Abstract
Toll-like receptors (TLRs) have a central role in innate immunity as they detect conserved pathogen-associated molecular patterns (PAMPs) on a range of microbes, including viruses, leading to innate immune activation and orchestration of the adaptive immune response. To date, a large number of viruses have been shown to trigger innate immunity via TLRs, suggesting that these receptors are likely to be important in the outcome to viral infection. This suggestion is supported by the observation that many viruses have evolved mechanisms not only to evade the innate immune system, but also to subvert it for the benefit of the virus. In this review we will discuss earlier evidence, mainly from knock-out mice studies, implicating TLRs in the innate immune response to viruses, in light of more recent clinical data demonstrating that TLRs are important for anti-viral immunity in humans.
Keywords: human studies, innate immunity, signal transduction, Toll-like receptor, viral evasion
Introduction
Innate immunity is made possible by a network of germ-line encoded pattern-recognition receptors (PRRs), which detect pathogen-associated molecular patterns (PAMPs) on invading microbes and trigger immunological responses. PRRs include the Nod-like receptors (NLRs), RIG-like receptors (RLRs), Toll-like receptors (TLRs) and the recently identified cytosolic DNA receptors [1–4].
TLRs are type 1 membrane spanning receptors that consist of an extracellular leucine-rich repeat (LRR) domain, a transmembrane-spanning domain and a cytoplasmic Toll-interleukin (IL)-1 receptor-resistance (TIR) domain. TLRs can be divided broadly into those that are located at the cell surface and those that are located to the intracellular endosomal compartment. TLR4 and 5 are expressed on the cell surface and detect lipopolysaccharide and bacterial flagellin, respectively, while TLR1/2 and TLR2/6 exist as heterodimers on the cell surface and detect bacterial triacylated and diacylated lipoprotein, respectively. In contrast, TLR3, 7, 8 and 9 are expressed on endosomes and detect microbial nucleic acids [5]. In order for signalling from these TLRs to occur endosomal acidification and maturation are required, leading to the production of proinflammatory cytokines and type I interferons (IFNs) [6].
Signalling downstream of the TLRs is made possible by the existence of cytosolic TIR domain-containing adaptor proteins MyD88 (myeloid differentiation factor 88), MAL (MyD88 adaptor-like, also known as TIRAP), TRIF (TIR-domain-containing adaptor protein inducing IFN-β, also known as TICAM1) and TRAM (TRIF-related adaptor molecule, also known as TICAM2) [7]. The fifth member of the group, SARM (sterile α- and armadillo-motif-containing protein), has been reported to be an inhibitor of TRIF [8]. Engagement of TLRs with these TIR adaptors results in the activation of cytosolic signalling complexes containing TRAF and IRAK proteins, leading ultimately to the activation of NF-κB (nuclear factor-kappa B) and the IRF (interferon regulatory factor) family of transcription factors. This triggers the production of proinflammatory cytokines and type I IFNs [7]. NF-κB is necessary for IL-6 and tumour necrosis factor (TNF) production, IFN-β requires both NF-κB and IRF3, while IRF7 is required for IFN-α production [9]. Recent studies on TLR signalling mechanisms have revealed that TLR-induced IRF3 and IRF7 activation is initiated only from the endosome, whereas other signals such as MAP kinase and NF-κB activation can be triggered from the plasma membrane or endosome [10].
Viral inhibitors of TLR signalling components
Early evidence indicating that TLRs might be important in sensing viruses came from observations that some viruses encoded proteins to target TLR signalling. For example, studies of vaccinia virus (VACV) revealed two proteins that target the TLR system for inhibition. A46R was shown to associate with the TIR adaptors to down-regulate TLR signalling, while A52R was shown to target IRAK2 to inhibit TLR-mediated NF-κB activation [11,12]. Another protein of VACV, K7 was shown to target DDX3, required for IFN-β induction [13]. Hepatitis C virus (HCV) is another virus that encodes proteins to inhibit TLR-mediated signalling, in that its protease NS3/4A cleaves TRIF, while NS5A inhibits MyD88 [14,15]. These and other TLR viral inhibitors contribute to virulence [16]. Figure 1 depicts the signalling events following TLR stimulation and the viral evasion strategies that continue to be identified which target these signalling proteins.
Fig. 1.
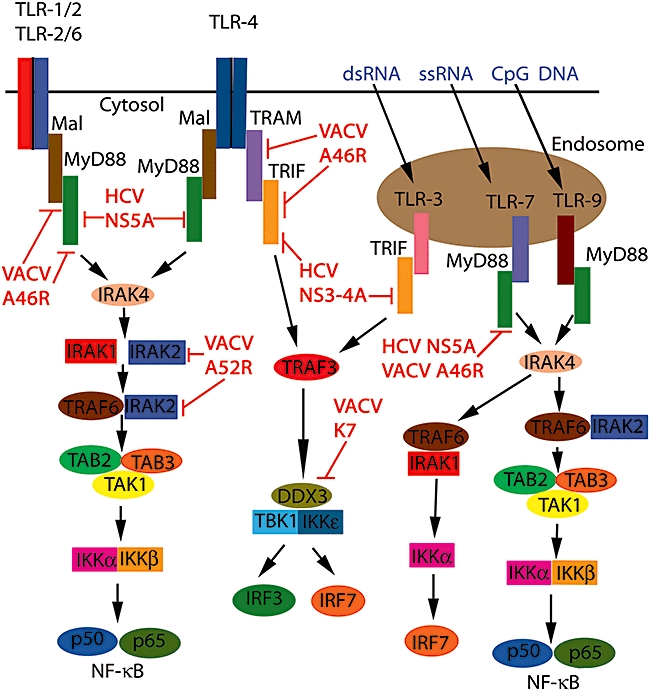
Toll-like receptor (TLR) signalling and inhibition by viral proteins. Upon ligation of the TLRs with their respective ligands, TIR domain-containing adaptors are recruited to the receptors. MyD88 is the prototypical member of the group being utilized by all TLRs except TLR3, which utilizes TRIF only. TLR2 requires MAL along with MyD88 and TLR4 requires all four adapters, including TRAM, as a bridging adapter to TRIF. Adaptor recruitment leads to the activation of interleukin-1 receptor-associated kinase (IRAK4) and the phosphorylation of IRAK1. IRAK1 and IRAK2 signal to tumour necrosis factor (TNF) receptor-associated factor (TRAF)6, which leads to the activation of the transforming growth factor-β-activated kinase 1 (TAK)1-containing complex. This stimulates the kinase activity of the IκB kinase (IKK) complex and triggers the activation of NFκB, allowing its movement into the nucleus and the expression of proinflammatory cytokines. The pathway to IRF3 and IRF7 activation following TRIF stimulation from either TLR3 or TLR4 requires TRAF3 and the IKKε/TRAF family member-associated NF-κB activator-binding kinase 1 (TBK1) complex, resulting in type I IFN production. TLR7 and TLR9 activate the IRAK1/TRAF6 complex that stimulates IKKα phosphorylation and activation of IRF7, allowing the production of type I IFNs. A46R from the vaccinia virus associates with and inhibits all four adaptor proteins, MyD88, MAL, TRAM and TRIF. In contrast, A52R from vaccinia virus (VACV) binds to and inhibits IRAK2, while K7 inhibits DDX3, a component of the IKKε/TBK1 complex. The hepatitis C virus (HCV) protease NS3-4A cleaves and inactivates TRIF, while NS5A, also from HCV, sequesters MyD88.
Members of the TLR family that have been shown to be involved in responses to viral infection are TLR1, TLR2, TLR3, TLR4, TLR6, TLR7, TLR8 and TLR9 [9]. The contribution of each of these receptors to viral infections is discussed below. In particular, we will discuss the evidence emerging that suggests a role for human TLRs in the anti-viral response.
TLR2
Immune responses against a number of DNA viruses such as human cytomegalovirus (HCMV), herpes simplex virus (HSV), Epstein–Barr virus (EBV), VACV and RNA viruses such as lymphocytic choriomeningitis virus (LCMV), HCV and respiratory syncytial virus (RSV), are at least partially dependent upon TLR2 [17–25] (see Table 1 for virus nomenclature). These TLR2-dependent anti-viral responses occur across many different cell types. For example, LCMV in glial cells of the central nervous system (CNS) [18], EBV in monocytes [19] and HSV in microglial cells [20] all elicit TLR2-dependent responses. In human fibroblasts a TLR1/2 heterodimer recognizes the envelope proteins of HCMV leading to the secretion of proinflammatory cytokines [21,26]. In leucocytes a role for TLR2 in anti-viral responses was demonstrated in a study of RSV [22]. It was shown that TLR2/6 heterodimers were important in cytokine responses to RSV and in controlling viral replication in vivo. In addition, neutrophil migration and dendritic cell activation in the lung were dependent upon TLR2–RSV interactions [22].
Table 1.
Virus nomenclature.
| Abbreviation | Virus | Family | Genome structure |
|---|---|---|---|
| HCMV | Human cytomegalovirus | Herpesviridae | dsDNA |
| HSV | Herpes simplex virus | Herpesviridae | dsDNA |
| EBV | Epstein–Barr virus | Herpesviridae | dsDNA |
| VACV | Vaccinia virus | Poxviridae | dsDNA |
| LCMV | Lymphocytic choriomeningitis virus | Arenaviridae | ssRNA (−) |
| HCV | Hepatitis C virus | Flaviviridae | ssRNA (+) |
| RSV | Respiratory syncytial virus | Paramyxoviridae | ssRNA (−) |
| PTV | Punta Toro virus | Bunyaviridae | ssRNA (−) |
| IAV | Influenza A virus | Orthomyxoviridae | ssRNA (−) |
| WNV | West Nile virus | Flaviviridae | ssRNA (+) |
| EBOV | Ebola virus | Filoviridae | ssRNA (−) |
| HIV | Human immunodeficiency virus | Retroviridae | ssRNA (RT) |
| VSV | Vesicular stomatitis virus | Rhabdoviridae | ssRNA (−) |
| MCMV | Murine cytomegalovirus | Herpesviridae | dsDNA |
| ADV | Adenovirus | Adenoviridae | dsDNA |
| MHV-68 | Murine gammaherpesvirus 68 | Herpesviridae | dsDNA |
| HBV | Hepatitis B virus | Hepadnaviridae | dsDNA-RT |
| KSHV | Kaposi's sarcoma-associated herpesvirus | Herpesviridae | dsDNA |
+: Positive strand RNA; −: negative-stranded RNA; RT: reverse transcribed.
A role for TLR2 in sensing VACV infection was first reported in a study using bone marrow-derived dendritic cells (DCs), where the proinflammatory cytokine response was shown to be TLR2 dependent, while the type I IFN response was shown to be TLR2 independent [23]. This was in agreement with a plethora of studies showing that MyD88-dependent TLR2 signalling was not involved in type I IFN production. Recently, however, this paradigm has been challenged, as it has been reported that TLR2, in a specialized group of monocytes called Ly6Chi inflammatory monocytes, can induce type I IFNs following sensing VACV and MCMV virus particles [24,27]. Interestingly, TLR2 internalization was required for this process to occur while bacterial TLR2 agonists were unable to elicit this response. This situation is reminiscent of that of TLR4, where receptor internalization to endosomes is required for IRF3 activation and type I IFN production [28]. Thus the type I IFN-inducing TLRs appear to be those capable of endosomal localization, compared to those TLRs capable only of triggering proinflammatory cytokines, which are located at the cell surface.
There are, however, instances of viral infection where TLR2 may be subverted for the benefit of the virus. In a report of patients with chronic HCV infection it was demonstrated that the HCV core protein induced the expression of IL-10 and TNF-α cytokines from monocytes. These cytokines caused both a reduction in IFN-α release from plasmacytoid DCs (pDCs) and also triggered pDC apoptosis. Using blocking antibodies it was shown that the HCV core protein triggered release of these cytokines through TLR2 [29]. Thus HCV core protein engaging TLR2 accounts for HCV-induced pDC loss and reduced IFN-α, which may lead to viral persistence and also explains in part why IFN-α is useful therapeutically in HCV infection [29] (Table 2).
Table 2.
The interaction of viruses with mouse and human Toll-like receptors (TLRs) in different model systems.
| TLR | Virus | Macromolecule detected | Model system used | Reference |
|---|---|---|---|---|
| TLR1 human | HCMV | Envelope proteins B and H | HEK-293s | [26] |
| TLR2 murine | HSV | Unknown | Knock-out mice | [17] |
| VACV | Unknown | Murine Ly6Chi monocytes | [24] | |
| VACV | Unknown | Knock-out mice | [23] | |
| LCMV | Unknown | Murine glia | [18] | |
| RSV | Unknown | Knock-out mice | [22] | |
| TLR2 human | HCV | Core protein, NS3 | Human monocytes and murine macrophages | [25] |
| EBV | Unknown | Human monocytes | [19] | |
| HCMV | Envelope proteins B and H | HEK-293s, human fibroblasts | [21,26] | |
| TLR3 murine | VACV | Unknown | Knock-out mice | [37] |
| PTV | Unknown | Knock-out mice | [36] | |
| IAV | Unknown | Knock-out mice | [38] | |
| WNV | Unknown | Knock-out mice | [39,40,62] | |
| TLR3 human | VACV | Unknown | Human keratinocytes | [94] |
| HSV | Unknown | Human polymorphisms | [43] | |
| EBV | dsRNA | Human PBMCs and lymphocytes | [44] | |
| TLR4 murine | RSV | Fusion F protein | Knock-out mice | [45] |
| VACV | Unknown | Knock-out mice | [21,47] | |
| TLR4 human | RSV | Unknown | Human polymorphisms | [52] |
| VACV | Unknown | Human keratinocytes | [94] | |
| EBOV | Glycoprotein | THP1 cells | [49] | |
| HIV | Unknown | Primary human microglia | [50] | |
| HIV | Unknown | Human polymorphisms | [51] | |
| HBV | Unknown | Primary human monocytes | [53] | |
| TLR6 murine | RSV | Unknown | Knock-out mice | [22] |
| TLR7 murine | IAV | ssRNA | Knock-out mice | [54] |
| HIV | ssRNA | Knock-out mice | [55] | |
| VSV | ssRNA | Knock-out mice | [56] | |
| WNV | Unknown | Knock-out mice | [39,40,62] | |
| TLR7 human | HIV | Unknown | Patient study | [63] |
| HIV | Unknown | Human polymorphisms | [64] | |
| KSHV | Unknown | Latent infected primary effusion lymphoma cell lines | [92] | |
| TLR8 human | HIV | ssRNA | HEK 293 cells | [55] |
| KSHV | Unknown | Latent infected primary effusion lymphoma cell lines | [92] | |
| TLR9 murine | HSV-1 | Unknown | Knock-out mice | [67] |
| MCMV | Unknown | Knock-out mice | [66] | |
| ADV | Unknown | Knock-out mice | [69] | |
| Poxviruses | Unknown | Knock-out mice | [70] | |
| MHV-68 | Unknown | Knock-out mice | [71] | |
| HIV | CpG | Transgenic mice | [80] | |
| TLR9 human | HIV | Unknown | Human B cells | [72] |
| HIV | CpG | Human B cells | [73] | |
| HIV | gp120 | Human pDCs | [74] | |
| HIV | Unknown | Human polymorphisms | [75] | |
| HBV | Unknown | Human pDCs | [76] | |
| HCV | Unknown | Patient liver samples | [77] | |
| RSV | Unknown | Human pDCs | [78] |
CpG: cytosine-guanine dinucleotide; HEK: human embryonic kidney; PBMC: peripheral blood mononuclear cells; pDC: plasmacytoid dendritic cell.
TLR3
In myeloid dendritic cells TLR3 is expressed in endosomes, like the other TLR receptors of nucleic acids; however, in fibroblasts and epithelial cells, TLR3 is expressed on the cell surface [30]. Among the TLR receptors, TLR3 is expressed most abundantly in the brain and is found in neurones [31], astrocytes [32] and microglia [33]. TLR3 was shown to be a receptor for dsRNA, and as well as sensing some RNA viruses can also sense DNA viruses that generate dsRNA during their life cycle [34]. On the other hand, immunity to LCMV, VSV, MCMV and Reovirus was unaffected in TLR3-deficient mice [30,35].
In other examples of viral infection, TLR3 was shown to mediate detrimental immunity to the host. Mice deficient in TLR3 exhibited greater resistance to infection with a number of viruses including Punta Toro, VACV and influenza virus, observations thought to be due to TLR3-mediated overproduction of inflammatory mediators [36–38]. These are examples where TLR3-mediated immunity favours the virus and may be viewed as viral subversion [16].
The relationship of TLR3 to West Nile virus (WNV) infection was also explored. Initially it was reported that TLR3 contributed to WNV lethality by promoting peripheral inflammation that led to the breakdown of the blood–brain barrier, resulting in an increased viral load in the brain. Therefore, mice lacking TLR3 were more resistant to lethal WNV infection compared to wild-type mice [39]. However, a subsequent study reported the opposite results, where TLR3 was shown to have a protective role against WNV infection whereby the absence of TLR3 decreased mice survival in response to WNV and enhanced viral load in the brain [40]. Although the data concerning TLR3 and West Nile virus is controversial, other evidence in support of a positive role for TLR3 in protective immunity against WNV comes from the observations that the WNV protein NS1 inhibits TLR3 signalling and the envelope protein inhibits RIP1, a protein required for signalling downstream of TLR3 [41,42].
Many studies that have reported TLR-dependent anti-viral responses have utilized knock-out mice, and the data regarding human TLRs in response to viral infection is more limited. However, a role for human TLR3 in providing protective immunity against herpes simplex encephalitis (HSE) was reported in two children who harboured a heterozygous mutation in TLR3 [43]. This mutation (C to T) resulted in replacement of a proline with a serine at amino acid 554 in leucine-rich repeat 20 of the extracellular domain. This mutation behaved in an autosomal dominant manner to predispose specifically to HSE, as immunity to other viruses was not impaired. This is the first report that showed conclusively a link between human TLR-3 and anti-viral immunity, particularly in the CNS [43]. In addition, RNA released from EBV-infected cells is capable of activating human TLR3 in a clinically relevant setting. Sera from patients chronically infected with EBV triggered TLR3 signalling in EBV-transformed lymphocytes and peripheral mononuclear cells [44] (Table 2).
TLR4
Initial evidence that TLR4 might be an anti-viral PRR was demonstrated in a study showing that the RSV fusion F protein stimulated cytokine production via TLR4 [45]. Further studies of RSV infection in TLR4 knock-out mice showed reduced natural killer (NK) cell function, impaired interleukin (IL)-12 expression and impaired virus clearance compared to wild-type mice [46]. In terms of VACV infection, mice lacking TLR4 exhibited greater viral replication and mortality compared to wild-type mice following respiratory infection [47]. It is thought that this TLR4-mediated protective immunity against VACV is due to the detection of a yet-to-be-identified viral ligand by TLR4 [47]. In macrophages it was shown that VSV activated the PI3 kinase pathway via TLR4, leading to type I IFN expression and thus conferring anti-viral immunity [48]. Other reports by contrast have shown that TLR4 contributes to detrimental immunity in response to some viral infections. The recognition of Ebola virus glycoprotein by human TLR4 leads to the production of proinflammatory cytokines and is thought to mediate viral immunopathogenesis [49].
Like TLR3, TLR4 has now been implicated in the human anti-viral response. Studies using human microglia demonstrated that TLR4 activation was shown to inhibit human immunodeficiency virus (HIV) replication in a pathway requiring IRF3 [50]. Related to this, it was found that polymorphisms in TLR4 were shown to influence viral load in HIV-infected individuals [51]. The importance of human TLR4 in RSV infection was highlighted in a study reporting that in infants the presence of the TLR4 mutations Asp299Gly or Thr399Ile were associated with increased risk of severe RSV bronchiolitis [52]. Finally, in patients with chronic hepatitis B infection, TLR4 (and TLR2) is overexpressed on monocytes and modulates the activities of regulated T cells (Tregs) which may contribute to immunotolerance [53] (Table 2).
TLR7/8
TLR7 and TLR8 are related phylogenetically and functionally and have been identified as important sensors of ssRNA from the viral genomes of influenza, VSV and HIV [54–56]. While it was once thought that TLR8 was non-functional in mice [57], it is now known that murine TLR8 can be activated under some circumstances [58]. The contribution of these receptors to immunity comes from both TLR7-expressing pDCs and TLR8-expressing myeloid DCs [59]. Both mouse and human TLR7-expressing pDCs are responsible for the production of high levels of type I IFNs, important in the induction of type I T helper responses, class switching of B cells and the promotion of cross-priming [59]. In addition, B cells expressing TLR7 respond to ssRNA viruses by the activation of co-stimulatory molecules and cytokine production [56].
An important contribution of TLR7 to immunity against WNV was demonstrated using knock-out mice. Both TLR7 and MyD88 knock-out mice had increased viral replication and mortality compared to control mice. Upon closer examination it was found that these knock-out mice exhibited reduced homing of macrophages, CD4+ and CD8+ T cells to the brain infected with WNV, which was shown to be dependent upon IL-23 [60,61]. Another study, however, cast doubt on the requirement for TLR7 in immunity against WNV. It was shown that susceptibility to the virus did not differ in wild-type or TLR7 knock-out mice following intradermal challenge, in contrast to the study where intraperitoneal infection was used [60]. In wild-type mice there were reduced numbers of CD11c+ Langerhans cells in the epidermis following intradermal infection, an effect not observed in TLR7-deficient mice [62]. The authors concluded that, upon cutaneous infection, the TLR7-mediated immune response contributes to viral pathogenesis by promoting WNV dissemination from the skin to other organs to initiate systemic infection. It was proposed that this process might reduce the TLR7-mediated protective immunity during the systemic stage of infection [62]. The case of WNV and TLR7 illustrates that defining the precise role of TLRs in viral infection is challenging and depends upon factors such as virus dose, passage history of the virus and route of administration [62].
New insights into the importance of human TLR7 in HIV pathogenesis have been demonstrated. It was shown recently that TLR7 may be a crucial factor in explaining why HIV-1-infected women have lower viral loads in early HIV infection but progress to acquired immunodeficiency syndrome (AIDS) more rapidly than men [63]. It was found that pDCs from women produced significantly more IFN-α in response to HIV-1 encoded TLR7 ligands when compared to pDCs from men, after adjusting for viral load. In addition, there were also higher levels of CD8+ T cell activation in women chronically infected with HIV-1. It is thought that this increased level of HIV immune activation in women may lead to heightened disease progression. These data suggest that inhibition of the TLR7 pathway in pDCs might represent a new approach to treating HIV-1 infections. Interestingly, a polymorphism in TLR7, Gln11Leu, has been associated with higher viral loads and accelerated disease progression in HIV-infected individuals [64] (Table 2).
TLR9
Immune activation induces the expression of TLR9 in a number of cell types, such as neutrophils, monocytes and CD4+ T cells. Non-immune cells such as epithelial cells also express TLR9. In contrast, TLR9 is expressed constitutively on B cells and plasmacytoid DCs [65]. Because TLR9 can sense hypomethylated cytosine-guanine dinucleotide (CpG) motifs of microbial DNA, immune responses to a number of DNA viruses are mediated by TLR9. Anti-viral responses dependent upon TLR9 are due largely to the production of large amounts of type I IFNs from pDCs. These responses occur in response to infection with HSV-1 [66–68], MCMV [66] adenovirus [69] and poxviruses [70]. An anti-viral role for TLR9 against murine gammaherpesvirus 68 infection was also identified [71]. Bone marrow-derived DCs deficient in TLR9 produced fewer proinflammatory cytokines and type I IFNs in response to this virus, and in infection models greater viral loads were observed in mice lacking TLR9 [71].
In terms of human TLR9 a number of reports suggest that this TLR is important in HIV infection. TLR9 is reduced in B cells of HIV-infected individuals, leading to impaired B cell responses to TLR9 agonists [72]. Furthermore, CpG oligonucleotides were found to enhance B cell responsiveness in HIV-infected individuals [73]. In addition, it has been shown that gp120 of HIV suppresses the activation of human pDCs following TLR9 stimulation [74]. Finally, polymorphisms in TLR9 affect the clinical outcome to HIV-1 infection, leading to a more rapid disease progression [75]. As HIV is an RNA virus it is not known if TLR9 senses HIV directly, or if immune responses to HIV mediated by TLR9 occurs by some secondary means.
Other examples of modulation of human TLR9 by viral infection include patients with chronic hepatitis B infection, where TLR9 expression is reduced in pDCs [76], and HCV-dependent cirrhosis, where TLR9 was found to be up-regulated, suggesting that TLR9 might be important during this infection [77]. Further, in RSV and measles infection, TLR9 (and TLR7)-mediated type I IFN production is inhibited in human PDCs [78].
Although it has been reported that TLR9 can mediate detrimental immunity during bacterial infection [79] and CpG DNA leads to HIV–LTR transactivation and HIV replication in murine splenic cells [80], there are as yet no reports indicating that TLR9 promotes detrimental immunity during viral infection, as has been reported for TLR3. In addition, there are no reports suggesting that TLR9 is manipulated by the virus as a means of systemic dissemination. In contrast, it has been reported that TLR7 and TLR8 on human neutrophils might contribute to the pathogenesis of influenza infection [81] (Table 2).
Co-operation of TLRs in viral recognition
In natural viral infections multiple PRRs are likely to be engaged in responding to a particular virus, and this appears to be the case for TLRs. The importance of such co-operation between different TLRs in providing anti-viral immunity is highlighted in a study by Sorensen et al. [17]. Using knock-out mice they showed that TLR2 and TLR9 were both required for immunity to HSV-2. Systemic infection-induced cytokine response was affected significantly in the double knock-outs while only partially affected in either single knock-out. Interestingly, viral loads in the brain were increased in the TLR2/9 double knock-outs compared to the single knock-outs or control mice. Similar findings were also reported in HSV-infected dendritic cells [82]. Another example of such co-operation occurs in MCMV infection, where it was shown that both TLR7 and TLR9 mediate the pDC response to this virus, and was the first study showing that TLR7 can respond to a DNA virus [83]. In other examples of viral infection it is clear that TLRs co-operate with other PRRs to provide anti-viral immunity. The modified vaccinia virus Ankara (MVA) strain is currently being developed as a vaccine vector against HIV/AIDS and innate immune sensing of this virus is mediated by TLR2/6, melanoma differentiation-associated gene 5 (MDA-5) and the NALP-3 inflammasome [84].
Therapeutic use of TLR agonists in anti-viral immunity
A number of studies have shown that a variety of TLR agonists may have a positive effect in anti-viral immunity. For example, a bacterial ligand of TLR2/6, FSl-1, was shown to induce significant resistance to experimental HSV-2 infection [85]. Ampligen, an analogue of the synthetic TLR3 agonist poly(I:C), has been explored for the treatment of HIV [86]. Preclinical studies have shown that another derivative of poly(I:C), poly ICLC, confers protective immunity to a range of viruses including influenza, RSV and SARS [87]. PIKA, another TLR3 agonist, has been proposed as a possible treatment in an influenza pandemic due to inhibition of influenza replication and potency as an adjuvant [88]. Agonists for TLR3 and TLR9 were shown to induce potent anti-viral responses to HSV-2 [89]. Interestingly, another group showed that this effect was dependent upon the release of Type III interferons which, like type I IFNs, display anti-viral activity [90]. Currently, imiquimod is used for the topical treatment of genital warts caused by human papilloma virus and for some skin cancer conditions such as basal cell carcinoma and actinic keratosis. In addition, the TLR7/8 ligand resiquimod (R848) was also examined in clinical studies of HSV and HCV, unfortunately with mixed results [91].
Recently, it has been shown that agonists for TLR7/8 may affect viral latency, as they and viruses acting via TLR7/8 have been shown to reactivate latent Kaposi's sarcoma-associated herpesvirus (KSHV), which sounds a note of caution in the therapeutic use of such agonists [92]. Finally, stimulation of TLR9 with CpG oligonucleotides provided protection against influenza [87] and TLR9 agonists have also been proposed to be useful in the treatment of HIV [93].
Unanswered questions and future perspectives
Huge strides have been made in understanding the immune response mediated by TLRs following infection with different viruses. However, not all the signalling events are defined fully and further details of the proteins required and how they are regulated are likely to be revealed. It is also clear that the outcome of a virus–TLR interaction is complex and depends upon the particular TLR and the virus in question as well as the host species. For example, the TLR4-dependent immune response to VACV in mice is beneficial while the TLR3 contribution is detrimental [37,47]. In contrast, TLR3 in human keratinocytes provides protective immunity against VACV infection [94]. Also it is now appreciated that immune responses for at least some viral infections requires the contribution of many PRRs. How all these signals are integrated in natural infection is unknown. Regarding TLR signalling, important differences in mice and humans have been observed, therefore therapeutic manipulation of TLRs will require a more complete understanding of the human system [16]. Because TLRs can mediate harmful immune responses following viral infection, further studies should reveal drug targets of TLR signalling pathways that may be modulated in order to improve outcome to different viral infections. It is possible that some of these drugs might be based on known viral inhibitors of TLR signalling. Conversely, given the many promising TLR agonists that have been reported to have anti-viral activities, there is reason to be optimistic that such strategies may be employed either as treatments for viral infection or possibly as vaccine adjuvants.
Acknowledgments
This work is supported by Science Foundation Ireland grant 07/IN1/B934 and the Health Research Board (Post-Doctoral Research Fellowship to M.C.).
Disclosure
Nothing to disclose.
References
- 1.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–37. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hornung V, Ablasser A, Charrel-Dennis M, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–18. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanai H, Savitsky D, Tamura T, Taniguchi T. Regulation of the cytosolic DNA-sensing system in innate immunity: a current view. Curr Opin Immunol. 2009;21:17–22. doi: 10.1016/j.coi.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 5.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388:621–5. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 6.Uematsu S, Akira S. Toll-like receptors and Type I interferons. J Biol Chem. 2007;282:15319–23. doi: 10.1074/jbc.R700009200. [DOI] [PubMed] [Google Scholar]
- 7.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–64. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 8.Carty M, Goodbody R, Schroder M, Stack J, Moynagh PN, Bowie AG. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signalling. Nat Immunol. 2006;7:1074–81. doi: 10.1038/ni1382. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–42. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stack J, Haga IR, Schroder M, et al. Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J Exp Med. 2005;201:1007–18. doi: 10.1084/jem.20041442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maloney G, Schroder M, Bowie AG. Vaccinia virus protein A52R activates p38 mitogen-activated protein kinase and potentiates lipopolysaccharide-induced interleukin-10. J Biol Chem. 2005;280:30838–44. doi: 10.1074/jbc.M501917200. [DOI] [PubMed] [Google Scholar]
- 13.Schroder M, Baran M, Bowie AG. Viral targeting of DEAD box protein 3 reveals its role in TBK1/IKKepsilon-mediated IRF activation. EMBO J. 2008;27:2147–57. doi: 10.1038/emboj.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li K, Foy E, Ferreon JC, et al. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci USA. 2005;102:2992–7. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abe T, Kaname Y, Hamamoto I, et al. Hepatitis C virus nonstructural protein 5A modulates the toll-like receptor-MyD88-dependent signalling pathway in macrophage cell lines. J Virol. 2007;81:8953–66. doi: 10.1128/JVI.00649-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowie AG, Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol. 2008;8:911–22. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorensen LN, Reinert LS, Malmgaard L, Bartholdy C, Thomsen AR, Paludan SR. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J Immunol. 2008;181:8604–12. doi: 10.4049/jimmunol.181.12.8604. [DOI] [PubMed] [Google Scholar]
- 18.Zhou S, Halle A, Kurt-Jones EA, et al. Lymphocytic choriomeningitis virus (LCMV) infection of CNS glial cells results in TLR2-MyD88/Mal-dependent inflammatory responses. J Neuroimmunol. 2008;194:70–82. doi: 10.1016/j.jneuroim.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaudreault E, Fiola S, Olivier M, Gosselin J. Epstein–Barr virus induces MCP-1 secretion by human monocytes via TLR2. J Virol. 2007;81:8016–24. doi: 10.1128/JVI.00403-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aravalli RN, Hu S, Lokensgard JR. Inhibition of toll-like receptor signalling in primary murine microglia. J Neuroimmune Pharmacol. 2008;3:5–11. doi: 10.1007/s11481-007-9097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juckem LK, Boehme KW, Feire AL, Compton T. Differential initiation of innate immune responses induced by human cytomegalovirus entry into fibroblast cells. J Immunol. 2008;180:4965–77. doi: 10.4049/jimmunol.180.7.4965. [DOI] [PubMed] [Google Scholar]
- 22.Murawski MR, Bowen GN, Cerny AM, et al. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J Virol. 2009;83:1492–500. doi: 10.1128/JVI.00671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J, Martinez J, Huang X, Yang Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood. 2007;109:619–25. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol. 2009;10:1200–7. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolganiuc A, Oak S, Kodys K, et al. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004;127:1513–24. doi: 10.1053/j.gastro.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 26.Boehme KW, Guerrero M, Compton T. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J Immunol. 2006;177:7094–102. doi: 10.4049/jimmunol.177.10.7094. [DOI] [PubMed] [Google Scholar]
- 27.Bauernfeind F, Hornung V. TLR2 joins the interferon gang. Nat Immunol. 2009;10:1139–41. doi: 10.1038/ni1109-1139. [DOI] [PubMed] [Google Scholar]
- 28.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–8. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolganiuc A, Chang S, Kodys K, et al. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol. 2006;177:6758–68. doi: 10.4049/jimmunol.177.10.6758. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto M, Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C) Adv Drug Deliv Rev. 2008;60:805–12. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Prehaud C, Megret F, Lafage M, Lafon M. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol. 2005;79:12893–904. doi: 10.1128/JVI.79.20.12893-12904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J Neuroimmunol. 2005;159:12–19. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–21. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 34.Yoneyama M, Fujita T. Recognition of viral nucleic acids in innate immunity. Rev Med Virol. 20:4–22. doi: 10.1002/rmv.633. [DOI] [PubMed] [Google Scholar]
- 35.Edelmann KH, Richardson-Burns S, Alexopoulou L, Tyler KL, Flavell RA, Oldstone MB. Does Toll-like receptor 3 play a biological role in virus infections? Virology. 2004;322:231–8. doi: 10.1016/j.virol.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 36.Gowen BB, Hoopes JD, Wong MH, et al. TLR3 deletion limits mortality and disease severity due to Phlebovirus infection. J Immunol. 2006;177:6301–7. doi: 10.4049/jimmunol.177.9.6301. [DOI] [PubMed] [Google Scholar]
- 37.Hutchens M, Luker KE, Sottile P, et al. TLR3 increases disease morbidity and mortality from vaccinia infection. J Immunol. 2008;180:483–91. doi: 10.4049/jimmunol.180.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Goffic R, Balloy V, Lagranderie M, et al. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2:e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–73. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 40.Daffis S, Samuel MA, Suthar MS, Gale M, Jr, Diamond MS. Toll-like receptor 3 has a protective role against West Nile virus infection. J Virol. 2008;82:10349–58. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson JR, de Sessions PF, Leon MA, Scholle F. West Nile virus nonstructural protein 1 inhibits TLR3 signal transduction. J Virol. 2008;82:8262–71. doi: 10.1128/JVI.00226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arjona A, Ledizet M, Anthony K, et al. West Nile virus envelope protein inhibits dsRNA-induced innate immune responses. J Immunol. 2007;179:8403–9. doi: 10.4049/jimmunol.179.12.8403. [DOI] [PubMed] [Google Scholar]
- 43.Zhang SY, Jouanguy E, Ugolini S, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–7. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 44.Iwakiri D, Zhou L, Samanta M, et al. Epstein–Barr virus (EBV)-encoded small RNA is released from EBV-infected cells and activates signalling from Toll-like receptor 3. J Exp Med. 2009;206:2091–9. doi: 10.1084/jem.20081761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurt-Jones EA, Popova L, Kwinn L, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 46.Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730–7. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hutchens MA, Luker KE, Sonstein J, Nunez G, Curtis JL, Luker GD. Protective effect of Toll-like receptor 4 in pulmonary vaccinia infection. PLoS Pathog. 2008;4:e1000153. doi: 10.1371/journal.ppat.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schabbauer G, Luyendyk J, Crozat K, et al. TLR4/CD14-mediated PI3K activation is an essential component of interferon-dependent VSV resistance in macrophages. Mol Immunol. 2008;45:2790–6. doi: 10.1016/j.molimm.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okumura A, Pitha PM, Yoshimura A, Harty RN. Interaction between Ebola virus glycoprotein and host toll-like receptor 4 leads to induction of proinflammatory cytokines and SOCS1. J Virol. 84:27–33. doi: 10.1128/JVI.01462-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suh HS, Zhao ML, Choi N, Belbin TJ, Brosnan CF, Lee SC. TLR3 and TLR4 are innate antiviral immune receptors in human microglia: role of IRF3 in modulating antiviral and inflammatory response in the CNS. Virology. 2009;392:246–59. doi: 10.1016/j.virol.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pine SO, McElrath MJ, Bochud PY. Polymorphisms in toll-like receptor 4 and toll-like receptor 9 influence viral load in a seroincident cohort of HIV-1-infected individuals. AIDS. 2009;23:2387–95. doi: 10.1097/QAD.0b013e328330b489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tal G, Mandelberg A, Dalal I, et al. Association between common Toll-like receptor 4 mutations and severe respiratory syncytial virus disease. J Infect Dis. 2004;189:2057–63. doi: 10.1086/420830. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Lian JQ, Huang CX, et al. Overexpression of Toll-like receptor 2/4 on monocytes modulates the activities of CD4(+)CD25(+) regulatory T cells in chronic hepatitis B virus infection. Virology. 397:34–42. doi: 10.1016/j.virol.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–31. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 55.Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 56.Lund JM, Alexopoulou L, Sato A, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101:5598–603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jurk M, Heil F, Vollmer J, et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 58.Gorden KK, Qiu XX, Binsfeld CC, Vasilakos JP, Alkan SS. Cutting edge: activation of murine TLR8 by a combination of imidazoquinoline immune response modifiers and polyT oligodeoxynucleotides. J Immunol. 2006;177:6584–7. doi: 10.4049/jimmunol.177.10.6584. [DOI] [PubMed] [Google Scholar]
- 59.Diebold SS. Recognition of viral single-stranded RNA by Toll-like receptors. Adv Drug Deliv Rev. 2008;60:813–23. doi: 10.1016/j.addr.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 60.Town T, Bai F, Wang T, et al. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity. 2009;30:242–53. doi: 10.1016/j.immuni.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Finberg RW, Wang JP. Antiviral responses: different roles for different tolls. Immunity. 2009;30:173–5. doi: 10.1016/j.immuni.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 62.Welte T, Reagan K, Fang H, et al. Toll-like receptor 7-induced immune response to cutaneous West Nile virus infection. J Gen Virol. 2009;90:2660–8. doi: 10.1099/vir.0.011783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meier A, Chang JJ, Chan ES, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–9. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oh DY, Baumann K, Hamouda O, et al. A frequent functional toll-like receptor 7 polymorphism is associated with accelerated HIV-1 disease progression. AIDS. 2009;23:297–307. doi: 10.1097/QAD.0b013e32831fb540. [DOI] [PubMed] [Google Scholar]
- 65.Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 66.Krug A, French AR, Barchet W, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–19. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 67.Hochrein H, Schlatter B, O'Keeffe M, et al. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc Natl Acad Sci USA. 2004;101:11416–21. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–20. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 2007;81:3170–80. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Samuelsson C, Hausmann J, Lauterbach H, et al. Survival of lethal poxvirus infection in mice depends on TLR9, and therapeutic vaccination provides protection. J Clin Invest. 2008;118:1776–84. doi: 10.1172/JCI33940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guggemoos S, Hangel D, Hamm S, Heit A, Bauer S, Adler H. TLR9 contributes to antiviral immunity during gammaherpesvirus infection. J Immunol. 2008;180:438–43. doi: 10.4049/jimmunol.180.1.438. [DOI] [PubMed] [Google Scholar]
- 72.Jiang W, Lederman MM, Mohner RJ, et al. Impaired naive and memory B-cell responsiveness to TLR9 stimulation in human immunodeficiency virus infection. J Virol. 2008;82:7837–45. doi: 10.1128/JVI.00660-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malaspina A, Moir S, DiPoto AC, et al. CpG oligonucleotides enhance proliferative and effector responses of B Cells in HIV-infected individuals. J Immunol. 2008;181:1199–206. doi: 10.4049/jimmunol.181.2.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martinelli E, Cicala C, Van Ryk D, et al. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{alpha} secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci USA. 2007;104:3396–401. doi: 10.1073/pnas.0611353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bochud PY, Hersberger M, Taffe P, et al. Polymorphisms in Toll-like receptor 9 influence the clinical course of HIV-1 infection. AIDS. 2007;21:441–6. doi: 10.1097/QAD.0b013e328012b8ac. [DOI] [PubMed] [Google Scholar]
- 76.Xie Q, Shen HC, Jia NN, et al. Patients with chronic hepatitis B infection display deficiency of plasmacytoid dendritic cells with reduced expression of TLR9. Microbes Infect. 2009;11:515–23. doi: 10.1016/j.micinf.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 77.Huang XX, McCaughan GW, Shackel NA, Gorrell MD. Up-regulation of proproliferative genes and the ligand/receptor pair placental growth factor and vascular endothelial growth factor receptor 1 in hepatitis C cirrhosis. Liver Int. 2007;27:960–8. doi: 10.1111/j.1478-3231.2007.01542.x. [DOI] [PubMed] [Google Scholar]
- 78.Schlender J, Hornung V, Finke S, et al. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J Virol. 2005;79:5507–15. doi: 10.1128/JVI.79.9.5507-5515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Plitas G, Burt BM, Nguyen HM, Bamboat ZM, DeMatteo RP. Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J Exp Med. 2008;205:1277–83. doi: 10.1084/jem.20080162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Equils O, Schito ML, Karahashi H, et al. Toll-like receptor 2 (TLR2) and TLR9 signalling results in HIV-long terminal repeat trans-activation and HIV replication in HIV-1 transgenic mouse spleen cells: implications of simultaneous activation of TLRs on HIV replication. J Immunol. 2003;170:5159–64. doi: 10.4049/jimmunol.170.10.5159. [DOI] [PubMed] [Google Scholar]
- 81.Wang JP, Bowen GN, Padden C, et al. Toll-like receptor-mediated activation of neutrophils by influenza A virus. Blood. 2008;112:2028–34. doi: 10.1182/blood-2008-01-132860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci USA. 2006;103:17343–8. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zucchini N, Bessou G, Traub S, et al. Cutting edge: overlapping functions of TLR7 and TLR9 for innate defense against a herpesvirus infection. J Immunol. 2008;180:5799–803. doi: 10.4049/jimmunol.180.9.5799. [DOI] [PubMed] [Google Scholar]
- 84.Delaloye J, Roger T, Steiner-Tardivel QG, et al. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 2009;5:e1000480. doi: 10.1371/journal.ppat.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Rose WA, II, McGowin CL, Pyles RB. FSL-1, a bacterial-derived toll-like receptor 2/6 agonist, enhances resistance to experimental HSV-2 infection. Virol J. 2009;6:195. doi: 10.1186/1743-422X-6-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gowen BB, Wong MH, Jung KH, et al. TLR3 is essential for the induction of protective immunity against Punta Toro Virus infection by the double-stranded RNA (dsRNA), poly(I:C12U), but not Poly(I:C): differential recognition of synthetic dsRNA molecules. J Immunol. 2007;178:5200–8. doi: 10.4049/jimmunol.178.8.5200. [DOI] [PubMed] [Google Scholar]
- 87.Wong JP, Christopher ME, Viswanathan S, et al. Antiviral role of toll-like receptor-3 agonists against seasonal and avian influenza viruses. Curr Pharm Des. 2009;15:1269–74. doi: 10.2174/138161209787846775. [DOI] [PubMed] [Google Scholar]
- 88.Lau YF, Tang LH, Ooi EE. A TLR3 ligand that exhibits potent inhibition of influenza virus replication and has strong adjuvant activity has the potential for dual applications in an influenza pandemic. Vaccine. 2009;27:1354–64. doi: 10.1016/j.vaccine.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gill N, Deacon PM, Lichty B, Mossman KL, Ashkar AA. Induction of innate immunity against herpes simplex virus type 2 infection via local delivery of Toll-like receptor ligands correlates with beta interferon production. J Virol. 2006;80:9943–50. doi: 10.1128/JVI.01036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ank N, Iversen MB, Bartholdy C, et al. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol. 2008;180:2474–85. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- 91.Miller RL, Meng TC, Tomai MA. The antiviral activity of Toll-like receptor 7 and 7/8 agonists. Drug News Perspect. 2008;21:69–87. doi: 10.1358/dnp.2008.21.2.1188193. [DOI] [PubMed] [Google Scholar]
- 92.Gregory SM, West JA, Dillon PJ, Hilscher C, Dittmer DP, Damania B. Toll-like receptor signalling controls reactivation of KSHV from latency. Proc Natl Acad Sci USA. 2009;106:11725–30. doi: 10.1073/pnas.0905316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Becker Y. A point of view: HIV-1/AIDS is an allergy but CpG ODN treatments may inhibit virus replication and reactivate the adaptive immunity – hypothesis and implications. Virus Genes. 2005;30:127–31. doi: 10.1007/s11262-004-4590-0. [DOI] [PubMed] [Google Scholar]
- 94.Howell MD, Gallo RL, Boguniewicz M, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–8. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]


