing the effects of weak signals and amplifying the influence of strong signals (i.e., increasing signal-to-noise ratio). Comparisons among direct (red blue), indirect (lion stripes), and unrelated (fork wave) priming conditions have been used to examine this question. As formulated by Kischka et al. (1996), an indirect priming condition is expected to elicit a wider, more unfocused spread of activation of the semantic network compared to direct priming, which is assumed to produce a narrower, more focused pattern of activation. If DA does increase the signal-to-noise ratio and does operate functionally to focus or narrow the spread of activation within the semantic network, then DA agonists should produce a reduction in indirect priming effects. Results to date for healthy individuals provide support for this set of assumptions and additionally implicate a role for D1 receptors. The indirect priming effect was reduced by levodopa (L-dopa) compared to placebo (Kischka et al., 1996) and by pergolide (D1/D2 agonist) compared to bromocriptine (D2 agonist) and placebo (Roesch-Ely et al., 2006). Moreover, presentation rate may modulate further the effect of DA (L-dopa) on both direct and indirect priming, with the chief effects of this unselective DA agonist occurring at the faster rate (250 ms SOA versus 500 and 1200 ms SOAs) (Angwin et al., 2004). This latter finding is suggestive with respect to the present findings of different patterns of correlation across pharmacological conditions and the N400 under different SOA conditions (350 versus 950 ms).
Rate of language (SOA), cognitive coordination, and clinical symptoms
Activation of linguistic codes for semantic meaning likely involves coordination with processing speed, perception, and executive functions. An assumption underlying the present work is that the extent to which each of these domains is dominant in its influence on semantic memory function is determined by the time available (presentation rate or SOA) to perform that function. A strong version of the processing timing-deficit hypothesis for semantic memory in schizophrenia involves timing as fundamental to the operational linkages between semantic memory and cognition, and between semantic memory and clinical symptomatology. As developed in the preceding section, dopamine may modulate such timing-based connectivity. The present data are consistent with this formulation in the variable patterns of association observed between semantic memory and cognition across presentation rates (SOA). The linkage between semantic memory and clinical symptoms also varied across SOA. These latter relationships are of interest for theoretical and clinical reasons. The relationship observed between paranoia and N400–350 ms SOA may be shaped by early perceptual processes that serve to alert animals of potential threat (e.g.: the formulation by Breitmeyer and Ganz (1976) of transient-channel visual system activity as an “early warning system”). The relationship observed between thought disturbance and N400–950 ms SOA may be influenced by executive/attention functions that require more time to engage and coordinate with semantic memory.
Membrane lipids, neurotransmission, and cognition
Findings suggest an association between membrane fatty acids and language and memory function for schizophrenia. Cell membrane polyunsaturated fatty acids (PUFAs), including arachidonic acid (AA), were associated with patients’ N400 priming response. Inverse relationships were observed with higher levels of total PUFAs and AA associated with reduced and abnormal N400 semantic priming. This effect was observed only for unmedicated patients. While the therapeutic significance of relationships between essential fatty acids and clinical disorder is promising although presently undemonstrated (Fenton et al., 2000), several lines of evidence indicate a complex interplay among cell membrane constituents and dynamics, neurotransmission, and behavior. One possibility concerns the effects of inflammation on brain function and behavior. Arachidonic acid is an omega-6 fatty acid that has been shown to enhance basal inflammatory response, increase serum corticosterone concentrations, and evoke anxiety behavior during maze performance in rats (Song et al., 2003). An additional possibility is that essential fatty acids may mediate neurotransmitter receptor function (Yehuda et al., 1999), including serotonergic responsivity (Reddy et al., 2007; Yao et al., 1996; Yao and van Kammen, 2004) and phospholipid turnover, the latter of which may be elevated in schizophrenia patients and individuals with dyslexia (reviewed in Skosnik and Yao, 2003). Disturbance to phospholipid breakdown may therefore influence semantic memory function reflected in N400 response.
N400, cognition, and clinical symptoms
Associations between N400 and cognition and clinical symptoms have been reported in prior work, although these relationships have not typically been of primary interest. An exception is Salisbury’s (2004) examination of the relationship between N400 and cognition in healthy volunteers, which showed N400 was associated with a measure of attention and executive function, but was not correlated with vocabulary knowledge. The N400 data for normal controls in this report are generally consistent with Salisbury’s data. More commonly reported are associations between clinical symptoms and N400, with results indicating the quality of medicated patients’ N400 response to semantic context, elicited by a variety of paradigms and SOAs, to be associated with thought disorder, paranoia, delusions, psychosis and conceptual disorganization (Debruille et al., 2007; Ditman and Kuperberg, in press; Kiang et al., 2007; Kostova et al., 2005; Kuperberg et al., 2006; Mathalon et al., 2002; Salisbury et al., 2000, 2002). The present findings provide information regarding the modulation of the relationship between N400 and clinical symptoms by SOA and dopamine. Findings also suggest cell membrane fatty acids may exert an indirect influence on clinical symptoms that is mediated by semantic memory function (N400).
Summary
N400 semantic priming was associated with cognition in patients and controls. These relationships were influenced by presentation rate in both groups, and by pharmacological condition in patients. Levels of RBC cell membrane fatty acids were associated with N400 in unmedicated patients. Clinical symptoms were associated with N400, but not with cognition or cell membrane fatty acids. Results suggest cell membrane fatty acid composition and dynamics are associated with semantic memory in schizophrenia. Findings also suggest a series of linkages that are modulated by dopamine: cell membrane fatty acids are associated with N400 semantic priming; N400 semantic priming is associated with clinical symptoms. Females were not included in the present study, and these interpretations must therefore be restricted to males.
Acknowledgments
Role of Funding Source
Funding for this work was provided by grants to authors from the National Institute of Mental Health (MH50631, MH55762, MH44841), Medical Research Service of the Department of Veterans Affairs, the Bataan and Corregidor Medical Fund, Inc., and with resources and use of facilities at the Veterans Affairs Pittsburgh Healthcare System, Highland Drive Division. The NIMH and the Medical Research Service of the Department of Veterans Affairs had no further role in the design or conduct of the study, in analyses or interpretation of data, in writing of the report, or in the decision to submit the paper for publication.
This research was supported by the National Institute of Mental Health (MH50631 (RC), MH55762 (SRS), MH44841 (DPvK)), the Medical Research Service of the Department of Veterans Affairs, the Bataan and Corregidor Medical Fund, Inc. (DPvK), and with resources and use of facilities at the Veterans Affairs Pittsburgh Healthcare System, Highland Drive Division. We thank the patients who served as study participants. We are also grateful to a number of individuals who provided valuable contributions to the study, including the clinical and nursing staff of the Veterans Affairs Pittsburgh Healthcare System, Highland Drive Division. The contributions of the anonymous reviewers are also appreciated.
Footnotes
Contributors
Dr. Condray was responsible for design of the semantic memory study, data collection and analyses, and manuscript preparation. Electrophysiological recording, data analyses, and interpretation of results were accomplished by Drs. Condray and Steinhauer. Biochemistry data collection and measurement were conducted by Dr. Yao. Clinical protocols conducted in the Schizophrenia Research Unit, Highland Drive VA Pittsburgh Healthcare System were designed and supervised by Dr. van Kammen. Dr. van Kammen also contributed to the interpretation of data related to neuropsychopharmacology. Drs. Yao, Reddy, and van Kammen contributed to interpretation of results for the cell membrane fatty acid data. Dr. Morrow contributed to interpretation of neuropsychological test results. All authors contributed to and have approved the final manuscript.
Conflict of Interest
No conflict declared.
References
- Aleman A, Hijman R, de Haan EHF, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. American Journal of Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. American Psychiatric Association; Washington, D.C: 2000. Text Revision. [Google Scholar]
- Andreasen NC, Paradiso S, O’Leary DS. Cognitive Dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophrenia Bulletin. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biological Psychiatry. 1999;46:908–920. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Angwin AJ, Chenery HJ, Copland DA, Arnott WL, Murdoch BE, Silburn PA. Dopamine and semantic activation: an investigation of masked direct and indirect priming. Journal of the International Neuropsychological Society. 2004;10:15–25. doi: 10.1017/S1355617704101033. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia praecox or the group of schizophrenias. International Universities; New York: 1911/1950. [Google Scholar]
- Braff DL, Freedman R, Schork NJ, Gottesman II. De-constructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophrenia Bulletin. 2007;33 (1):21–32. doi: 10.1093/schbul/sbl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitmeyer BG, Ganz L. Implications of sustained and transient channels for theories of visualpattern masking, saccadic suppression, and information processing. Psychological Review. 1976;83:1–36. [PubMed] [Google Scholar]
- Cannon M, Caspi A, Moffitt TE, Harrington HL, Taylor A, Murray R, Poulton R. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Archives of General Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287:2020–2022. doi: 10.1126/science.287.5460.2020. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, Brown JW. Computational perspectives on dopamine function in prefrontal cortex. Current Opinion in Neurobiology. 2002;12:223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Condray R. Language disorder in schizophrenia as a developmental learning disorder. Schizophrenia Research. 2005;73:5–20. doi: 10.1016/j.schres.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Condray R, Steinhauer SR, Cohen JD, van Kammen DP, Kasparek A. Modulation of language processing in schizophrenia: effects of context and haloperidol on the event-related potential. Biological Psychiatry. 1999;45:1336–1355. doi: 10.1016/s0006-3223(98)00317-5. [DOI] [PubMed] [Google Scholar]
- Condray R, Siegle GJ, Cohen JD, van Kammen DP, Steinhauer SR. Automatic activation of the semantic network in schizophrenia: evidence from event-related potentials. Biological Psychiatary. 2003;54:1134–1148. doi: 10.1016/s0006-3223(03)00699-1. [DOI] [PubMed] [Google Scholar]
- Connor WE, Neuringer M, Lin DS. Dietary effects on brain fatty acid composition: the reversibility of n-3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. Journal of Lipid Research. 1990;3:237–247. [PubMed] [Google Scholar]
- Crow TJ, Done DJ, Sacker A. Childhood precursors of psychosis as clues to its evolutionary origins. European Archives of Psychiatry and Clinical Neuroscience. 1995;245:61–69. doi: 10.1007/BF02190732. [DOI] [PubMed] [Google Scholar]
- Debruille JB, Kumar N, Saheb D, Chintoh A, Gharghi D, Lionnet C, King S. Delusions and processing of discrepant information: an event-related brain potential study. Schizophrenia Research. 2007;89:261–277. doi: 10.1016/j.schres.2006.07.014. [DOI] [PubMed] [Google Scholar]
- DeLisi LE. Speech disorder in schizophrenia: review of the literature and exploration of its relation to the uniquely human capacity for language. Schizophrenia Bulletin. 2001;27 (3):481–496. doi: 10.1093/oxfordjournals.schbul.a006889. [DOI] [PubMed] [Google Scholar]
- Ditman T, Kuperberg GR. The time course of building discourse coherence in schizophrenia: an erp investigation. Psychophysiology. doi: 10.1111/j.1469-8986.2007.00565.x. in press. [DOI] [PubMed] [Google Scholar]
- Dodge JT, Mitchell Cl, Hanahan DJ. The preparation and chemical characteristics of hemoglobin-free ghosts on human erythrocytes. Archives of Biochemistry and Biophysics. 1963;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Feldberg W. Possible association of schizophrenia with a disturbance in prostaglandin metabolism, a physiological hypothesis. Psychological Medicine. 1976;6:359–369. doi: 10.1017/s0033291700015798. [DOI] [PubMed] [Google Scholar]
- Fenton WS, Hibbeln J, Knable M. Essential fatty acids, lipid membrane abnormalities, and the diagnosis and treatment of schizophrenia. Biological Psychiatry. 2000;47:8–21. doi: 10.1016/s0006-3223(99)00092-x. [DOI] [PubMed] [Google Scholar]
- Fodor J. The modularity of mind. The MIT Press; Cambridge, MA: 1983. [Google Scholar]
- Ford J, Krystal JH, Mathalon DH. Neural synchrony in schizophrenia: from networks to new treatments. Schizophrenia Bulletin. 2007;33:848–852. doi: 10.1093/schbul/sbm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frishkoff GA, Tucker DM, Davey C, Scherg M. Frontal and posterior sources of event-related potentials in semantic comprehension. Cognitive Brain Research. 2004;20:329–354. doi: 10.1016/j.cogbrainres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, III, Williams GV. D1 receptors in prefrontal cells and circuits. Brain Research Reviews. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Glazer WM. N400 and semantic categorization in schizophrenia. Biological Psychiatry. 1991;29 (5):467–480. doi: 10.1016/0006-3223(91)90269-r. [DOI] [PubMed] [Google Scholar]
- Halgren E, Dhond RP, Christensen N, Van Petten C, Marinkovic K, Lewine JD, Dale AM. N400-like magnetoencephalography responses modulated by semantic context, word frequency, and lexical class in sentences. NeuroImage. 2002;17:1101–1116. doi: 10.1006/nimg.2002.1268. [DOI] [PubMed] [Google Scholar]
- Horrobin DF. Schizophrenia as a prostaglandin deficiency disease. Lancet. 1977;1:936–937. doi: 10.1016/s0140-6736(77)92228-0. [DOI] [PubMed] [Google Scholar]
- Ichihara K, Shibahara A, Yamamoto K, Nakayama T. An improved method for rapid analysis of the fatty acids of glycerolipids. Lipids. 1996;31:535–539. doi: 10.1007/BF02522648. [DOI] [PubMed] [Google Scholar]
- Jastak S, Wilkinson GS. WRAT-R: Wide range achievement test administration manual. Western Psychological Services; Los Angeles: 1984. [Google Scholar]
- Johnstone B, Callahan CD, Kapila CJ, Bouman DE. The comparability of the WRAT-R Reading Test and NAART as estimates of premorbid intelligence in neurologically impaired patients. Archives of Clinical Neuropsychology. 1996;11:513–519. [PubMed] [Google Scholar]
- Joy S, Fein D, Kaplan E. Decoding digit symbol: speed, memory, and visual scanning. Assessment. 2003;10 (1):56–65. doi: 10.1177/0095399702250335. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Gur RC, Saykin AJ. Reading on the Wide Range Achievement Test-Revised and parental education as predictors of IQ: comparison with the Barona formula. Archives of Clinical Neuropsychology. 1995;10:147–157. [PubMed] [Google Scholar]
- Keppel G. Design and analysis: a researcher’s handbook. 3. Prentice Hall; New Jersey: 1991. [Google Scholar]
- Kiang M, Kutas M, Light GA, Braff DL. Electrophysiological insights into conceptual disorganization in schizophrenia. Schizophrenia Research. 2007;92:225–236. doi: 10.1016/j.schres.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kischka U, Kammer TH, Maier S, Weisbrod M, Thimm M, Spitzer M. Dopaminergic modulation of semantic network activation. Neuropsychologia. 1996;34:1107–1113. doi: 10.1016/0028-3932(96)00024-3. [DOI] [PubMed] [Google Scholar]
- Kostova M, Passerieux C, Laurent JP, Hardy-Baylé MC. N400 anomalies in schizophrenia are correlated with the severity of formal thought disorder. Schizophrenia Research. 2005;78:285–291. doi: 10.1016/j.schres.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Dementia praecox and paraphrenia. Chicago Medical Book Company; Chicago: 1896/1971. [Google Scholar]
- Kremen WS, Seidman LJ, Pepple JR, Lyons MJ, Tsuang MT, Faraone SV. Neuropsychological risk indicators for schizophrenia: a review of family studies. Schizophrenia Bulletin. 1994;20 (1):103–119. doi: 10.1093/schbul/20.1.103. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Sitnikova T, Goff D, Holcomb PJ. Making sense of sentences in schizophrenia: electrophysiological evidence for abnormal interactions between semantic and syntactic processing. Journal of Abnormal Psychology. 2006;115 (2):251–265. doi: 10.1037/0021-843X.115.2.251. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends in Cognitive Science. 2000;4:463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Llinás R. Is dyslexia a dyschronia? Annals of New York Academy of Sciences. 1993;682:48–56. doi: 10.1111/j.1749-6632.1993.tb22958.x. [DOI] [PubMed] [Google Scholar]
- Mahadik SP, Yao JK. Phospholipids in schizophrenia. In: Lieberman JA, editor. Textbook of Schizophrenia. American Psychiatric Press, Inc; 2006. pp. 117–135. [Google Scholar]
- Mathalon DH, Faustman WO, Ford JM. N400 and automatic semantic processing abnormalities in patients with schizophrenia. Archives of General Psychiatry. 2002;59:641–648. doi: 10.1001/archpsyc.59.7.641. [DOI] [PubMed] [Google Scholar]
- McDonnell LE, Skinner FK, Ward PE, Glen AI, Glen AC, MacDonald DJ, Boyle RM, Horrobin DF. Increased levels of cytosolic phospholipase A2 in dyslexics. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2000;63:37–39. doi: 10.1054/plef.2000.0189. [DOI] [PubMed] [Google Scholar]
- McNamera RK, Jandacek R, Rider T, Tso P, Hahn CG, Richtand NM, Stanford KE. Abnormalities in the fatty acid composition of the postmortem orbitofrontal cortex of schizophrenic patients: gender differences and partial normalization with antipsychotic medications. Schizophrenia Research. 2007;91:37–50. doi: 10.1016/j.schres.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Ober B, Vinogradov S. Semantic priming in schizophrenia: a review and synthesis. Journal of the International Neuropsychological Society. 2002;8:699–720. doi: 10.1017/s1355617702801357. [DOI] [PubMed] [Google Scholar]
- Neely JH. Semantic priming effects in visual word recognition: a selective review of current findings and theories. In: Besner D, Humphreys GW, editors. Basic processes in reading: visual word recognition. Erlbaum Associates; New Jersey: 1991. pp. 264–336. [Google Scholar]
- Nobre AC, Coull JT, Frith CD, Mesulam MM. Orbitofrontal cortex is activated during breaches of expectation in tasks of visual attention. Nature Neuroscience. 1999;2:11–12. doi: 10.1038/4513. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME, Gitlin M, Ventura J, Goldstein MJ, Snyder KS, Yee CM, Mintz J. Developmental processes in schizophrenic disorders: longitudinal studies of vulnerability and stress. Schizophrenia Bulletin. 1992;18:387–425. doi: 10.1093/schbul/18.3.387. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Pedhazur EJ. Multiple-regregression in behavioral research: Explanation and prediction. 3. Wadsworth Publishing Company; US: 1997. [Google Scholar]
- Phillips WA, Silverstein SM. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behavioral and Brain Sciences. 2003;26:65–138. doi: 10.1017/s0140525x03000025. [DOI] [PubMed] [Google Scholar]
- Reddy RD, Keshavan MS, Yao JK. Blunted serotonergic responsivity in neuroleptic-naïve patients at first-episode of schizophrenia. Schizophrenia Research. 2007;90:81–85. doi: 10.1016/j.schres.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Richardson AJ, Cox IJ, Sargentoini J, Puri BK. Abnormal cerebral phospholipid metabolism in dyslexia indicated by phosphorus-31 magnetic resonance spectroscopy. NMR in Biomedicine. 1997;10:309–314. doi: 10.1002/(sici)1099-1492(199710)10:7<309::aid-nbm484>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Richardson AJ, Allen SJ, Hajnal JV, Cox IJ, Easton T, Puri BK. Associations between central and peripheral measures of phospholipids breakdown revealed by cerebral 31-phosphorus magnetic resonance spectroscopy and fatty acid composition of erythrocyte membranes. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2001;25:513–1521. doi: 10.1016/s0278-5846(01)00211-1. [DOI] [PubMed] [Google Scholar]
- Roesch-Ely D, Weiland S, Scheffel H, Schwaninger M, Hundemer H-P, Kolter T, Weisbrod M. Dopaminergic modulation of semantic priming in healthy volunteers. Biological Psychiatry. 2006;60:604–611. doi: 10.1016/j.biopsych.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Rose HG, Oklander M. Improved procedure for the extraction of lipids from human erythrocytes. Journal of Lipid Research. 1965;6:428–431. [PubMed] [Google Scholar]
- Ryan CM, Morrow LA, Bromet EJ, Parkinson DK. Assessment of neuropsychological dysfunction in the workplace: normative data from the Pittsburgh Occupational Exposures Test Battery. Journal Clinical and Experimental Neuropsychology. 1987;9 (6):665–679. doi: 10.1080/01688638708405209. [DOI] [PubMed] [Google Scholar]
- Salisbury DF. Semantic memory and verbal working memory correlates of N400 to subordinate homographs. Brain and Cognition. 2004;55:396–399. doi: 10.1016/j.bandc.2004.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, O’Donnell BF, McCarley RW, Nestor PG, Shenton ME. Event-related potentials elicited during a context-free homograph task in normal versus schizophrenic subjects. Psychophysiology. 2000;37:456–463. [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, Nestor PG, McCarley RW. Semantic bias, homograph comprehension, and event-related potentials in schizophrenia. Clinical Neurophysiology. 2002;113:383–395. doi: 10.1016/s1388-2457(02)00003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD, Buckner RL. Memory systems of 1999. In: Tulving E, Craik FIM, editors. The Oxford Handbook of Memory. Oxford University Press; New York: 2000. pp. 627–643. [Google Scholar]
- Servan-Schreiber D, Printz H, Cohen JD. A network model of catecholamine effects: gain, signal-to-noise ratio, and behavior. Science. 1990;249:892–895. doi: 10.1126/science.2392679. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Yao JK. From membrane phospholipids defects to altered neurotransmission: is arachidonic acid a nexus in the pathophysiology of schizophrenia? Prostaglandins, Leukotrienes and Essential Fatty Acids. 2003;69:367–384. doi: 10.1016/j.plefa.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Song C, Li X, Leonard BE, Horrobin DF. Effects of dietary n-3 or n-6 acids on interleukin-1 B-induced anxiety, stress, and inflammatory responses in rats. Journal of Lipid Research. 2003;44:1984–1991. doi: 10.1194/jlr.M300217-JLR200. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured clinical interview for DSM-III-R. Biometrics Research Department, New York State Psychiatric Institute; New York: 1989. [Google Scholar]
- Thompson JL, Pogue-Geile MF, Grace AA. Developmental pathology, dopamine, and stress: a model for the age of onset of schizophrenia symptoms. Schizophrenia Bulletin. 2004;30:875–900. doi: 10.1093/oxfordjournals.schbul.a007139. [DOI] [PubMed] [Google Scholar]
- van Kammen DP, Kelley ME, Gurklis JA, Gilbertson MW, Yao JK, Condray R, Peters JL. Predicting duration of clinical stability following haloperidol withdrawal in schizophrenic patients. Neuropsychopharmacology. 1996;14:275–283. doi: 10.1016/0893-133X(95)00135-Z. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale – Revised. The Psychological Corporation; New York: 1981. [Google Scholar]
- Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of cognitive change with haloperidol in clinical trials of atypical antipsychotics: dose effects and comparison to practice effects. Schizophrenia Research. 2007;89:211–224. doi: 10.1016/j.schres.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Yao JK, Reddy R. Fatty acids and psychiatric disorders. In: Chow CK, editor. Fatty acids in foods and their health implications. Marcel Dekker; New York: 2000. pp. 995–1012. [Google Scholar]
- Yao JK, van Kammen DP. Membrane phospholipids and cytokine interaction in schizophrenia. International Review of Neurobiology. 2004;59:297–326. doi: 10.1016/S0074-7742(04)59012-8. [DOI] [PubMed] [Google Scholar]
- Yao JK, van Kammen DP, Welker JA. Red blood cell membrane dynamics in schizophrenia. II. Fatty acid composition. Schizophrenia Research. 1994;13 (3):217–226. doi: 10.1016/0920-9964(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Yao JK, van Kammen DP, Moss HB, Sokulski DE. Decreased serotonergic responsivity in platelets of drug-free patients with schizophrenia. Psychiatry Research. 1996;63:123–132. doi: 10.1016/0165-1781(96)02862-4. [DOI] [PubMed] [Google Scholar]
- Yao JK, Stanley JA, Reddy RD, Keshavan M, Pettegrew JW. Correlations between peripheral polyunsaturated fatty acid content and in vivo membrane phospholipids metabolites. Biological Psychiatry. 2002;52:823–830. doi: 10.1016/s0006-3223(02)01397-5. [DOI] [PubMed] [Google Scholar]
- Yehuda S, Rabinovitz S, Mostofsky DI. Essential fatty acids are mediators of brain biochemistry and cognitive functions. Journal of Neuroscience Research. 1999;56:565–570. doi: 10.1002/(SICI)1097-4547(19990615)56:6<565::AID-JNR2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Zubin J, Spring B. Vulnerability: a new view of schizophrenia. Journal of Abnormal Psychology. 1977;86:103–126. doi: 10.1037//0021-843x.86.2.103. [DOI] [PubMed] [Google Scholar]
- Zubin J, Steinhauer SR. How to break the logjam in schizophrenia: a look beyond genetics. The Journal of Nervous and Mental Disease. 1981;169:477–492. doi: 10.1097/00005053-198108000-00002. [DOI] [PubMed] [Google Scholar]
Further reading
- Reddy RD, Keshavan MS, Yao JK. Reduced red blood cell membrane essential polyunsaturated fatty acids in first episode schizophrenia at neuroleptic-naïve baseline. Schizophrenia Bulletin. 2004;30 (4):901–911. doi: 10.1093/oxfordjournals.schbul.a007140. [DOI] [PubMed] [Google Scholar]
- Yao JK, van Kammen DP. Red blood cell dynamics in schizophrenia. I. Membrane fluidity. Schizophrenia Research. 1994;11 (3):209–216. doi: 10.1016/0920-9964(94)90014-0. [DOI] [PubMed] [Google Scholar]