Abstract
We have identified previously a nuclear fluorescence reactivity (NFR) pattern on monkey oesophagus sections exposed to coeliac disease (CD) patients' sera positive for anti-endomysium antibodies (EMA). The aim of the present work was to characterize the NFR, study the time–course of NFR-positive results in relation to gluten withdrawal and evaluate the potential role of NFR in the follow-up of CD. Twenty untreated, 87 treated CD patients and 15 healthy controls were recruited and followed for 12 months. Their sera were incubated on monkey oesophagus sections to evaluate the presence of NFR by indirect immunofluorescence analysis. Duodenal mucosa samples from treated CD patients were challenged with gliadin peptides, and thus the occurrence of NFR in culture supernatants was assessed. The NFR immunoglobulins (Igs) reactivity with the nuclear extract of a human intestinal cell line was investigated. Serum NFR was present in all untreated CD patients, persisted up to 151 ± 37 days from gluten withdrawal and reappeared in treated CD patients under dietary transgressions. Serum NFR was also detected in two healthy controls. In culture supernatants of coeliac intestinal mucosa challenged with gliadin peptides, NFR appeared before EMA. The Igs responsible for NFR were identified as belonging to the IgA2 subclass. The NFR resulted differently from EMA and anti-nuclear antibodies, but reacted with two nuclear antigens of 65 and 49 kDa. A new autoantibody, named NFR related to CD, was described. Furthermore, NFR detection might become a valuable tool in monitoring adherence to a gluten-free diet and identifying slight dietary transgressions.
Keywords: anti-endomysium, coeliac disease, follow-up, gluten-free diet, nuclear fluorescence reactivity
Introduction
Coeliac disease (CD) is a chronic inflammatory disorder triggered by the ingestion of wheat gluten and other storage proteins in rye and barley [1], while the role of oat is still debated [2]. This condition represents the most frequent food intolerance worldwide [3].
A T cell-mediated immune response against gluten fractions (gliadins and glutenins), that takes shape in the small bowel mucosa of individuals bearing the human leucocyte antigen (HLA) alleles DQ2/8 [4], is considered the pivotal event in the pathogenesis of CD [5–7]. As well as the cellular immune response, CD patients show antibodies against gliadin itself (anti-gliadin: AGA; anti-deamidated gliadin peptides: DGP) [8,9] as well as against muscolaris mucosae of the primate oesophagus (anti-endomysium: EMA) [10]. The enzyme tissue transglutaminase (tTG) has been identified as the main endomysial antigen [11]. However, it has been demonstrated that tTG is not the only autoantigen associated with CD, and other tissue components are considered to be involved in the CD-related autoimmunity [12–15].
Circulating EMA and anti-tTG antibodies are used currently for the diagnosis and follow-up of CD patients because of their high sensitivity and specificity [16,17]. In addition, CD patients showing EMA/anti-tTG-positive results also show villous atrophy, crypt hyperplasia and/or intraepithelial lymphocytosis in their duodenal biopsies [18,19] and, in most cases, serum antibodies disappear within 6–12 months after gluten withdrawal from their diet [20–22].
During the last two decades, the intestinal mucosa has been identified as a site of EMA/anti-tTG antibody production [23–25]. These antibodies are indeed detectable in supernatants of duodenal biopsies from CD patients after in vitro culture with and/or without gliadin peptides [23,26]. Furthermore, it was shown that EMA appear in vitro earlier than changes in duodenal mucosa morphology when a gluten-free diet (GFD) is not followed strictly [27].
Some investigations on the appearance of serum antibodies in early childhood CD or during in vivo gluten challenge have reported that EMA/anti-tTG may emerge later than AGA/DGP, suggesting that EMA and anti-tTG are not the first antibodies produced at CD onset or during its relapse [28,29]. However, as yet there is no serological test powerful enough to assess compliance to a GFD and/or the occurrence of dietary transgressions [20,30].
Nine years ago the occurrence of a gluten-dependent serum immunoglobulin (Ig)A cross-reactivity between wheat proteins and a 55-kDa nuclear antigen expressed in human fibroblasts, intestinal and endothelial cells has been related to CD [31]. Testing sera of CD patients recently in remission and still positive for EMA, we observed a nuclear fluorescence reactivity (NFR) pattern on monkey oesophagus sections, of as yet unknown significance, that disappears after a GFD [32]. Consistently, Storch et al. have described a new autoantibody in CD patients' serum that, reacting with monkey oesophagus sections, designs a punctiform pattern [33].
Based upon these observations, the aim of the present study was: (i) to characterize the NFR and its role in CD; (ii) to assess the time–course of NFR-positive results in relation to gluten withdrawal from the diet and EMA persistence; and (iii) to evaluate the potential role of NFR in identifying dietary transgressions. For these purposes, the presence of IgA NFR in sera from untreated and treated CD patients and healthy controls was assessed, the ability of coeliac intestinal mucosa to produce IgA NFR was evaluated and, finally, the serum IgA reactivity with the nuclear extract of a human intestinal cell line was investigated.
Materials and methods
Patients
A total of 122 study participants was divided into three groups, as follows.
Group 1
Group 1 comprised untreated CD patients (seven male/13 female, mean age 22·3, range 18–46 years) with duodenal villous atrophy (grades IIIa–c of the modified Marsh classification) and serum EMA-positive results. During the study, all patients were put onto a GFD and monitored for 12 months. Compliance with the GFD was assessed every 15 days by careful examination of a patient's food diary (control level 1) followed, whenever possible, by a specific medical interview (control level 2). At the same time-points, a blood sample was obtained to detect EMA as a further index of adherence to the GFD (control level 3). All patients in this group presented excellent compliance with the GFD and completed the clinical phase of the study. Conversely, the NFR characterization was performed exclusively on 11 of 20 patients in this group who, after a reasonable period on a GFD, agreed to undergo a second duodenal biopsy. By preliminary evaluation, the subgroup of 11 patients appeared to be gender- and age-reflective of the overall group.
Group 2
Group 2 comprised treated CD patients (31 male/56 female, mean age 31·3, range 19–54 years) on a GFD from at least 12 months, and showing serum EMA-negative results. During the study, all patients continued to take a GFD and were followed regularly for 12 months. Compliance with the GFD was assessed every 15 days as described for group 1.
Group 3
Group 3 comprised healthy subjects (five male/10 female, mean age 28·7, range 18–55 years) not affected by CD or other autoimmune disease, and with no consanguinity with CD patients. At study entry their sera were collected and stored at −70°C until tested. Two of the subjects in this group showed an NFR-like pattern in the absence of serum EMA. For ethical reasons, the latter two subjects were not submitted to duodenal biopsy to exclude a subclinical form of CD. However, they agreed to undergo a GFD and to be monitored for 12 months. Adherence to the GFD was assessed every month as described for group 1. Both treated subjects presented excellent compliance to the GFD and completed the study.
CD patients were selected from among the out-patients admitted to our gastrointestinal unit from January 2006 to December 2007 who showed clinical features described for groups 1 or 2, and who agreed to undergo the study protocol. The diagnosis of CD was made in accordance with the procedure adopted worldwide [34], based on clinical case identification, serological screening and duodenal biopsy histology. Healthy subjects were selected among the blood donors admitted to our hospital from January 2006 to December 2007 who showed clinical features described for group 3, and who agreed to undergo the study protocol. The diagnosis of CD was excluded in individuals not clinically suspicious, with serum EMA-negative results.
Because the suitability of oat as part of a GFD is still controversial [2], all the GFDs administered in this study included the withdrawal of any oat-based product.
All procedures followed in this study were in accordance with the ethical standards of the institutional committee responsible for human experimentation. Furthermore, informed consent was obtained from each study participant.
Anti-endomysium and NFR circulating antibodies
To assess the occurrence of NFR in relation to EMA, IgA EMA/NFR antibodies were searched in sera of all study participants at baseline and during a GFD. Furthermore, to ascertain if EMA and NFR belonged to distinct IgA subclasses, IgA1 and IgA2 EMA/NFR antibodies were searched in sera of the 11 patients in group 1 subjected to NFR characterization.
Total IgA, IgA1 and IgA2 EMA/NFR antibodies were evaluated in sera diluted 1:5 by indirect immunofluorescence analysis (IFA) on cryostat sections of monkey oesophagus (Eurospital, Trieste, Italy). After sera incubation, the sections were stained by means of fluorescein isothiocyanate (FITC)-conjugated anti-human IgA (Sigma, St Louis, MO, USA; diluted 1:100) and IgA1 (Sigma; diluted 1:20) monoclonal antibodies (mAbs), non-conjugated anti-human IgA2 mAb (ICN Biomedicals, Aurora, OH, USA; diluted 1:10) and its tetramethylrhodamine isothiocyanate (TRITC)-conjugated detector (Sigma; diluted 1:20), all used according to the manufacturer's instructions. Fluorescence for EMA (Fig. 1a) and NFR (Fig. 1b) was evaluated blindly by three trained observers, whose agreement rate was 99·6%.
Fig. 1.
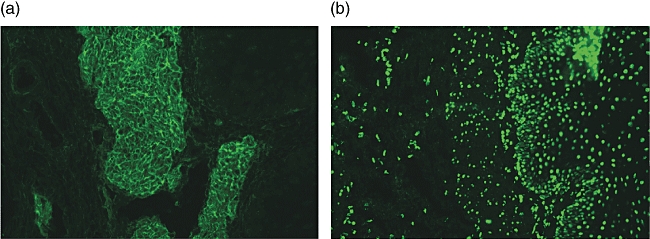
Serum immunoglobulin (Ig)A endomysium antibodies (EMA) and nuclear fluorescence reactivity (NFR) antibody patterns (Olympus microscope, 400×). (a) Serum IgA EMA-positive. A fluorescence feature of EMA is a honeycomb-like staining pattern along muscolaris mucosae (endomysium) in cryostat sections of the third distal portion of monkey oesophagus. Specifically, EMA react against the collagenous matrix of type 3 connective tissue surrounding the smooth muscle fibres of the primate oesophagus. (b) Serum IgA NFR-positive. Fluorescence feature of NFR is a dot-shaped staining pattern on nuclei of muscular and epithelial cells in the same sections.
All FITC-conjugated and non-conjugated secondary mAbs, as well as the TRITC-conjugated anti-IgA2 mAb detector, were incubated further, alone or combined variously, on sections not exposed previously to serum antibodies. No fluorescence signal was observed after any of these control incubations, ensuring that there was no non-specific binding.
Double-staining assay
To establish if EMA and NFR fluorescence patterns were related to distinct antibodies, and if the latter could be present simultaneously in the bloodstream, an indirect IFA-based double-staining assay was performed on monkey oesophagus sections (Eurospital) incubated first with sera of the 11 patients in group 1 subjected to NFR characterization. Because it was shown during this study that EMA and NFR belong, respectively, to IgA1 and IgA2 isotypes (see below), the subsequent incubations with two different secondary mAbs (anti-human IgA1 and IgA2) detected by two different fluorochromes (FITC and TRITC, respectively) allowed the development, on every section, of a double-staining pattern. For interpretation, the appearance of two different and not overlapping fluorescence signals was considered indicative for the simultaneous presence of two distinct antibodies in CD patients' sera.
Anti-nuclear circulating antibodies
To investigate the possible contribution of anti-nuclear antibodies (ANA) in determining the NFR fluorescence pattern, classical ANA were searched in sera of all patients in group 1 using an indirect IFA-based commercial kit (Sigma) on both rat liver sections and human epithelial-2 (HEp-2) cell substrates. Results, evaluated blindly by three observers, were compared with positive controls presenting homogeneous (ANA-H), nucleolar (ANA-N) and speckled (ANA-S) antibody patterns. The occurrence of centromeric (ANA-C), peripheral (ANA-P) and cytoplasmic (Golgi apparatus, lysosomal, mitochondrial, ribosomal, speckled) HEp-2 antibody patterns, as well as nuclear subpatterns (e.g. coarse speckled, diffuse grainy, fine speckled) was also investigated.
Organ culture system
To verify if the small bowel mucosa was able to produce NFR antibodies, duodenal mucosa samples were obtained from the 11 patients in group 1 who, after a reasonable period on a GFD, agreed to undergo a second upper endoscopy with biopsy sampling.
The culture medium, prepared with 17 ml RPMI-1640 medium, 3 ml fetal calf serum (FCS), 0·2 ml l-glutamine (200 mM), 2 ml penicillin (10 000 UI/ml)–streptomycin (10 000 µg/ml) and 0·04 ml gentamycin (10 mg/ml) (Gibco /Invitrogen, Carlsbad, CA, USA), was stabilized preventively at pH 7·4 and was then sterilized by filtration with a 0·22 µm pore size filter (Sigma). The duodenal mucosa samples, washed first in physiological solution (NaCl, 9 g/l), were placed into sterile tubes containing 500 µl of medium and then cultured, with and without a peptic–tryptic digest of gliadin (PT–gliadin; 1 mg/ml), at 37°C from 30 min to 48 h. Thereafter, supernatants were collected and stored at −70°C until tested. All operations were performed in a sterile environment.
Total IgA, IgA1 and IgA2 EMA/NFR antibodies were evaluated in undiluted culture supernatants by indirect IFA on monkey oesophagus sections (Eurospital), as described for sera.
Caco2 cell culture and total cell protein extraction
The human colorectal cancer cells Caco2 were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FCS, 2 mM l-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco /Invitrogen) under 95% air and 5% CO2, at 37°C up to cell confluence. Subsequently, cells were washed twice in phosphate-buffered saline (PBS) to remove culture medium-derived proteins and total cell proteins were extracted by incubation with a TNE extraction buffer [50 mM Tris/HCl at pH 7·8, 150 mM NaCl, 1 mM ethylenediamine tetraacetic acid (EDTA), 1% TRITON X-100] containing protease inhibitors on ice for 30 min. Extracted total cell proteins were collected and stored at −70°C until used.
Cell fractionation procedures
The cytosolic and nuclear protein fractions of Caco2 cells were prepared by a standard method. Briefly, after Caco2 cell culture and washing, the cell pellet was resuspended in 3 ml RBS medium [10 mM Tris/HCl at pH 7·4, 10 mM NaCl, 1·5 mM MgCl2, 1 mM phenylmethylsulphonyl fluoride (PMSF)] and incubated on ice for 10 min. Cells were broken by incubation with NP-40 and Na-Deoxicholate detergents (0·5% and 0·15%, respectively), on ice for 30 min. Thereafter, cells were homogenized with a glass–glass potter and the homogenate was centrifuged (800 g for 10 min) at 4°C. The supernatant representing the cytosolic protein fraction was collected and stored at −70°C until used. The pellet containing the crude nuclear protein fraction was resuspended in 3 ml RBS medium and centrifuged (1000 g for 30 min) through a sucrose cushion (30% sucrose in RBS medium) at 4°C. Subsequently, the pellet was resuspended in 500 µl extraction buffer (10 mM Tris/HCl at pH 7·4, 3 mM MgCl2, 2 mM mercaptoethanol, 1 mM PMSF) containing 0·4 M NaCl and incubated on ice for 20 min. After centrifugation (14 500 g for 5 min) at 4°C the pellet was resuspended in 500 µl extraction buffer containing 1 M NaCl, incubated on ice for 20 min and centrifuged (14 500 g for 5 min) at 4°C. The supernatant representing the nuclear protein fraction was collected and stored at −70°C until used.
Characterization of serum IgA-defined autoantigens by immunoblotting
To characterize the NFR further, sera of the 11 patients in group 1 subjected to molecular study were analysed for IgA reactivity with nitrocellulose-blotted Caco2 cell proteins.
Total cell protein extract, as well as its cytosolic and nuclear fractions, were boiled for 3 min and submitted to denaturing 10% preparative sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). Gel-separated proteins were blotted onto nitrocellulose membranes (Protran nitrocellulose transfer membrane; Schleicher & Schuell Whatman group, Dassel, Germany). Nitrocellulose strips (width 2 cm) were cut from the membranes and were then blocked twice for 5 min and once for 30 min in buffer A [50 mM sodium phosphate buffer at pH 7·4, containing 0·5% Tween 20 and 0·5% bovine serum albumin (BSA)]. Blocked strips were probed overnight at 4°C with sera diluted 1:500 in the same buffer. Thereafter, strips were washed twice for 5 min and once for 15 min with buffer B (50 mM sodium phosphate buffer at pH 7·4, containing 0·5% Tween 20) and incubated overnight at room temperature with a peroxidase-conjugated anti-human IgA polyclonal antibody (Chemicon, Temecula, CA, USA) diluted 1:8000 in buffer A. Strips were finally washed and dried before exposition to Hyperfilms ECL (Amersham Pharmacia Biotech, Uppsala, Sweden) for approximately 3–5 s.
The purity of nuclear and cytosolic protein fractions was assessed by exposing the nitrocellulose-blotted total cell protein extract and its fractions to anti-human histone H2B anti-serum (Chemicon).
Statistical analysis
Significant statistical differences between EMA and NFR antibodies, detected as total IgA, IgA1 and IgA2 in sera of the 11 patients in group 1 subjected to NFR characterization, were calculated by χ2 test for qualitative and independent data. The P-values ≤0·05 were considered significant.
Results
Time–course of serum EMA and NFR antibodies
At baseline, all 20 untreated CD patients in group 1 showed serum IgA EMA-positive and NFR-negative results. Serum EMA disappeared after 76 ± 34 days from starting the GFD while, at the same time, serum NFR antibodies became apparent. The NFR antibodies cleared completely from sera in the following 75 ± 41 days for a total of 151 ± 37 days from starting the GFD (Fig. 2).
Fig. 2.
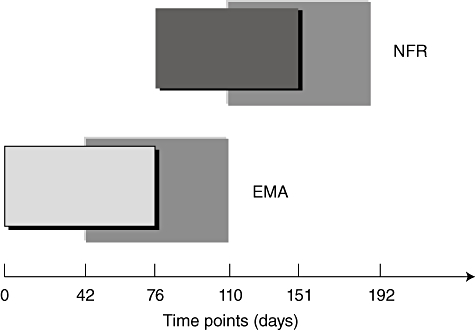
Time–course of serum immunoglobulin (Ig)A endomysium antibodies (EMA) and nuclear fluorescence reactivity (NFR) antibodies. The timing of serum IgA EMA and NFR antibody appearance/disappearance are plotted in the graph. The small boxes refer to the time–course of serum antibodies, from their appearance to disappearance (mean values). The great boxes refer to standard deviations of serum antibody disappearance times. At baseline, EMA are detectable in coeliac disease (CD) patients' sera and disappear after 76 ± 34 days from starting the gluten-free diet (GFD). At the latter time-point, serum NFR antibodies become observable and disappear in the following 75 ± 41 days, for a total of 151 ± 37 days from starting the GFD.
At the time of monitoring, 24 of 87 treated CD patients in group 2 showed serum IgA EMA-negative and NFR-positive results, while the remaining 63 patients displayed negative results for both circulating antibodies. The combination of three GFD control levels (self-reported, dietetic assessment and serum EMA determination) highlighted that, during the previous months, the 24 patients presenting serum NFR-positive results were introducing small amounts of gluten. Ten of these patients continued to consume gluten occasionally and, thus, their sera became EMA-positive within the following 3 months. The remaining 14 patients, who began to follow a strict GFD, showed the disappearance of serum NFR antibodies in the following 2 months.
Characterization of serum NFR antibodies
Based on the timing of serum antibodies reported in the above section, IgA1 and IgA2 EMA were evaluated in sera of 11 of 20 untreated CD patients in group 1, while IgA1 and IgA2 NFR antibodies were searched in sera of the same patients on a GFD from at least 3 months. As a result, serum NFR antibodies were linked to the IgA2 subclass in all the 11 patients evaluated, while serum EMA were associated with IgA1 isotype in all except three of these patients, who presented simultaneously EMA of both IgA1 and IgA2 subclasses (Table 1).
Table 1.
Isotypic characterization of serum endomysium antibodies (EMA) and nuclear fluorescence reactivity (NFR) Abs.
| Serum antibody-positive results |
Statistical analysis |
|||
|---|---|---|---|---|
| Isotypes | EMA | NFR | χ2 | P |
| Total IgA | 11/11 | 11/11 | n.s. | n.s. |
| IgA1 | 11/11 | 0/11 | 18·182 | <0·001 |
| IgA2 | 3/11 | 11/11 | 9·625 | 0·002 |
Total immunoglobulin (Ig)A, IgA1 and IgA2 EMA were evaluated in sera of untreated coeliac disease (CD) patients, while total IgA, IgA1 and IgA2 NFR antibodies were searched in sera of the same CD patients on a gluten-free diet (GFD) for at least 3 months. Statistical analysis refer to χ2 test for qualitative and independent data calculated between serum EMA and NFR antibodies detected as total IgA, IgA1 and IgA2; n.s.: not significant.
A double-staining assay was performed by exploiting the ability of FITC-detected IgA1 EMA and TRITC-detected IgA2 NFR to bind tissue structures on monkey oesophagus sections. In this manner it was shown that serum EMA and NFR antibodies reacted with two different and not overlapping tissue structures, and that these antibodies were present simultaneously in sera of all the 11 untreated CD patients evaluated (Fig. 3a–c).
Fig. 3.
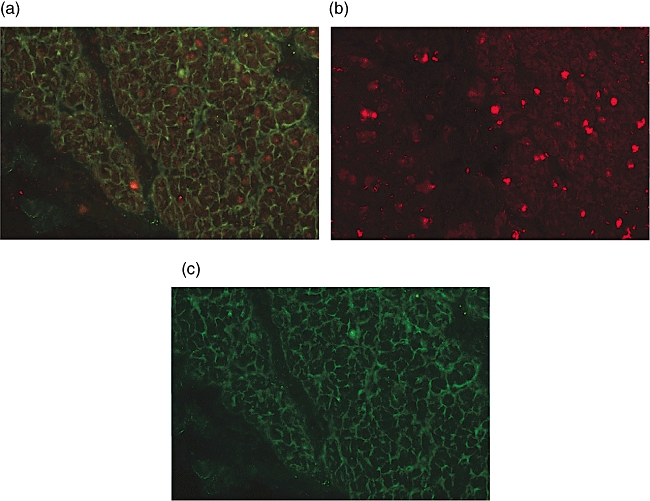
Double staining assay for serum immunoglobulin (Ig)A1 endomysium antibodies (EMA) and IgA2 nuclear fluorescence reactivity (NFR) antibodies (Olympus microscope, 400×). (a) Double staining for serum EMA (green) and NFR antibodies (red) on a monkey oesophagus section. EMA are stained with a fluorescein isothiocyanate (FITC)-conjugated anti-human IgA1 monoclonal antibody (mAb), NFR is stained with a non-conjugated anti-human IgA2 mAb and revealed by a tetramethylrhodamine isothiocyanate (TRITC)-conjugated anti-IgA2 mAb detector. Serum EMA and NFR antibodies, belonging to distinct IgA subclasses, react with two different and not overlapping tissue structures. Furthermore, these antibodies may be simultaneously present in sera of untreated coeliac disease (CD) patients. (b) The same section stained for serum IgA2 NFR antibodies only (red). (c) The same section stained for serum IgA1 EMA only (green).
Sera analysed for IgA reactivity with nitrocellulose-blotted Caco2 cell proteins were obtained from each of the 11 CD patients evaluated at two time-points. The first serum sample was collected when NFR antibodies were still present, while the second sample was taken when NFR antibodies were no longer detectable. Consistently, a serum IgA reactivity with 65- and 49-kDa proteins was observed at the first time-point while, in the second serum sample, the same reactivity was not longer detectable. Cell fractionation experiments showed that serum IgA reactivity with 65- and 49-kDa proteins was observable in total cell protein extract and in its nuclear fraction, but not in cytosolic fraction (Fig. 4a). The purity of cell protein fractions was confirmed by the reaction of anti-human histone H2B anti-serum with total cell protein extract and its nuclear fraction, but not with the cytosolic fraction (Fig. 4b).
Fig. 4.
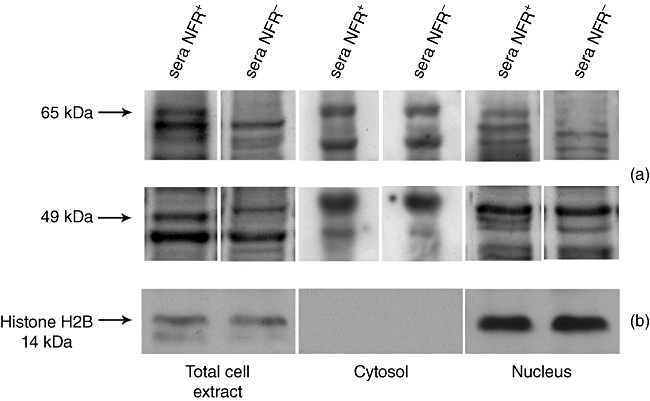
Serum immunoglobulin (Ig)A reactivity with nitrocellulose-blotted Caco2 cell proteins. (a) Nitrocellulose-blotted Caco2 cell protein strips are incubated with coeliac disease (CD) patients' sera positive for nuclear fluorescence reactivity (NFR) antibodies, as well as with sera of the same patients at NFR disappearance after a gluten-free diet (GFD). Arrows indicate the position of the 65- and 49-kDa-reactive components. Molecular masses are indicated on the left side. Serum IgA reactivity is found in total cell protein extract and in its nuclear fraction but not in cytosolic fraction and, after a GFD, disappear with the disappearance of NFR itself. (b) Nitrocellulose-blotted total cell protein extract, as well as its nuclear and cytosolic fractions are also probed with anti-human histone H2B anti-serum. As a result, the reaction of this anti-serum with total cell protein extract and its nuclear fraction, but not with cytosolic fraction, ensures the purity of cell protein fractions.
Kinetics of EMA and NFR antibody production in organ culture system
In four of 11 treated CD patients in group 1, duodenal NFR antibodies appeared after 4 h from starting the in vitro gliadin challenge and became detectable in all supernatants after 6 h of biopsy culture. At the same time-points, no duodenal EMA were detectable. At 24 and 48 h from starting the in vitro gliadin challenge, EMA and NFR antibodies were present simultaneously in culture supernatants (Fig. 5). At any time-point, neither EMA nor NFR antibodies were detectable in supernatants when the biopsy samples were cultured in medium alone.
Fig. 5.
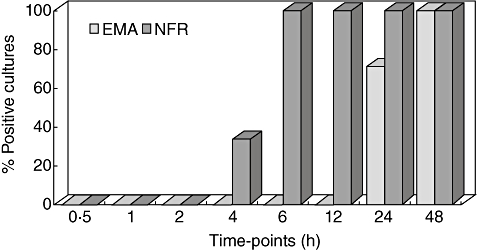
Kinetics of endomysium antibodies (EMA) and nuclear fluorescence reactivity (NFR) antibody production in the organ culture system. Duodenal IgA1 EMA and IgA2 NFR antibody results, obtained during in vitro gliadin challenge of biopsy samples from treated CD patients, are plotted in the graph. In detail, NFR antibodies are produced/secreted by all biopsy samples after 6 h, whereas EMA appear in supernatants later than 12 h from starting the biopsy culture.
Twelve of 24 treated CD patients in group 2, who at a certain point of their GFD presented serum EMA-negative and NFR-positive results, were submitted to upper endoscopy and their biopsy samples were cultured in the presence and absence of PT–gliadin. EMA and NFR antibodies were detectable in all culture supernatants, irrespective of the in vitro gliadin challenge.
Serum ANA evaluation
Because of the timing of serum EMA and NFR antibodies, circulating ANA were evaluated at three time-points: during EMA-positive results, under EMA disappearance/NFR-positive results and after NFR disappearance. At all time-points, serum ANA were positive in two of 20 CD patients in group 1. In both cases, an ANA-S antibody pattern (subpattern: fine speckled) was visible.
Healthy controls
None of the 15 subjects in group 3 presented serum EMA-positive results, while two showed an NFR-like pattern on monkey oesophagus sections. The latter two subjects were put on a GFD for 12 months. Serum EMA and NFR antibodies were evaluated each month, showing no changes in the NFR-like pattern. The characterization of this NFR-like pattern showed that it belonged simultaneously to IgA1 and IgA2 subclasses, and that it was localized in the nucleus.
Discussion
The results of the present study demonstrate that serum IgA from CD patients are able to react with two nuclear antigens determining the appearance of a nuclear fluorescence reactivity (NFR) antibody pattern on monkey oesophagus sections used routinely for EMA detection. Moreover, as NFR antibodies are detectable in serum as long as the CD patients consume gluten and disappear after gluten withdrawal from the diet, they are gluten-dependent and related strictly to CD.
The autoimmune nature of CD is understood clearly [5–7], and the main autoantigen is well known to be tTG [11]. However, tTG is not the only CD-related autoantigen, as other tissue components have been shown to be a target of coeliac autoimmunity [12–15]. In serum of active CD patients, antibodies against thyroid and pancreas structures, cytoskeleton molecules and central nervous system-related antigens have been found previously [14]. The present study adds a new antigen type to the list, as we found that serum IgA from untreated CD patients react with two NFR-related nuclear antigens of 65 and 49 kDa. The identity of NFR-related autoantigens is as yet unknown, but based on the different distribution of EMA and NFR reaction sites on monkey oesophagus sections it is reasonable to hypothesize that these reactivities are due to distinct antigenic specificity. Indeed, EMA and NFR antibody patterns are never observable simultaneously during total IgA EMA detection but, using secondary mAbs against IgA subclasses (IgA1 and IgA2) coupled with different fluorochromes (FITC and TRITC), the presence of two different and not overlapping fluorescence signals becomes evident. That the main endomysial antigen, known to be tTG [11], has a different molecular weight with respect to the newly identified autoantigens (85 versus 65 and 49 kDa), further confirms the hypothesis that EMA and NFR are two distinct antibodies. Finally, the observation that EMA are associated mainly with the IgA1 isotype and NFR is always related to the IgA2 subclass suggests, for these antibodies, a molecular recombination based on two distinct isotypic-switching pathways. However, further investigations are necessary to understand the biological significance of this finding.
The nuclear nature of NFR-related 65- and 49-kDa antigens has been evidenced by cell fractionation experiments. In fact, sera collected from CD patients when NFR antibodies are observable show IgA reactivity in total cell protein extract and in its nuclear fraction that is absent in the cytosolic fraction. Serum IgA reactivity with 65- and 49-kDa antigens has been detected on lysates of the human Caco2 cell line, and is therefore definable as autoimmune. Moreover, we also show that this autoreactivity is gluten-dependent, and therefore related strictly to CD. Indeed, it is present in CD patients' sera up to NFR antibodies are observable and disappear on a GFD, with the clearance of NFR antibodies themselves.
Circulating autoantibodies CD patients provide an important tool in screening, diagnosing and monitoring the disease. In detail, serum EMA and anti-tTG antibodies are used currently in clinical practice on account of their high sensitivity and specificity [16,17]. Furthermore, serum EMA disappear upon the mucosal healing subsequent to a GFD [21], while after gluten reintroduction into the diet their reappearance may predict mucosal relapse [28]. The kinetics of EMA, however, is not well known and it is not investigated widely. In the present study, we show that EMA disappearance in sera from treated CD patients is complete within 76 ± 34 days after starting the GFD. At this time-point, serum NFR antibodies become observable and persist for a further 75 ± 41 days for a total of 151 ± 37 days from starting the GFD. Our data also show that, after the reintroduction of small amounts of gluten in the diet, NFR antibodies reappear within a few days, much earlier than serum EMA. The biopsy culture study shows that NFR antibodies are produced early (4–6 h), while EMA appear after more than 12 h from starting the in vitro gliadin challenge. This in vitro finding is consistent with result of the in vivo gluten-induced reactivation of CD. Consequently, given that NFR seems to be more sensitive than EMA as an early marker of CD reactivation, NFR antibody detection in serum from treated CD patients might become a valuable tool in monitoring adherence to GFD and identifying slight dietary transgressions. The appearance of serum NFR during gluten withdrawal, together with the persistence of symptoms when these antibodies are still positive but EMA are already negative, also suggest that NFR assessment could be an useful tool to determine the right time to perform a second duodenal biopsy. However, before applying these suggestions, our data need to be confirmed by large clinical trials.
The presence of a serum NFR-like pattern in some healthy controls evaluated in this study could suggest a low specificity for NFR antibody detection in CD monitoring. However, it is noteworthy that NFR belongs to a different IgA isotype with respect to that of the NFR-like pattern observed in controls. In addition, this NFR-like pattern is never associated with EMA and, in the controls treated with 12 months of gluten withdrawal, it did not disappear, showing the absence of a gluten dependency.
On the other hand, as only two of 20 CD patients evaluated in this study show serum ANA-positive results, it is possible to conclude that NFR antibodies are different from the classics ANA. Incidentally, the ANA prevalence observed in our CD patients does not exceed the frequency reported currently for different classes of healthy individuals [35].
In conclusion, this is an early translational study describing a new autoantibody named NFR related to CD. In fact, the presence of NFR antibodies in CD patients' serum is gluten-dependent and, accordingly, they could be considered to be CD-specific. The identity of NFR-related 65- and 49-kDa autoantigens is yet unknown, and therefore further investigations should be addressed to either obtain new knowledge on the humoral response of CD or to facilitate the development of a novel and promising serological test. In this regard, if our data are confirmed by large clinical trials, serum NFR antibody detection might to become a useful tool to monitor treated CD patients.
Acknowledgments
The present study was supported by research funds assigned to Antonio Picarelli MD from the Sapienza University, Rome, Italy.
Disclosure
Authors declare that there are no financial or other relationships that might lead to conflicts of interest.
References
- 1.Di Sabatino A, Corazza GR. Celiac disease. Lancet. 2009;373:1480–93. doi: 10.1016/S0140-6736(09)60254-3. [DOI] [PubMed] [Google Scholar]
- 2.Ellis HJ, Ciclitira PJ. Should celiac sufferers be allowed their oats? Eur J Gastroenterol Hepatol. 2008;20:492–3. doi: 10.1097/MEG.0b013e3282f465b0. [DOI] [PubMed] [Google Scholar]
- 3.Accomando S, Cataldo F. The global village of celiac disease. Dig Liver Dis. 2004;36:492–8. doi: 10.1016/j.dld.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 4.Heap GA, van Heel DA. Genetics and pathogenesis of celiac disease. Semin Immunol. 2009;21:346–54. doi: 10.1016/j.smim.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Jabri B, Kasarda DD, Green PH. Innate and adaptive immunity: the yin and yang of celiac disease. Immunol Rev. 2005;206:219–31. doi: 10.1111/j.0105-2896.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- 6.Kagnoff MF. Celiac disease: pathogenesis of a model immunogenetic disease. J Clin Invest. 2007;117:41–9. doi: 10.1172/JCI30253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jabri B, Sollid LM. Tissue-mediated control of immunopathology in celiac disease. Nat Rev Immunol. 2009;9:858–70. doi: 10.1038/nri2670. [DOI] [PubMed] [Google Scholar]
- 8.Savilahti E, Viander M, Perkkiö M, Vainio E, Kalimo K, Reunala T. IgA antigliadin antibodies: a marker of mucosal damage in childhood celiac disease. Lancet. 1983;1:320–2. doi: 10.1016/s0140-6736(83)91627-6. [DOI] [PubMed] [Google Scholar]
- 9.Aleanzi M, Demonte AM, Esper C, Garcilazo S, Waggener M. Celiac disease: antibody recognition against native and selectively deamidated gliadin peptides. Clin Chem. 2001;47:2023–8. [PubMed] [Google Scholar]
- 10.Chorzelski TP, Sulej J, Tchorzewska H, Jablonska S, Beutner EH, Kumar V. IgA class endomysium antibodies in dermatitis herpetiformis and celiac disease. Ann NY Acad Sci. 1983;420:325–34. doi: 10.1111/j.1749-6632.1983.tb22220.x. [DOI] [PubMed] [Google Scholar]
- 11.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 12.Uhlig HH, Lichtenfeld J, Osman AA, Richter T, Mothes T. Evidence for existence of celiac disease autoantigens apart from tissue transglutaminase. Eur J Gastroenterol Hepatol. 2000;12:1017–20. doi: 10.1097/00042737-200012090-00009. [DOI] [PubMed] [Google Scholar]
- 13.Stulik J, Hernychova L, Porkertova S, et al. Identification of new celiac disease autoantigens using proteomic analysis. Proteomics. 2003;3:951–6. doi: 10.1002/pmic.200300370. [DOI] [PubMed] [Google Scholar]
- 14.Shaoul R, Lerner A. Associated autoantibodies in celiac disease. Autoimmun Rev. 2007;6:559–65. doi: 10.1016/j.autrev.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Alaedini A, Green PH. Autoantibodies in celiac disease. Autoimmunity. 2008;41:19–26. doi: 10.1080/08916930701619219. [DOI] [PubMed] [Google Scholar]
- 16.Rostom A, Dubé C, Cranney A, et al. The diagnostic accuracy of serologic tests for celiac disease: a systematic review. Gastroenterology. 2005;128:S38–46. doi: 10.1053/j.gastro.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 17.Lewis NR, Scott BB. Systematic review: the use of serology to exclude or diagnose celiac disease (a comparison of the endomysial and tissue transglutaminase antibody tests) Aliment Pharmacol Ther. 2006;24:47–54. doi: 10.1111/j.1365-2036.2006.02967.x. [DOI] [PubMed] [Google Scholar]
- 18.Dickson BC, Streutker CJ, Chetty R. Celiac disease: an update for pathologists. J Clin Pathol. 2006;59:1008–16. doi: 10.1136/jcp.2005.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurppa K, Collin P, Viljamaa M, et al. Diagnosing mild enteropathy celiac disease: a randomized, controlled clinical study. Gastroenterology. 2009;136:816–23. doi: 10.1053/j.gastro.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 20.Leffler DA, Edwards George JB, Dennis M, Cook EF, Schuppan D, Kelly CP. A prospective comparative study of five measures of gluten-free diet adherence in adults with celiac disease. Aliment Pharmacol Ther. 2007;26:1227–35. doi: 10.1111/j.1365-2036.2007.03501.x. [DOI] [PubMed] [Google Scholar]
- 21.Setty M, Hormaza L, Guandalini S. Celiac disease: risk assessment, diagnosis, and monitoring. Mol Diagn Ther. 2008;12:289–98. doi: 10.1007/BF03256294. [DOI] [PubMed] [Google Scholar]
- 22.Dipper CR, Maitra S, Thomas R, et al. Anti-tissue transglutaminase antibodies in the follow-up of adult celiac disease. Aliment Pharmacol Ther. 2009;30:236–44. doi: 10.1111/j.1365-2036.2009.04039.x. [DOI] [PubMed] [Google Scholar]
- 23.Picarelli A, Maiuri L, Frate A, Greco M, Auricchio S, Londei M. Production of antiendomysial antibodies after in-vitro gliadin challenge of small intestine biopsy samples from patients with celiac disease. Lancet. 1996;348:1065–7. doi: 10.1016/S0140-6736(96)03060-7. [DOI] [PubMed] [Google Scholar]
- 24.Sblattero D, Florian F, Not T, et al. The gut as site of production of autoimmune antibodies. J Pediatr Gastroenterol Nutr. 2004;39:S730–1. doi: 10.1097/00005176-200406003-00007. [DOI] [PubMed] [Google Scholar]
- 25.Stenman SM, Lindfors K, Korponay-Szabo IR, et al. Secretion of celiac disease autoantibodies after in vitro gliadin challenge is dependent on small-bowel mucosal transglutaminase 2-specific IgA deposits. BMC Immunol. 2008;9:6. doi: 10.1186/1471-2172-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carroccio A, Di Prima L, Pirrone G, et al. Anti-transglutaminase antibody assay of the culture medium of intestinal biopsy specimens can improve the accuracy of celiac disease diagnosis. Clin Chem. 2006;52:1175–80. doi: 10.1373/clinchem.2005.061366. [DOI] [PubMed] [Google Scholar]
- 27.Bonamico M, Sabbatella L, Di Tola M, et al. Antiendomysial antibody detection in biopsy culture allows avoidance of gluten challenge in celiac children. J Pediatr Gastroenterol Nutr. 2005;40:165–9. doi: 10.1097/00005176-200502000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Troncone R, Caputo N, Micillo M, Maiuri L, Poggi V. Immunologic and intestinal permeability tests as predictors of relapse during gluten challenge in childhood celiac disease. Scand J Gastroenterol. 1994;29:144–7. doi: 10.3109/00365529409090453. [DOI] [PubMed] [Google Scholar]
- 29.Liu E, Li M, Emery L, et al. Natural history of antibodies to deamidated gliadin peptides and transglutaminase in early childhood celiac disease. J Pediatr Gastroenterol Nutr. 2007;45:293–300. doi: 10.1097/MPG.0b013e31806c7b34. [DOI] [PubMed] [Google Scholar]
- 30.Troncone R, Mayer M, Spagnuolo F, Maiuri L, Greco L. Endomysial antibodies as unreliable markers for slight dietary transgressions in adolescents with celiac disease. J Pediatr Gastroenterol Nutr. 1995;21:69–72. doi: 10.1097/00005176-199507000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Natter S, Granditsch G, Reichel GL, et al. IgA cross-reactivity between a nuclear autoantigen and wheat proteins suggests molecular mimicry as a possible pathomechanism in celiac disease. Eur J Immunol. 2001;31:918–28. doi: 10.1002/1521-4141(200103)31:3<918::aid-immu918>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 32.Sabbatella L, Di Tola M, Di Cello T, et al. Nuclear fluorescence reactivity: a novel sensitive marker in the fine follow-up of celiac disease. Dig Liver Dis. 2001;33:A66. [Google Scholar]
- 33.Storch WB, Schloeder U. A new autoantibody in celiac disease. Clin Lab. 2002;48:19–23. [PubMed] [Google Scholar]
- 34.Fasano A, Araya M, Bhatnagar S, et al. Federation of International Societies of Pediatric Gastroenterology, Hepatology, and Nutrition consensus report on celiac disease. J Pediatr Gastroenterol Nutr. 2008;47:214–19. doi: 10.1097/MPG.0b013e318181afed. [DOI] [PubMed] [Google Scholar]
- 35.Marin GG, Cardiel MH, Cornejo H, Viveros ME. Prevalence of antinuclear antibodies in 3 groups of healthy individuals: blood donors, hospital personnel, and relatives of patients with autoimmune diseases. J Clin Rheumatol. 2009;15:325–9. doi: 10.1097/RHU.0b013e3181bb971b. [DOI] [PubMed] [Google Scholar]


