Abstract
The association between recovery of brain function and behavior after transient cerebral ischemia in animals and humans is incompletely characterized. Quantitative diffusion- (DWI), perfusion- (PWI), T2-weighted (T2WI), and functional magnetic resonance imaging (fMRI) were performed before, during, and up to 1 day after 20-mins transient middle cerebral artery occlusion (tMCAO; n = 6) or sham operation (n = 6) in male Sprague–Dawley rats. Viability thresholds were employed to calculate diffusion, perfusion, and T2 lesion volumes. Region of interest analysis was used to evaluate structural and functional MR signal changes within the sensorimotor network, which were then related to corresponding behavioral measures. Post-mortem 2,3,5-triphenyltetrazolium chloride (TTC) staining was performed 24 h after ischemia. Transient middle cerebral artery occlusion produced lesions on DWI and PWI, which fully recovered by 30 mins after reperfusion. Ipsilesional fMRI responses to hypercapnia and forepaw stimulation were significantly impaired after ischemia and did not fully normalize until 3 and 24 h after tMCAO, respectively. No abnormalities were observed on imaging or TTC at 24 h despite significant behavioral dysfunctions including contralesional forelimb impairment and ipsilesional neglect. No MRI, behavioral, or TTC anomalies were observed in sham-operated rats. There were no significant correlations between MRI parameters, behavior, and TTC in either group. Together, these results suggest that normal findings on diffusion, perfusion, and T2 imaging shortly after transient ischemia may not indicate normal tissue status as indicated by fMRI and behavior, which may help explain the persistence of neurologic deficits in patients with normal conventional MRI after cerebral ischemia.
Keywords: CBF, DWI, fMRI, MCAO, PWI, sensorimotor
Introduction
Cerebral ischemia is a main cause of sensorimotor morbidity in modern society and substantially increases short- and long-term risks for adverse cardiovascular and cerebrovascular events (Daffertshofer et al, 2004). Yet despite persistent symptoms, many patients present with no abnormalities during the hyperacute stage on conventional T1- and T2-weighted magnetic resonance imaging (MRI). Attempts have recently been made to use diffusion-(DWI) and perfusion-weighted imaging (PWI) to evaluate potential brain pathology after transient focal ischemia both clinically and experimentally (Daffertshofer et al, 2004; Kidwell et al, 1999). Earlier studies conducted in our lab revealed ischemic changes on multimodal MRI after periods of transient middle cerebral artery occlusion (tMCAO) in the rat and correlated these findings with histopathologic outcomes in regions where imaging abnormalities were permanently reversible (Li et al, 2000a). It was found that resolution of DWI lesions does not necessarily indicate tissue salvage from ischemia as selective neural necrosis was seen in such regions. This observation may help explain the persistence of neurologic deficits in patients who have normal DWI and PWI results after cerebral ischemia (Ay et al, 1999).
Although histologic analysis is useful in animal modeling, it cannot be replicated in clinical studies and, along with conventional imaging, fails to assess the postischemic functional integrity of brain tissue in vivo. Alternate surrogate techniques have therefore been developed to better understand neural function after transient ischemia, including positron emission tomography (PET), magnetoencephalography (MEG), transcranial magnetic stimulation (TMS), electroencephalography (EEG), and functional MRI (fMRI) (Kim et al, 2005). However, only the latter enables simultaneous acquisition of high-resolution structural and functional information in a completely noninvasive manner and, for this reason, has been widely used to map brain responses to external stimuli after stroke (Dijkhuizen et al, 2001). Surprisingly, fMRI has not—to our knowledge—been employed to investigate brain activity after transient cerebrovascular insults characterized by permanent resolution of ischemic lesions. Repeated fMRI measurements performed acutely after brief ischemia could provide useful information about regional hemodynamic and metabolic processes, which may contribute to developing a means for monitoring and understanding recovery processes.
Measures of behavioral outcome are gaining widespread popularity in animal studies and are arguably necessary to better assess the full range of consequences of cerebral ischemia (Virley et al, 2000). A few experimental stroke studies have attempted to correlate behavioral recovery with restitution of MR parameters, but a clear causal link between the two has not been established (Dijkhuizen et al, 2003; Rogers et al, 1997). It has been postulated that such a relationship may be found if the employed behavioral tests inform exclusively on the brain regions being examined (Virley et al, 2000). This supposition is supported by clinical studies showing that postischemic clinical syndromes vary in patients depending on the location of cerebral infarction (Gavrilescu and Kase, 1995), which implies that the type of functional impairment is contingent on damage within a particular region of the brain and that a stronger relationship between restorations of brain activity and behavioral function may be found when neural and functional measures are matched more specifically. Several recent clinical stroke studies utilizing MRI have begun to address these issues (Baron and Warach, 2005), but similar progress in animal models is lacking and there remains a paucity of information in either field with respect to transient ischemia.
Thus, the goal of this study was to correlate changes in ischemic tissue injury, hemodynamics, and activity within structures of the cerebral sensorimotor network with changes in the specific behavioral functions that they subserve in rats acutely recovering from 20-mins tMCAO. It was hypothesized that (1) the extent of ischemic damage is linked to the degree of loss of brain functions and (2) recovery of brain functions within the ipsilesional sensorimotor network is correlated with recovery of sensorimotor behavioral deficits. To this end, (1) multiparametric MRI was used to map and dynamically measure signatures of tissue damage and function within the brain and (2) adhesive label removal and forepaw placement tests were employed to evaluate sensorimotor function with general neurologic status assessed via Bederson scoring.
Materials and methods
Animal Preparation
All procedures were in accordance with institutional guidelines. Twelve male Sprague–Dawley rats (Taconic Farms, Hudson, NY, USA) weighing 300 to 350 g were divided into Groups 1 (n = 6) and 2 (n = 6) undergoing 20-mins tMCAO with reperfusion or sham operation, respectively, using the intraluminal suture method (Li et al, 1998). After surgery, animals were immediately placed into the magnet for imaging. Anesthesia was induced with 5.0% isoflurane under spontaneous respiration and maintained at 2.0% during surgery and 1.0% during MRI at a flow rate of 1.5L/min (Shen et al, 2005). Needle electrodes were subcutaneously inserted into both forepaws and connected in series for forepaw stimulation during imaging (Liu et al, 2004; Shen et al, 2005; Sicard and Duong, 2005). Physiologic parameters (respiration rate (RR), heart rate (HR), arterial oxyhemoglobin saturation, and end-tidal CO2 (EtCO2)) were noninvasively monitored and recorded throughout imaging onto a computer via the Biopac System (Santa Barbara, CA, USA) (Sicard and Duong, 2005). Temperature was continuously monitored with a rectal probe and maintained at 37.0 ± 0.5°C with a feedback-controlled heating pad (Sicard and Duong, 2005).
Hypercapnic Challenge and Forepaw Stimulation
Hypercapnic challenges tested cerebrovascular reactivity and consisted of a premixed gas of 5% CO2 with 21% O2 and balance N2 equilibrated for ~3 mins before imaging initiation (Sicard et al, 2003; Sicard and Duong, 2005). Tissue function was assessed with previously optimized bilateral somatosensory forepaw stimulation parameters (6 mA current with 0.3 ms pulse duration at 3 Hz) designed to yield robust fMRI responses without inducing significant changes in HR, RR, blood pressure, and blood gases (Liu et al, 2004; Sicard and Duong, 2005). Two forepaw stimulation and hypercapnic challenge trials were performed per imaging block, each trial consisting of a 2 mins baseline period followed by 2 mins of forepaw stimulation or hypercapnia (Sicard and Duong, 2005).
Magnetic Resonance Imaging Measurements
Magnetic resonance imaging experiments were performed on a 4.7 T/40 cm horizontal magnet equipped with a Biospec Bruker console (Billerica, MA, USA) and a 20G/cm gradient insert (ID = 12 cm and rise time = 120 µs). A surface coil (ID = 2.3 cm) was used for brain imaging and an actively decoupled neck coil for perfusion labeling (Meng et al, 2004). Animals were imaged before and midway during occlusion, as well as 15, 30, 60, 90, 120, 150, 180 mins, and 24 h after reperfusion. A complete imaging block lasted 30 mins where the apparent diffusion coefficient (ADC) of water, basal cerebral blood flow (bCBF), as well as hypercapnia- and forepaw stimulation-induced changes in CBF and the blood oxygenation level-dependent (BOLD) fMRI signals were recorded. Basal cerebral blood flow and ADC were the only parameters recorded during occlusion because of time limitations. Lastly, T2-weighted images (T2WIs) were acquired at preocclusion, 180 mins, and 24 h time points.
Three ADC maps were separately acquired with diffusion-sensitive gradients applied along the x, y, or z direction (Shen et al, 2003). Single-shot, echo-planar images (EPI) were acquired over 2.5 mins with matrix = 64 × 64, spectral width = 200 kHz, time to repetition (TR) = 2 secs (90° flip-angle), time to echo (TE) = 37.5 ms, b = 4 and 1,170 secs/mm2, Δ = 24 ms, δ = 4.75 ms, field of view (FOV) = 2.56 × 2.56 cm, seven 1.5 mm slices, and 16 averages. T2-weighted imaging was performed using a fast spin-echo pulse sequence (Sicard and Duong, 2005) with matrix = 128 × 128, TR = 2 secs, effective TE = 20 or 104 ms, FOV= 2.56 × 2.56 cm, seven 1.5 mm slices, and 16 averages.
Simultaneous CBF and BOLD measurements were made using the continuous arterial spin-labeling technique with single-shot, gradient-echo, EPI acquisition (Sicard and Duong, 2005). Paired images were acquired alternately—one with arterial spin labeling and the other without. BOLD images were obtained from the nonlabeled images of the CBF measurements. Continuous arterial spin-labeling employed a 1.78 secs square radiofrequency pulse to the labeling coil in the presence of a 1.0 G/cm gradient along the flow direction to satisfy the condition of adiabatic inversion and the sign of the frequency offset was switched for nonlabeled images (Shen et al, 2005). Magnetic resonance parameters were as follows: matrix = 64 × 64, TR = 2 secs (90° flip-angle), TE = 20 ms, FOV= 2.56 × 2.56 cm, seven 1.5 mm slices, and 60 pairs of images.
Magnetic Resonance Data Analysis
Image analysis employed codes written in Matlab (Math-Works Inc., Natick, MA, USA) and STIMULATE (University of Minnesota, Minneapolis, MN, USA) software (Sicard and Duong, 2005). Coregistration of images was performed using in-house software involving manual and automatic alignment without spatial interpolation (Liu et al, 2004). Quantitative average ADC maps, in units of mm2/secs, were calculated using the three separately acquired ADC maps using the Stejskal–Tanner equation: ADC = −ln(S1/S0)/(b1−b0), where , ln is the natural logarithm, and S0 and S1 are the signal intensities obtained with b0 and b1, respectively (Stejskal and Tanner, 1965). B values are proportional to the gradient strength (G), magnetogyric ratio (γ), duration (δ), and time between application (Δ) of gradient pulses. Quantitative T2-maps, in units of ms, were constructed using a linear least-squares regression performed on the two acquired T2WIs (Li et al, 2000a). Quantitative bCBF maps, in units of mL/g/min, were calculated using: SCBF = λ/T1(SC−SL)/(SL + [2α−1]SC), where SC and SL are the signal intensities of the control and labeled images, respectively, λ is the water brain–blood partition coefficient set to 0.9, α is the spin-labeling efficiency set to 0.75, and tissue T1 set to 1.5 secs (Duong et al, 2000; Silva et al, 1999). In vivo ADC, bCBF, and T2 lesion volumes were calculated utilizing viability thresholds and methodology previously derived for this model as described in detail elsewhere (Dijkhuizen et al, 2003; Shen et al, 2003). These thresholds were used to identify all pixels with abnormal ADC, CBF, or T2 characteristics on each image slice at each time point, with corresponding lesion volumes calculated by summing the abnormal areas and multiplying by slice thickness.
Crosscorrelation analysis was performed on fMRI data to calculate CBF and BOLD activation maps used to determine hypercapnia (ΔBOLDCO2 and ΔCBFCO2 ) and forepaw stimulation (ΔBOLDFS and ΔCBFFS) evoked signal changes relative to baseline (Liu et al, 2004; Shen et al, 2005; Sicard and Duong, 2005). Reported ΔBOLDCO2 and ΔCBFCO2 values are averages of two hypercapnia trials performed during each imaging block; the same is true for reported ΔBOLDFS and ΔCBFFS values.
Region of Interest Analysis of the Sensorimotor Network
Regions of interest (ROIs) were positioned bilaterally on MR maps and encompassed structures of the cerebral sensorimotor network, namely: forepaw region of primary somatosensory cortex (Sf1), secondary somatosensory cortex (S2), primary motor cortex (M1), ventral thalamus (thalamus), and caudatoputamen (CPu) (Dijkhuizen et al, 2001; Sicard and Duong, 2005; Virley et al, 2000; Weiller, 1998). ROIs were drawn carefully and conservatively with regard to anatomic images and a stereotaxic atlas of the rat brain (Paxinos and Watson, 1997) to ensure correct placement and to minimize partial-volume effects (Liu et al, 2004; Sicard et al, 2003; Sicard and Duong, 2005). ROIs of the ischemic hemisphere were obtained by symmetrically reflecting the contralesional ROIs along the midline (Shen et al, 2005). This approach was used to measure ADC, bCBF, ΔBOLDCO2, ΔCBFCO2, ΔBOLDFS, and ΔCBFFS values within ROIs, with the latter two parameters investigated solely within Sf1 (Liu et al, 2004; Sicard and Duong, 2005).
Neurologic and Histologic Evaluation
Animals underwent objective adhesive removal and forepaw placement sensorimotor behavioral testing before, 5 and 24 h after tMCAO or sham operation as fully described elsewhere (De Ryck et al, 1992; Virley et al, 2000). Briefly, the bilateral adhesive label removal test assessed contralesional neglect and ipsilesional bias by recording latency to contact and remove labels (in secs) as well as order of label contact and removal. The forepaw placement test examined sensorimotor integration scored on the following scale: 0 if the placing response was immediate and normal, 1 if the response was slow or delayed, and 2 if the response did not occur within 2 secs. These specific behavioral measures were used because they are fulfilled by the anatomic structures probed by MRI, and also because they reflect a quantitative assessment, which makes them more appropriate parameters to correlate with quantitative MR changes for investigating the relationship between functional outcome and brain pathology/function (Virley et al, 2000). Lastly, general neurologic status was monitored via Bederson scoring as this qualitative grading system is commonly used to assesses behavior in experimental models of cerebral ischemia (De Ryck et al, 1992).
At 24 h after tMCAO, subsequent to MRI and behavioral testing, animals were killed under anesthesia and their brains sectioned coronally into seven 1.5 mm-thick slices (corresponding to the MR slices), which were then stained with 2,3,5-triphenyltetrazolium chloride (TTC) for postmortem infarct volume calculation with edema correction (Meng et al, 2004).
Statistical Analysis
Data are presented as mean ± s.d. unless otherwise indicated, and were analyzed with SigmaStat software (Rockware Inc., Golden, CO, USA). Statistical comparisons were performed using analysis of variance (with repeated measures where appropriate) with post hoc Dunn’s or Tukey’s test for multiple comparisons and two-tailed paired or unpaired Student’s t-test, where appropriate. Correlation analyses employed Pearson’s product moment- or the Spearman rank-order tests. P < 0.05 was considered significant.
Results
Physiologic Measurements
All basal physiologic parameters did not significantly differ between groups or before and after tMCAO/sham operation (P > 0.05; data not shown), and were consistent with previous data obtained in normal isoflurane-anesthetized rats respiring spontaneously (Sicard et al, 2003). There were no statistically significant changes in recorded measures during forepaw stimulation in both groups at all time points (P > 0.05). Transient increases in RR and EtCO2 were recorded during hypercapnic challenge (P < 0.05), the magnitudes of which being similar between groups and time points and consistent with changes observed in normal rats under similar experimental conditions (Sicard and Duong, 2005). All proceeding shown data are exclusive to the tMCAO group.
Ischemic Damage
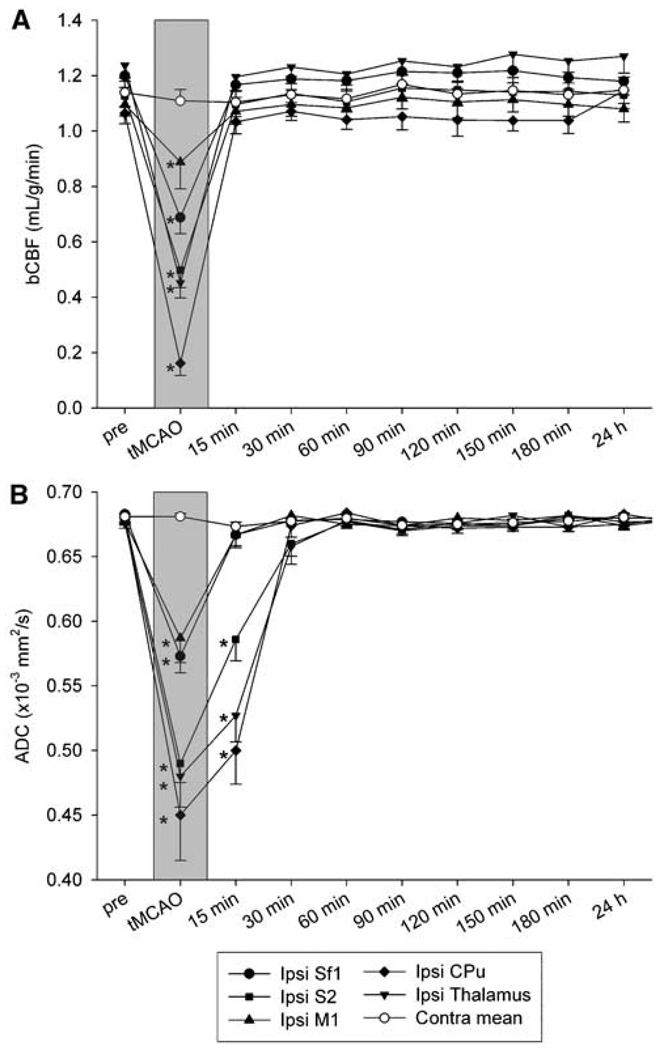
CBF and ADC lesions (197 ± 30 and 110 ± 26 mm3 volumes, respectively) were present during occlusion in the right MCA territory as shown in Figure 1. The temporal evolution of local quantitative bCBF and ADC changes in tMCAO rats is given in Figure 2. During tMCAO, bCBF and ADC values within investigated anatomic areas were significantly and heterogeneously reduced compared with corresponding preocclusion or contralesional regions (P < 0.05), with the greatest decreases found in subcortical structures such as CPu and milder perturbations occurring within lateral borderzone regions such as primary somatosensory cortex, which obtain collateral perfusion from the anterior communicating artery (Shen et al, 2005). All perfusion and diffusion abnormalities fully resolved by 15 and 30 mins postreperfusion, respectively, and no rats developed secondary lesions. At all postocclusion time points, ADC and bCBF values within contralesional regions did not significantly differ from preocclusion or sham-group values of corresponding localities (P > 0.05). Swelling or lesions were not present on T2 and TTC in either hemisphere 24 h after tMCAO (Figure 1). In sham-operated rats, there were no statistically significant differences in ADC, bCBF, and T2 values between hemispheres throughout the period of observation (P > 0.05), nor were TTC anomalies present.
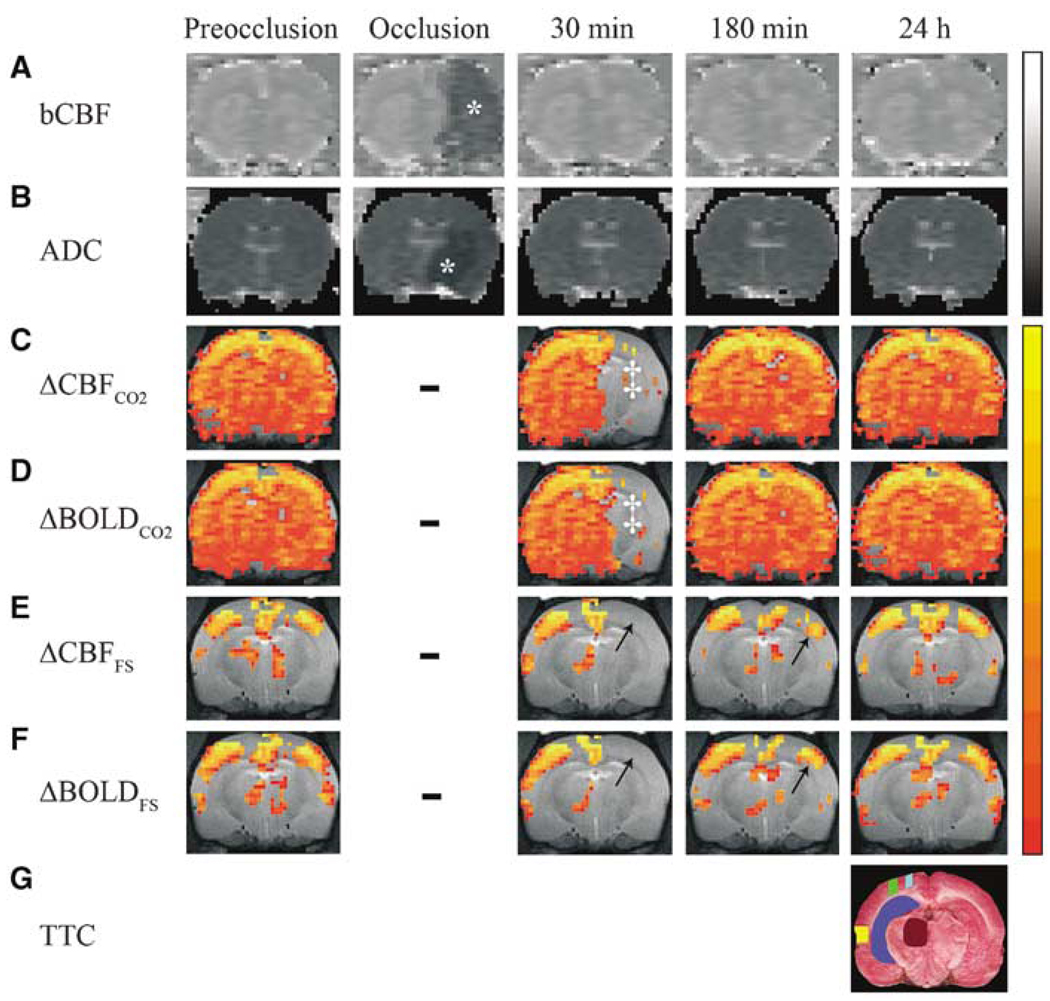
Figure 1.
Representative magnetic resonance (MR) and histological images of a 20-mins transient middle cerebral artery occlusion (tMCAO) rat at selected time points showing (A) basal cerebral blood flow (bCBF) (B) apparent diffusion coefficient (ADC), (C) ΔCBFCO2, (D) ΔBOLDCO2, (E) ΔCBFFS, and (F) ΔBOLDFS maps as well as (G) 2,3,5-triphenyltetrazolium chloride (TTC) staining. CO2 and FS subscripts refer to functional magnetic resonance imaging (fMRI) signal changes evoked by hypercapnic challenge and forepaw stimulation, respectively. Gray-scale bar indicates ADC ranging from 0 to 0.001 mm2/sec and bCBF from 0 to 3 mL/g/min. Color-scale bar indicates ΔCBF ranging from 10 to 200% and ΔBOLD from 1% to 5%. *ADC and CBF lesions. ‡Area of impaired response to 5% CO2. Arrows indicate impaired response to forepaw stimulation. Dashes indicate fMRI not performed during occlusion. Activation maps were overlaid on anatomic images, and a select slice (i.e., fourth-most anterior, corresponding to −2.12 mm from bregma) and percent-thresholds were chosen for visual clarity. ROIs overlaid on TTC are CPu (dark blue), M1 (light blue), thalamus (brown), Sf1 (green), and S2 (yellow).
Figure 2.
Temporal evolution of (A) basal cerebral blood flow (bCBF) and (B) apparent diffusion coefficient (ADC) within ROIs in 20-mins transient middle cerebral artery occlusion (tMCAO ) rats (n = 6; mean ± s.e.m.). Contralesional bCBF and ADC did not differ significantly between ROIs or time points before and after ischemia and were averaged into their respective contralesional means (Contra mean). *P < 0.05 versus ipsilesional (Ipsi) preocclusion (pre).
Functional Magnetic Resonance Imaging
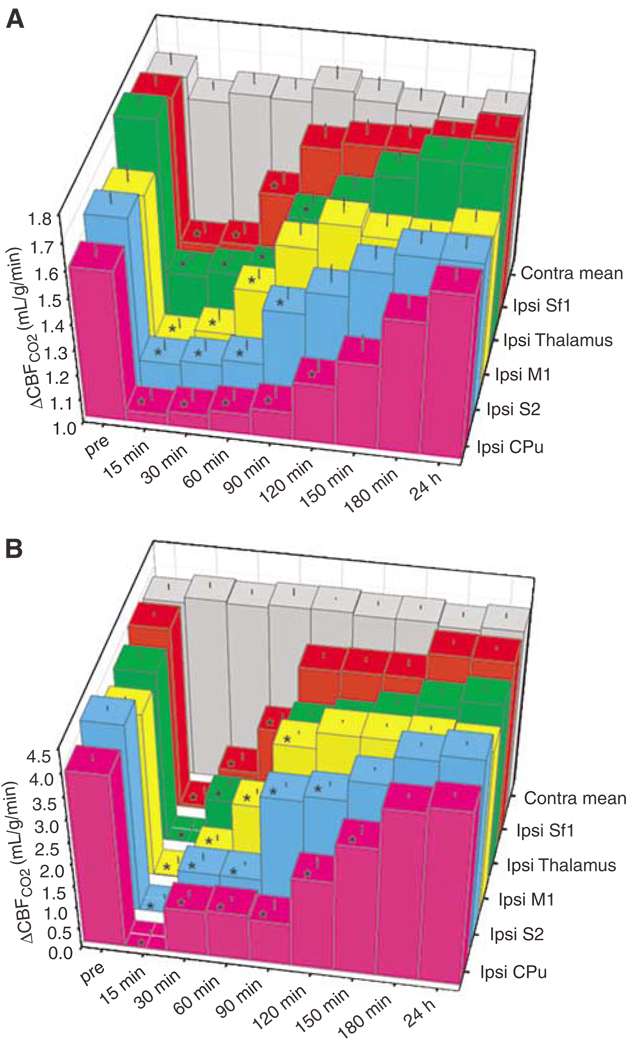
Hypercapnic challenges were employed to assess cerebrovascular reactivity. Hypercapnia fMRI maps and region-specific temporal evolution of ΔCBFCO2 and ΔBOLDCO2 responses of tMCAO rats are shown in Figures 1 and 3, respectively. Before occlusion, hypercapnic challenge produced robust increases in BOLD and CBF, which did not significantly differ between ROIs intra- or interhemispherically (P > 0.05). Shortly after ischemia, ΔBOLDCO2 and ΔCBFCO2 were nearly zero in ipsilesional ROIs and thereafter showed a differential recovery rate across localities with, for example, Sf1 and CPu renormalizing first (90 mins) and last (180 mins), respectively. There were no significant differences in ΔBOLDCO2 and ΔCBFCO2 across ROIs, hemispheres, or time in sham animals (P > 0.05).
Figure 3.
Temporal evolution of cerebrovascular reactivity in terms of hypercapnia-evoked (A) ΔCBFCO2 and (B) ΔBOLDCO2 within ROIs in 20-mins transient middle cerebral artery occlusion (tMCAO) rats (n = 6; mean + s.e.m.). All ipsilesional (Ipsi) regions showed significant reductions of ΔCBFCO2 and ΔBOLDCO2 after reperfusion, which recovered between 90 to 180 mins. Contralesional ΔCBFCO2 and ΔBOLDCO2 did not differ significantly between ROIs or time points before and after ischemia and were averaged into their respective contralesional means (Contra mean). *P < 0.05 versus ipsilesional (Ipsi) preocclusion (pre).
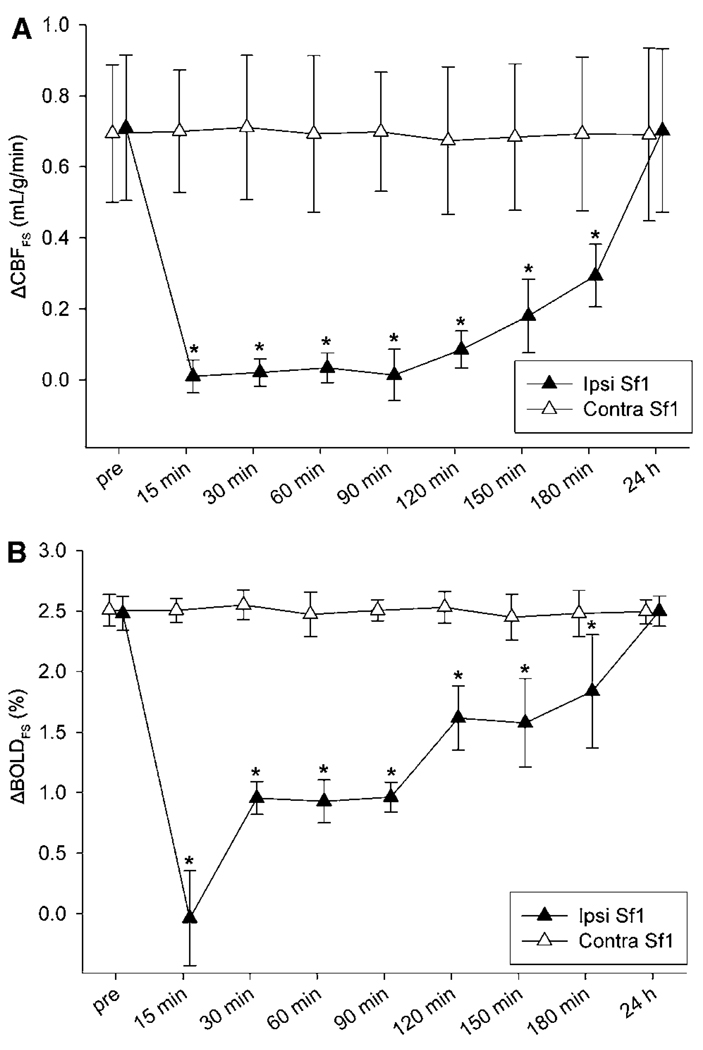
Forepaw stimulation fMRI maps and the temporal profile of ΔCBFFS and ΔBOLDFS responses within Sf1 are shown in Figures 1 and 4, respectively. Before tMCAO, there were no significant interhemispheric differences in ΔBOLDFS (2.5% ± 0.1% (ipsilesional) versus 2.4% ± 0.2% (contralesional); P > 0.05) and ΔCBFFS (0.70 ± 0.20 mL/g/min (ipsilesional) versus 0.68 ± 0.17 mL/g/min (contralesional); P > 0.05). However, immediately after transient ischemia, ipsilesional ΔCBFFS and ΔBOLDFS were negligible and differed significantly from preocclusion and contralesional values (P < 0.05), suggesting an uncoupling of metabolic activity and blood flow. ΔCBFFS and ΔBOLDFS responses were found to be normal at the 24 h time point. Notably, recovery of activation responses to somatosensory forepaw stimulation lagged significantly behind that of hypercapnic challenges within ipsilesional Sf1. Contralesional ΔCBFFS and ΔBOLDFS activations did not significantly differ between pre- and postocclusion time points, nor from corresponding sham values (P > 0.05). In sham-operated rats, there were no statistically significant differences in ΔBOLDFS and ΔCBFFS between sides and time points. Lastly, in both groups, occasional activations during forepaw stimulation were observed in M1, S2, and subcortical areas at time points before and after reperfusion as is typical for rats under isoflurane anesthesia (Dijkhuizen et al, 2001; Sicard and Duong, 2005).
Figure 4.
Temporal evolution of forepaw stimulation-evoked (A) ΔCBFFS and (B) ΔBOLDFS within ipsilesional (Ipsi) and contralesional (Contra) Sf1 in 20-mins tMCAO rats (n = 6; mean ± s.d.). *P < 0.05 versus preocclusion (pre).
Behavioral Status
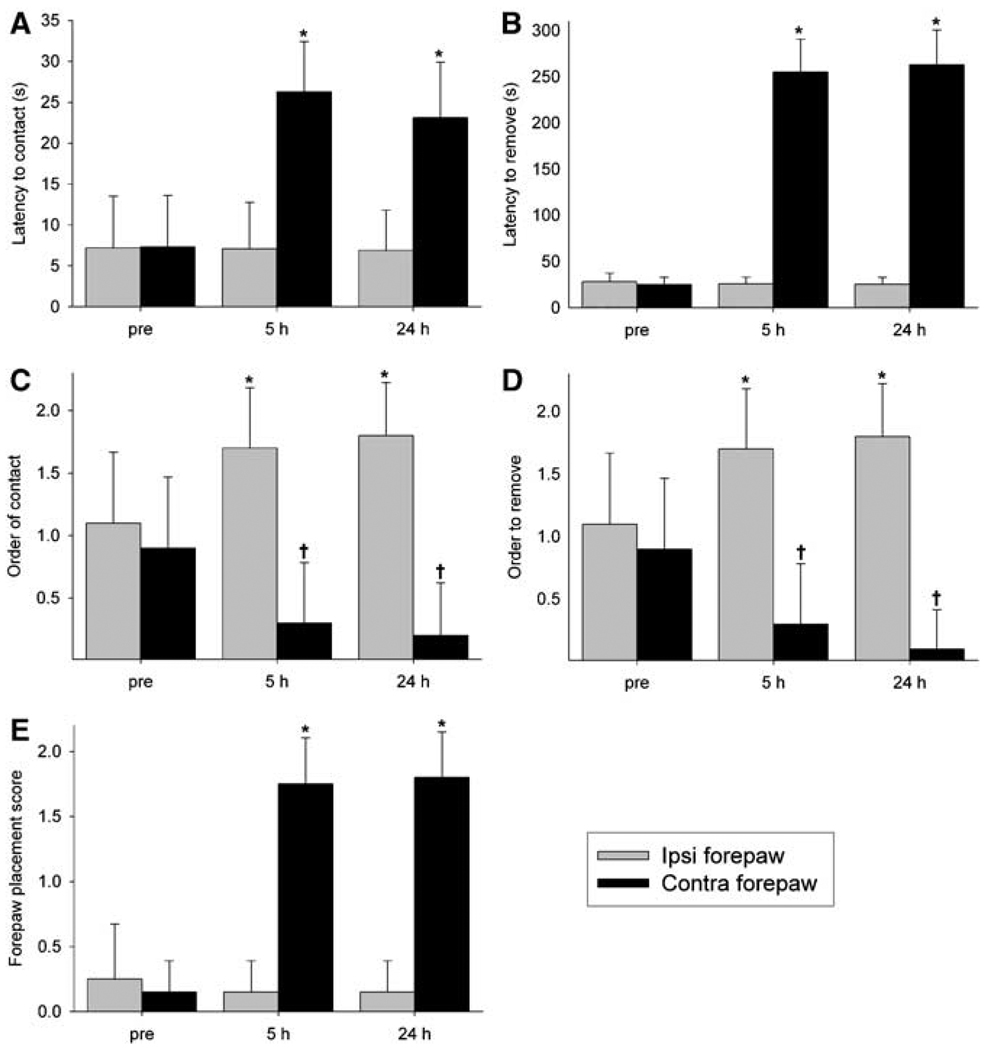
Figure 5 summarizes behavioral test results of tMCAO rats. Groups did not differ significantly in performance before surgery (P > 0.05; data not shown), indicating that they were matched for functional ability (Virley et al, 2000). At 5 h after tMCAO, rats displayed side-specific sensorimotor abnormalities relative to sham and preocclusion testing, which remained significant at 24 h (P < 0.05). Specifically, adhesive removal testing revealed increases in latency to contact and remove labels from the contralesional forepaw, indicating contralesional neglect (Virley et al, 2000). This test also showed a significant group × side × time interaction for order of label contact and removal, which further reflected a postischemic contralesional neglect and ipsilesional bias to sensory stimuli (Virley et al, 2000). The forepaw placement test showed preserved ipsilesional function with significantly increased contralesional limb placing scores. Lastly, Bederson scoring was normal (score = 0) at all investigated time points, which is not surprising given this test grades generalized behavior shown to be largely unaffected by short periods of transient ischemia (De Ryck et al, 1992). In sham rats, there were no statistically significant differences in behavioral parameters between sides and across time (P > 0.05).
Figure 5.
Mean latency to (A) contact and (B) remove as well as order to (C) contact and (D) remove adhesive labels placed around ipsilesional (Ipsi) and contralesional (Contra) forepaws, and (E) forepaw placement scores in 20-mins transient middle cerebral artery occlusion (tMCAO) rats (n = 6; mean + s.e.m). *P < 0.05 versus preocclusion (pre) Contra forepaw and †P < 0.05 versus pre Ipsi forepaw.
Correlation Analyses
We attempted to compare behavioral responses versus a wide array of functional (ΔCBFCO2, ΔBOLDCO2, ΔCBFFS, and ΔBOLDFS) and structural (ADC, bCBF, and T2) MRI parameters within ROIs after tMCAO. There were no significant correlations between MRI values within ipsilesional ROIs with any contralesional behavioral test measure at all individual time points and when temporally matched data were grouped together (R ranging from −0.41 to 0.79; P > 0.05 for all).
Discussion
This study serially tracked regional changes in the spatiotemporal evolution of quantitative ADC, bCBF, T2, and fMRI tissue signatures after transient MCAO in the rat and correlate these with appropriately specific sensorimotor behavioral measures. The major results of this study are: (1) 20 mins of transient focal cerebral ischemia produces ADC and CBF lesions that resolve permanently by 30 mins after reperfusion with no significant changes on T2 or TTC, (2) ipsilesional cerebrovascular reactivity and activation responses to somatosensory stimulation—assessed via fMRI—remain significantly perturbed in otherwise normal-appearing tissue for up to 3 and 24 h after reperfusion, respectively, (3) behavioral dysfunctions persist at 24 h despite complete renormalization of all MR parameters, and thus (4) there appears to be no significant association between the normalization of imaging modalities and recovery of behavior acutely after 20 mins of tMCAO.
Ischemic Damage
Perfusion-weighted imaging is widely used to measure cerebral blood flow during and after ischemia (Li et al, 2000a; Shen et al, 2005) with the technique used herein being sufficiently accurate and sensitive to quantitatively estimate bCBF reductions during and after transient and permanent ischemia produced by the employed MCAO model (Li et al, 2000a; Shen et al, 2005). Our reported region-specific variability in the magnitude of bCBF reduction during occlusion has been recognized in experimental focal ischemia (Dijkhuizen et al, 1998; Hakim et al, 1992; Marcoux et al, 1982), with perfusion deficits below a critical threshold producing metabolic energy failure and subsequent cellular swelling (Shen et al, 2005). The permanent renormalization of bCBF subsequent to reperfusion is also in agreement with previous studies (Dijkhuizen et al, 1998; Li et al, 2000a) and suggests that any postischemic injury in this study may not be because of a secondary compromise of blood flow.
Diffusion-weighted imaging is capable of mapping reversible and irreversible injury from the associated reduction of ADC and is widely recognized as a powerful tool for early detection and evaluation of transient cerebral ischemic damage in both animal models and humans (Coutts et al, 2005; Li et al, 2000a). Prior studies indicate that ADC lesions are potentially reversible when reperfusion is performed quickly after ischemia, with the permanency of reversal dependent on the duration of ischemia (Li et al, 2000a). Indeed, rats undergoing less than 30 mins of ischemia typically have complete reversal of initial ADC lesion within 15 to 60 mins after reperfusion (Li et al, 2000a). The present study corroborates this finding and further showed that certain brain regions appear more susceptible to ischemic insult than others. For example, the severity of ADC decline within the CPu during occlusion was significantly greater than in cortical regions. This selective vulnerability is likely because of heterogeneities in local bCBF during tMCAO (Dijkhuizen et al, 1998; Pulsinelli et al, 1982). However, other factors such as differences in postsynaptic organization (Mitani et al, 1992) as well as variations in mitochondrial capacity (Ter Horst et al, 1994) and the activity of free radical defense systems (Dijkhuizen et al, 2003) may also be involved.
Brain Function
Functional magnetic resonance imaging is gaining wide acceptance as a method for assessing the spatial and temporal dynamics of tissue function and how it relates to behavioral recovery and pathophysiology after ischemic insult to the mammalian brain, yet a clear relationship between these parameters remains unknown (Dijkhuizen et al, 2001, 2003; Nhan et al, 2004; Shen et al, 2005). Here, we found that transient focal ischemia led to dysfunctions of cerebrovascular responsivity to CO2 and neurovascular coupling response to somatosensory stimulation that were associated with perfusion/diffusion declines but remained abnormal far longer. These findings are in accord with previous animal studies reporting loss of stimulus-evoked cerebral hemodynamic responses that slowly recovered but were still significantly depressed up to 24 h after full renormalization of ADC (Reese et al, 2000; Schmitz et al, 1997). They are also supported by a human study showing persistently dysfunctional metabolic activity within the affected hemisphere of patients with clinically diagnosed transient ischemic attack (TIA) subsequently lacking ischemic lesions or hypoperfusion (Bisschops et al, 2002). Together, our findings suggest that fMRI is capable of identifying dysfunctional neural tissue that appears completely normal on diffusion-, perfusion-, and T2-weighted imaging and, thus, could potentially serve as a more sensitive and complementary indicator to such techniques for detecting ischemic brain injury.
Different, potentially interacting pathophysiologic mechanisms may be responsible for the recalcitrance of functional recovery within the sensorimotor network, including damage to its efferent and/or afferent projections (Andrews, 1991), tissue injury secondary to edematous swelling (Fagan et al, 2005), alterations in F-actin filaments in vascular smooth muscle cells (Kwon et al, 2002), selective neuronal necrosis (Li et al, 2000a), and ischemia below the threshold for irreversible damage but above the threshold for neuronal dysfunction (Astrup et al, 1981). The former two possibilities are remote given the apparently normal MR findings in nonsensorimotor network brain regions and no evidence of brain swelling on TTC or MRI, respectively. However, the latter three remain viable candidates. For instance, cytoskeletal disruption of vascular smooth muscle F-actin has been shown to occur after 15 or 45 mins transient ischemia in a duration-dependent manner (incapacitating its ability to maintain function in response to extrinsic stimuli) and tended to recover within 3 h after reperfusion (Kwon et al, 2002), potentially explaining the temporal profile of fMRI responses acutely after 20 mins tMCAO. Lastly, on visual inspection of fMRI maps, we did not find altered perilesional or contralesional activity thought to be associated with behavioral recovery as reported in some human and animal stroke studies (Dijkhuizen et al, 2003; Rossini and Pauri, 2000). This is plausibly owing to an interstudy difference in ischemic duration (among other methodological considerations), which is known to produce differences in postischemic pathologic processes (Dijkhuizen et al, 2003; Garcia et al, 1995) or such phenomena may simply occur after the end point of this study (Jones and Schallert, 1992).
Interestingly, there was a differential recovery rate in cerebrovascular reactivity to CO2 and neurovascular coupling response to forepaw somatosensory stimulation within ipsilesional Sf1; that is, at several time points, the structure responded normally to CO2 challenge but not to forepaw stimulation. This suggests vasoreactivity must be preserved for neural activity-coupled vascular responses to occur. Given this, one explanation for the lagging recovery of hemodynamic responses to forepaw stimulation in Sf1 is that the tissue itself was functionally silenced or subtly damaged by ischemia. Another possibility is that the thalamus or its afferent projections to Sf1 were damaged, resulting in a ‘secondary’ silencing of an otherwise uninjured cortex. These scenarios are not mutually exclusive and both may have contributed to the observed disconnect in recovery rates, as supported by a prior study (Li et al, 2000b) showing subtle histologic damage in both cortical and subcortical structures after brief focal cerebral ischemia.
Behavioral Status
Functional outcome is necessary to assess the consequences of cerebral ischemia and it is arguable that neurologic status is more relevant than underlying pathophysiology when predicting ‘quality of life’ (Virley et al, 2000). However, no animal experimental studies to date have attempted to relate performance on specific behavioral tasks with pathologic and functional changes occurring within their cerebroanatomic correlates as visualized by multimodal MRI. It has been postulated that such an analysis could yield valuable information on the exact interactions between postischemic events occurring in the brain and outward behavior (Dijkhuizen et al, 2003; Virley et al, 2000). For these reasons, the battery of behavioral tests used herein was chosen as it is sensitive to the effects of tMCAO for up to a period of 90 days (Lindner et al, 2003), models the impairments seen in human stroke patients (Rose et al, 1994), and informs exclusively on the observable activities subserved by the sensorimotor network, thereby permitting a more precise investigation of the relations between changes in MRI and behavioral parameters (Virley et al, 2000).
The persistent contralesional neglect and ipsilesional bias found after tMCAO is consistent with a prior animal study employing similar tests and experimental conditions (Virley et al, 2000) as well as in patients recovering from cerebral ischemia. Surprisingly, however, no significant associations were found between contralesional behavioral parameters and ipsilesional MR findings at all investigated time points; these results are similar to those of prior studies (Dijkhuizen et al, 2001, 2003; Kim et al, 2005) and show that a clear causal relationship between changes in brain and behavior remains to be found. We believe the lack of correlation between MR parameters and neurologic status reported herein to be valid but this finding could be at least partly attributable to other factors. Firstly, MRI may have been unable to detect subtle pathologic changes occurring after brief focal ischemia owing to partial volume effects resulting from inadequate spatial resolution (Li et al, 2000a). Other imaging techniques may be able to detect these changes of cellular integrity, including PET mapping of benzodiazepine receptors (Sette et al, 1993) or a combination of T1 and T2 MRI at 1 week after the onset of ischemia (Fujioka et al, 1999). Secondly, long-term postischemic plastic changes in the brain such as dendritic outgrowth and synaptogenesis (Weiller, 1998) may not have occurred within the time frame of this study as they require days to weeks to develop (Jones and Schallert, 1992). Thus, a correlation between fMRI and behavior may be found chronically after 20 mins tMCAO.
Experimental Limitations
Although this study contains several novel findings, it has some unavoidable design limitations, which will be mitigated by future studies. The persistence of behavioral deficits at 24 h despite normal MR findings suggests that cytologic damage may be present as found by other investigators (Li et al, 2000a), but the current study design provides little insight into this point as TTC staining was performed in place of more sensitive histologic stains. However, this choice was intentional as TTC is an excellent method for correlating in vivo with post-mortem infarct volumes (Chen et al, 2005) as well as for calculating viability thresholds (Bardutzky et al, 2005; Meng et al, 2004; Shen et al, 2003). Now that the MR and TTC results have been shown to be in good agreement, a histologic analysis (i.e., microscopic evaluation of hematoxylin–eosin stained brain tissue) more capable of detecting subtle postischemic pathology is currently being performed serially from 24 h to 21 days after tMCAO. Lastly, the purpose of this study was to examine the evolution of changes in multimodal MRI and behavioral parameters during hyperacute to acute time points after transient cerebral ischemia, which is why a longer experimental endpoint was not chosen. Retrospectively, however, our findings warrant a lengthier observation period that may enhance the ability to find significant correlations between MRI and behavior as well as show whether imaging parameters and brain pathology remain—or do not remain—negative during more chronic stages.
Although consistent with many established methodologies, the functional imaging performed in this study could be subject to several deficiencies and errors. First, bilateral—rather than unilateral—forepaw stimulation was chosen, making it relatively more difficult to assess contralesional activation responses in response to stimulation of a particular forepaw. However, bilateral stimulation is appropriate for functional imaging conducted hyperacutely after ischemia as it enables an equal number of trials to be performed in a shorter duration, thereby enhancing temporal resolution, and also reduces the likelihood of misinterpreting activation data that may be confounded by fluctuations in basal physiologic conditions (Sicard and Duong, 2005) known to occur during and shortly after ischemia (Shen et al, 2005). Secondly, no MRI measurements were made between 3 and 24 h, making it impossible to determine when fMRI responses to forepaw stimulation first renormalized. However, most MRI experiments do not continue past several hours postischemia because of increased mortality associated with prolonged anesthesia (Forrest et al, 1992)—therefore, we opted to terminate imaging at 3 h to improve next-day survivability for TTC staining and follow-up MRI. Lastly, the accuracy of the employed CBF technique may be subject to errors from magnetization transfer, transit time, and water exchange (Zhou et al, 2001), although these effects are negligible in setups such as ours utilizing actively decoupled two-coil systems and small animals (Shen et al, 2005). Even with this potential error, our quantitative fMRI responses arguably serve as better measures considering alternative techniques that index relative percent-changes, which may incorrectly reflect neural activity in light of aforementioned potential changes in baseline physiology.
Clinical Implications
Irrespective of its limitations, this study may provide clinicians with at least two pieces of important information. First, the data seem to uphold the notion that time is critical in the context of cerebrovascular disease (Coutts et al, 2005; Hacke et al, 2004). Acute brain imaging has been proven to be useful in the differential diagnosis, triage, and management of patients with TIA or stroke (Coutts et al, 2005; Hacke et al, 2004). However, this study suggests that the utility of acute imaging may be compromised by the passage of time periods as short as half an hour in, for example, cases where clinically significant cerebral ischemia results in readily discernible yet transient alterations in ADC. Secondly, the data argue that normal DWI, PWI, and T2 imaging after brief periods of ischemia may not indicate normal tissue status as fMRI and behavior remain impaired and, for this reason, fMRI may be a useful adjunct for evaluating the efficacy of potential neuroprotective therapies. Lastly, this model may help explain persisting neurologic deficits in subsets of patients with a normal MRI battery after cerebral ischemia (Ay et al, 1999).
In conclusion, the present study shows that 20 mins tMCAO produces sensorimotor behavioral deficits that continue despite recovery of multimodal MR parameters in their cerebroanatomic correlates. These findings may be relevant to a clinical subset of patients with negative MRI results but persistent neurologic morbidities.
Acknowledgements
We acknowledge our colleagues for their help with this study, including Drs JG Dobson, JA Harder, KG Helmer, JA King, and Q Shen as well as J Bouley and KF Schmidt
This work was supported in part by an NIH (NINDS, R01-DA01915802) grant to CF, and NIH (NINDS, R01-NS45879) and American Heart Association (SDG-0430020N) grants to TQD.
References
- Andrews RJ. Transhemispheric diaschisis. A review and comment. Stroke. 1991;22:943–949. doi: 10.1161/01.str.22.7.943. [DOI] [PubMed] [Google Scholar]
- Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- Ay H, Buonanno FS, Rordorf G, Schaefer PW, Schwamm LH, Wu O, Gonzalez RG, Yamada K, Sorensen GA, Koroshetz WJ. Normal diffusion-weighted MRI during stroke-like deficits. Neurology. 1999;52:1784–1792. doi: 10.1212/wnl.52.9.1784. [DOI] [PubMed] [Google Scholar]
- Bardutzky J, Shen Q, Henninger N, Bouley J, Duong TQ, Fisher M. Differences in ischemic lesion evolution in different rat strains using diffusion and perfusion imaging. Stroke. 2005;36:2000–2005. doi: 10.1161/01.STR.0000177486.85508.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JC, Warach S. Imaging. Stroke. 2005;36:196–199. doi: 10.1161/01.STR.0000154559.03784.db. [DOI] [PubMed] [Google Scholar]
- Bisschops RH, Kappelle LJ, Mali WP, van der Grond J. Hemodynamic and metabolic changes in transient ischemic attack patients—a magnetic resonance angiography and (1)H-magnetic resonance spectroscopy study performed within 3 days of onset of a transient ischemic attack. Stroke. 2002;33:110–115. doi: 10.1161/hs0102.100879. [DOI] [PubMed] [Google Scholar]
- Chen F, Suzuki Y, Nagai N, Peeters R, Marchal G, Ni Y. Dynamic susceptibility contrast-enhanced perfusion MR imaging at 1.5 T predicts final infarct size in a rat stroke model. J Neurosci Methods. 2005;141:55–60. doi: 10.1016/j.jneumeth.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Coutts SB, Simon JE, Eliasziw M, Sohn CH, Hill MD, Barber PA, Palumbo V, Kennedy J, Roy J, Gagnon A, Scott JN, Buchan AM, Demchuk AM. Triaging transient ischemic attack and minor stroke patients using acute magnetic resonance imaging. Ann Neurol. 2005;57:848–854. doi: 10.1002/ana.20497. [DOI] [PubMed] [Google Scholar]
- Daffertshofer M, Mielke O, Pullwitt A, Felsenstein M, Hennerici M. Transient ischemic attacks are more than ‘ministrokes’. Stroke. 2004;35:2453–2458. doi: 10.1161/01.STR.0000144050.90132.8e. [DOI] [PubMed] [Google Scholar]
- De Ryck M, Van Reempts J, Duytschaever H, Van Deuren B, Clincke G. Neocortical localization of tactile/proprioceptive limb placing reactions in the rat. Brain Res. 1992;573:44–60. doi: 10.1016/0006-8993(92)90112-m. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Knollema S, van der Worp HB, Ter Horst GJ, De Wildt DJ, Berkelbach van der Sprenkel JW, Tulleken KA, Nicolay K. Dynamics of cerebral tissue injury and perfusion after temporary hypoxia–ischemia in the rat—evidence for region-specific sensitivity and delayed damage. Stroke. 1998;29:695–704. doi: 10.1161/01.str.29.3.695. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Ren J, Mandeville JB, Wu O, Ozdag FM, Moskowitz MA, Rosen BR, Finklestein SP. Functional magnetic resonance imaging of reorganization in rat brain after stroke. Proc Natl Acad Sci USA. 2001;98:12766–12771. doi: 10.1073/pnas.231235598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkhuizen RM, Singhal AB, Mandeville JB, Wu O, Halpern EF, Finklestein SP, Rosen BR, Lo EH. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats—a functional magnetic resonance imaging study. J Neurosci. 2003;23:510–517. doi: 10.1523/JNEUROSCI.23-02-00510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong TQ, Silva AC, Lee SP, Kim SG. Functional MRI of calcium-dependent synaptic activity—cross correlation with CBF and BOLD measurements. Magn Reson Med. 2000;43:383–392. doi: 10.1002/(sici)1522-2594(200003)43:3<383::aid-mrm10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Fagan SC, Hess DC, Machado LS, Hohnadel EJ, Pollock DM, Ergul A. Tactics for vascular protection after acute ischemic stroke. Pharmacotherapy. 2005;25:387–395. doi: 10.1592/phco.25.3.387.61592. [DOI] [PubMed] [Google Scholar]
- Forrest JB, Rehder K, Cahalan MK, Goldsmith CH. Multicenter study of general anesthesia. III. Predictors of severe perioperative adverse outcomes. Anesthesiology. 1992;76:3–15. doi: 10.1097/00000542-199201000-00002. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Taoka T, Matsuo Y, Hiramatsu KI, Sakaki T. Novel brain ischemic change on MRI. Delayed ischemic hyperintensity on T1-weighted images and selective neuronal death in the caudoputamen of rats after brief focal ischemia. Stroke. 1999;30:1043–1046. doi: 10.1161/01.str.30.5.1043. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Liu KF, Ho KL. Neuronal necrosis after middle cerebral artery occlusion in Wistar rats progresses at different time intervals in the caudoputamen and the cortex. Stroke. 1995;26:636–642. doi: 10.1161/01.str.26.4.636. (discussion 643) [DOI] [PubMed] [Google Scholar]
- Gavrilescu T, Kase CS. Clinical stroke syndromes—clinical–anatomical correlations. Cerebrovasc Brain Metab Rev. 1995;7:218–239. [PubMed] [Google Scholar]
- Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, Brott T, Frankel M, Grotta JC, Haley EC, Jr, Kwiatkowski T, Levine SR, Lewandowski C, Lu M, Lyden P, Marler JR, Patel S, Tilley BC, Albers G, Bluhmki E, Wilhelm M, Hamilton S. Association of outcome with early stroke treatment—pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- Hakim AM, Hogan MJ, Carpenter S. Time course of cerebral blood flow and histological outcome after focal cerebral ischemia in rats. Stroke. 1992;23:1138–1143. doi: 10.1161/01.str.23.8.1138. discussion 1143–1134. [DOI] [PubMed] [Google Scholar]
- Jones TA, Schallert T. Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Res. 1992;581:156–160. doi: 10.1016/0006-8993(92)90356-e. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Alger JR, Di Salle F, Starkman S, Villablanca P, Bentson J, Saver JL. Diffusion MRI in patients with transient ischemic attacks. Stroke. 1999;30:1174–1180. doi: 10.1161/01.str.30.6.1174. [DOI] [PubMed] [Google Scholar]
- Kim YR, Huang IJ, Lee SR, Tejima E, Mandeville JB, van Meer MP, Dai G, Choi YW, Dijkhuizen RM, Lo EH, Rosen BR. Measurements of BOLD/CBV ratio show altered fMRI hemodynamics during stroke recovery in rats. J Cereb Blood Flow Metab. 2005;25:820–829. doi: 10.1038/sj.jcbfm.9600084. [DOI] [PubMed] [Google Scholar]
- Kwon O, Phillips CL, Molitoris BA. Ischemia induces alterations in actin filaments in renal vascular smooth muscle cells. Am J Physiol Renal Physiol. 2002;282:F1012–F1019. doi: 10.1152/ajprenal.00294.2001. [DOI] [PubMed] [Google Scholar]
- Li F, Han S, Tatlisumak T, Carano RA, Irie K, Sotak CH, Fisher M. A new method to improve in-bore middle cerebral artery occlusion in rats—demonstration with diffusion- and perfusion-weighted imaging. Stroke. 1998;29:1715–1719. doi: 10.1161/01.str.29.8.1715. (discussion 1719–1720) [DOI] [PubMed] [Google Scholar]
- Li F, Liu KF, Silva MD, Omae T, Sotak CH, Fenstermacher JD, Fisher M, Hsu CY, Lin W. Transient and permanent resolution of ischemic lesions on diffusion-weighted imaging after brief periods of focal ischemia in rats—correlation with histopathology. Stroke. 2000a;31:946–954. doi: 10.1161/01.str.31.4.946. [DOI] [PubMed] [Google Scholar]
- Li F, Silva MD, Sotak CH, Fisher M. Temporal evolution of ischemic injury evaluated with diffusion-, perfusion-, and T2-weighted MRI. Neurology. 2000b;54:689–696. doi: 10.1212/wnl.54.3.689. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Gribkoff VK, Donlan NA, Jones TA. Long-lasting functional disabilities in middle-aged rats with small cerebral infarcts. J Neurosci. 2003;23:10913–10922. doi: 10.1523/JNEUROSCI.23-34-10913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZM, Schmidt KF, Sicard KM, Duong TQ. Imaging oxygen consumption in forepaw somatosensory stimulation in rats under isoflurane anesthesia. Magn Reson Med. 2004;52:277–285. doi: 10.1002/mrm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcoux FW, Morawetz RB, Crowell RM, DeGirolami U, Halsey JH., Jr Differential regional vulnerability in transient focal cerebral ischemia. Stroke. 1982;13:339–346. doi: 10.1161/01.str.13.3.339. [DOI] [PubMed] [Google Scholar]
- Meng X, Fisher M, Shen Q, Sotak CH, Duong TQ. Characterizing the diffusion/perfusion mismatch in experimental focal cerebral ischemia. Ann Neurol. 2004;55:207–212. doi: 10.1002/ana.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani A, Andou Y, Kataoka K. Selective vulnerability of hippocampal CA1 neurons cannot be explained in terms of an increase in glutamate concentration during ischemia in the gerbil—brain microdialysis study. Neuroscience. 1992;48:307–313. doi: 10.1016/0306-4522(92)90492-k. [DOI] [PubMed] [Google Scholar]
- Nhan H, Barquist K, Bell K, Esselman P, Odderson IR, Cramer SC. Brain function early after stroke in relation to subsequent recovery. J Cereb Blood Flow Metab. 2004;24:756–763. doi: 10.1097/01.WCB.0000122744.72175.9C. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Boston: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Levy DE, Duffy TE. Regional cerebral blood flow and glucose metabolism following transient forebrain ischemia. Ann Neurol. 1982;11:499–502. doi: 10.1002/ana.410110510. [DOI] [PubMed] [Google Scholar]
- Reese T, Bjelke B, Porszasz R, Baumann D, Bochelen D, Sauter A, Rudin M. Regional brain activation by bicuculline visualized by functional magnetic resonance imaging. Time-resolved assessment of bicuculline-induced changes in local cerebral blood volume using an intravascular contrast agent. NMR Biomed. 2000;13:43–49. doi: 10.1002/(sici)1099-1492(200002)13:1<43::aid-nbm608>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28:2060–2065. doi: 10.1161/01.str.28.10.2060. (discussion 2066) [DOI] [PubMed] [Google Scholar]
- Rose L, Bakal DA, Fung TS, Farn P, Weaver LE. Tactile extinction and functional status after stroke. A preliminary investigation. Stroke. 1994;25:1973–1976. doi: 10.1161/01.str.25.10.1973. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Pauri F. Neuromagnetic integrated methods tracking human brain mechanisms of sensorimotor areas ‘plastic’ reorganisation. Brain Res Brain Res Rev. 2000;33:131–154. doi: 10.1016/s0169-328x(00)00090-5. [DOI] [PubMed] [Google Scholar]
- Schmitz B, Bottiger BW, Hossmann KA. Functional activation of cerebral blood flow after cardiac arrest in rat. J Cereb Blood Flow Metab. 1997;17:1202–1209. doi: 10.1097/00004647-199711000-00009. [DOI] [PubMed] [Google Scholar]
- Sette G, Baron JC, Young AR, Miyazawa H, Tillet I, Barre L, Travere JM, Derlon JM, MacKenzie ET. In vivo mapping of brain benzodiazepine receptor changes by positron emission tomography after focal ischemia in the anesthetized baboon. Stroke. 1993;24:2046–2057. doi: 10.1161/01.str.24.12.2046. (discussion 2057–2048) [DOI] [PubMed] [Google Scholar]
- Shen Q, Meng X, Fisher M, Sotak CH, Duong TQ. Pixel-by-pixel spatiotemporal progression of focal ischemia derived using quantitative perfusion and diffusion imaging. J Cereb Blood Flow Metab. 2003;23:1479–1488. doi: 10.1097/01.WCB.0000100064.36077.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Ren H, Cheng H, Fisher M, Duong TQ. Functional, perfusion and diffusion MRI of acute focal ischemic brain injury. J Cereb Blood Flow Metab. 2005;25:1265–1279. doi: 10.1038/sj.jcbfm.9600132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard KM, Duong TQ. Effects of hypoxia, hyperoxia, and hypercapnia on baseline and stimulus-evoked BOLD, CBF, and CMRO2 in spontaneously breathing animals. Neuroimage. 2005;25:850–858. doi: 10.1016/j.neuroimage.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard K, Shen Q, Brevard ME, Sullivan R, Ferris CF, King JA, Duong TQ. Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions—implications for functional MRI studies. J Cereb Blood Flow Metab. 2003;23:472–481. doi: 10.1097/01.WCB.0000054755.93668.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Lee SP, Yang G, Iadecola C, Kim SG. Simultaneous blood oxygenation level-dependent and cerebral blood flow functional magnetic resonance imaging during forepaw stimulation in the rat. J Cereb Blood Flow Metab. 1999;19:871–879. doi: 10.1097/00004647-199908000-00006. [DOI] [PubMed] [Google Scholar]
- Stejskal EO, Tanner JE. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Phys. 1965;42:288–292. [Google Scholar]
- Ter Horst GJ, Knollema S, Stuiver B, Hom H, Yoshimura S, Ruiters MH, Korf J. Differential glutathione peroxidase mRNA up-regulations in rat forebrain areas after transient hypoxia–ischemia. Ann NY Acad Sci. 1994;738:329–333. doi: 10.1111/j.1749-6632.1994.tb21819.x. [DOI] [PubMed] [Google Scholar]
- Virley D, Beech JS, Smart SC, Williams SC, Hodges H, Hunter AJ. A temporal MRI assessment of neuropathology after transient middle cerebral artery occlusion in the rat—correlations with behavior. J Cereb Blood Flow Metab. 2000;20:563–582. doi: 10.1097/00004647-200003000-00015. [DOI] [PubMed] [Google Scholar]
- Weiller C. Imaging recovery from stroke. Exp Brain Res. 1998;123:13–17. doi: 10.1007/s002210050539. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wilson DA, Ulatowski JA, Traystman RJ, van Zijl PC. Two-compartment exchange model for perfusion quantification using arterial spin tagging. J Cereb Blood Flow Metab. 2001;21:440–455. doi: 10.1097/00004647-200104000-00013. [DOI] [PubMed] [Google Scholar]