Abstract
Anti-endothelial cell antibodies (AECA) have been frequently detected in systemic vasculitis, which affects blood vessels of various sizes. To understand the pathogenic roles of AECA in systemic vasculitis, we attempted to identify target antigens for AECA comprehensively by a proteomic approach. Proteins extracted from human umbilical vein endothelial cells (HUVEC) were separated by two-dimensional electrophoresis, and Western blotting was subsequently conducted using sera from patients with systemic vasculitis. As a result, 53 autoantigenic protein spots for AECA were detected, nine of which were identified by mass spectrometry. One of the identified proteins was peroxiredoxin 2 (Prx2), an anti-oxidant enzyme. Frequency of anti-Prx2 autoantibodies, measured by enzyme-linked immunosorbent assay (ELISA), was significantly higher in systemic vasculitis (60%) compared to those in collagen diseases without clinical vasculitis (7%, P < 0·01) and healthy individuals (0%, P < 0·01). Further, the titres changed in parallel with the disease activity during time–courses. The presence of anti-Prx2 autoantibodies correlated significantly with elevation of serum d-dimers and thrombin–antithrombin complex (P < 0·05). Immunocytochemical analysis revealed that live endothelial cells expressed Prx2 on their surface. Interestingly, stimulation of HUVEC with rabbit anti-Prx2 antibodies increased secretion of interleukin (IL)-6, IL-1β, IL-1ra, growth regulated oncogene (GRO)-α, granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM–CSF), IL-8 and monocyte chemoattractant protein (MCP)-1 more than twofold compared to that of with rabbit immunoglobulin (Ig)G. Taken together, our data suggest that anti-Prx2 autoantibodies would be a useful marker for systemic vasculitis and would be involved in the inflammatory processes of systemic vasculitis.
Keywords: two-dimensional electrophoresis, anti-endothelial cell antibodies, peroxiredoxin 2, proteomics, systemic vasculitis
Introduction
Systemic vasculitis, characterized by chronic inflammation within the walls of blood vessels, is a heterogeneous disorder. Primary systemic vasculitis is classified into three groups according to the size of the affected blood vessels, as follows: (i) large blood vessels, i.e. Takayasu's arteritis (TA) and giant cell arteritis (GCA), (ii) middle-sized blood vessels, i.e. polyarteritis nodosa (PN) and Kawasaki's disease (KD) and (iii) small blood vessels, i.e. Wegener's granulomatosis (WG), microscopic polyangitis (MPA), allergic granulomatous angitis (AGA), cryoglobulinaemic vasculitis (CV) and Henoch–Schonlein purpura (HS) [1,2]. On the other hand, systemic vasculitis is associated with collagen diseases, malignancies and infectious diseases (secondary systemic vasculitis) [3]. Among collagen diseases, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and Behçet's disease (BD) are often associated with systemic vasculitis [3–5].
The pathogenesis of systemic vasculitis remains to be fully solved. At present, autoantibodies (AGA, MPA and WG), immune complexes (CV, HS and SLE) and pathogenic T cell responses (AGA, GCA, TA, WG and vasculo-BD, vBD) are considered to be candidates for the pathogenic factors [6,7]. Autoantibodies produced specifically in patients with systemic vasculitis may cause vascular inflammation directly or through the formation of immune complexes [2]. The representative autoantibodies are anti-neutrophil cytoplasmic antibodies (ANCA) and anti-endothelial cell antibodies (AECA) [7]. Two major autoantigens of proteinase 3 (PR3) and myeloperoxidase (MPO) for ANCA have been identified [8,9]. PR3-ANCA is detected specifically in WG and thereby used as a disease marker for WG, whereas MPO-ANCA is detected frequently in MPA, AGA and other autoimmune diseases [6]. It is hypothesized that ANCA trigger degranulation, activation and apoptosis of neutrophils which then cause endothelial cell damage [8].
In contrast with ANCA, AECA is detected widely in various types of systemic vasculitis [10–18]. The presence of AECA is known to be correlated with the activity of vasculitis [12,15,19]. Further, AECA is reported to be associated with particular clinical manifestations; for example, acute thrombotic events, retinal vasculitis and involvement of the central nervous system in BD [16,17], and vascular lesions, nephropathy and production of anti-cardiolipin antibodies in SLE [15,20]. AECA has been reported to be involved in endothelial cell damage and vascular injury by its binding to endothelial cell surface antigens [10,20]. Binding activity of AECA was increased by pretreatment of HUVEC with tumour necrosis factor (TNF), interleukin (IL)-1 or interferon (IFN)-γ[18]. Moreover, a part of AECA-containing sera showed antibody-dependent cellular cytotoxicity against HUVEC with unfractionated peripheral blood mononuclear cells [18]. Thus, AECA would react to constitutively expressed and/or cytokine-induced determinants on the surface of endothelial cells, which would contribute to vascular injury.
The significance of AECA in the diagnosis and pathogenesis of systemic vasculitis has not been identified fully. One of the reasons could be that target antigens for AECA were not established. Thereby, in most studies, detection of AECA was conducted by cellular enzyme-linked immunosorbent assay (ELISA), in which autoantibodies to various antigens were measured together. Immunoprecipitation and Western blotting (WB) were also used for investigation of AECA; however, the antigens remained unidentified [11,21]. To evaluate the roles of AECA precisely, it is essential to identify the target antigens for AECA in systemic vasculitis. Recently, techniques in proteomic studies have progressed rapidly, facilitating screening and identification of autoantigens by two-dimensional electrophoresis and WB followed by mass spectrometry. Several target antigens for AECA in secondary systemic vasculitis have been identified recently using such techniques, e.g. heat shock protein 60 in SLE and α-enolase and selenium binding protein in BD [20,22,23].
In this study, we comprehensively detected target antigens for AECA in patients with primary and secondary systemic vasculitis by the proteomic techniques. We identified several novel antigens for AECA, then focused upon one of the identified antigens, peroxiredoxin 2 (Prx2) of a redox enzyme. Anti-Prx2 antibodies up-regulated secretion of inflammatory cytokines and chemokines from vascular endothelial cells. Titres of the anti-Prx2 autoantibodies were correlated with the disease activity of systemic vasculitis. Thus, novel AECA, anti-Prx2 autoantibodies, may play a role in vascular injury in systemic vasculitis.
Methods
Patients
Forty patients with systemic vasculitis (25 females and 15 males) were enrolled into the study. The mean age was 53·1 years, with a range of 13–88 years. The patients comprised three with WG, eight with MPA, six with AGA, one with PN, two with HS, seven with TA, two with GCA, four with vBD, six with rheumatoid vasculitis (RV) and one with vasculitis associated with SLE. Fifty-five patients with collagen diseases without clinical vasculitis (43 females and 12 males) were enrolled as a non-vasculitis group. The mean age was 49·0 years with a range of 24–81 years. The patients comprised 20 with SLE, 20 with RA and 15 with BD. All the patients were diagnosed according to the respective classification criteria of American College of Rheumatology [24–33]. Twenty-five age- and sex-matched healthy donors were enrolled as a control group. Serum samples were obtained from the patients and healthy donors with informed consent. This study was approved by the ethics committee of St Marianna University School of Medicine.
Cell culture
Normal human umbilical vein endothelial cells (HUVEC) and HeLa cells were obtained from the Human Science Research Resources Bank (Sennan, Japan). Normal human aortic endothelial cells (HAEC) and normal human lung microvascular endothelial cells (HMVEC-L) and culture media and supplements for HUVEC, HAEC and HMVEC-L were obtained from Lonza (Walkersville, MD, USA). Normal human pulmonary artery endothelial cells (HPAEC) and normal human microvascular endothelial cells from adult dermis (HMVEC) were obtained from Life Technologies (Carlsbad, CA, USA). Culture media and supplements for HPAEC and HMVEC were obtained from Kurabo Industries (Osaka, Japan). All endothelial cells were cultured in the mixture of their respective media and supplements as described previously [34]. HeLa cells were cultured in Dulbecco's modified Eagle's medium (Sigma-Aldrich, St Louis, MO, USA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA). Cells at passages 3–9 were harvested by incubation in Ca- and Mg-free phosphate-buffered saline (PBS) containing 0·05% Trypsin–ethylenediamine tetraacetic acid (EDTA) (Gibco, Carlsbad, CA, USA) for 5 min at 37°C. The cells were then washed in PBS and stored at −80°C until use for two-dimensional electrophoresis (2DE) or sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE).
2DE
2DE was conducted as described previously [23]. Briefly, the harvested HUVEC and HeLa cells were lysed in a lysis buffer (7 M urea, 2 M thiourea and 4% 3-(3-cholamidepropyl)dimethylammonio-l-propanesulphonate, CHAPS). One hundred µg of the cell lysate was loaded onto Immobiline Drystrip Gels (pH 3–10; GE Healthcare, Uppsala, Sweden). As the first step of 2DE, isoelectric focusing electrophoresis was conducted using MultiPhor II (GE Healthcare). Next, the drystrip gels after the electrophoresis were placed onto a 12·5% SDS-PAGE gel, and SDS-PAGE was conducted as the second step of 2DE. After SDS-PAGE, the gels were stained with Coomassie brilliant blue (CBB) or Sypro Ruby (Invitrogen, Carlsbad, CA, USA) for visualizing proteins separated into spots. 2DE was conducted in duplicate. After electrophoresis, one gel was used for CBB staining and the other gel was used for transfer of the separated proteins onto a nitrocellulose membrane for WB.
SDS-PAGE
SDS-PAGE was conducted as described previously [23]. To detect cellular Prx2, HUVEC at the third to ninth passages and HeLa cells were used for SDS-PAGE and WB. Proteins were extracted from the two types of cells adjusted to be identical in cell number. Each of the protein solutions was mixed with an equal volume of 2× SDS-dithiothreitol (DTT) buffer (125 mM Tris, 20% glycerol, 160 mM SDS, 74·6 µM bromophenol blue, 50 mM DTT). The mixtures were boiled at 95°C and were centrifuged at 15 000 g. An identical volume of the supernatants was applied to 12·5% SDS-PAGE gels.
To detect anti-Prx2 autoantibodies, recombinant Prx2 protein fused with maltose binding protein (MBP) and MBP alone were prepared as described below. The recombinant proteins were applied to 12·5% SDS-PAGE gel.
To compare expression of Prx2 among HUVEC, HAEC, HPAEC, HMVEC and HMVEC-L, these cells were adjusted to be identical in cell number and were suspended in PBS. Each of the cell solutions were mixed with a equal volume of 2× SDS-DTT buffer, boiled at 95°C and centrifuged at 15 000 g. An identical volume of the supernatants was applied to a 15·0% SDS-PAGE gel.
To determine subcellular localization of Prx2, proteins were extracted into PBS from membrane and organelle fractions of these cells using the ProteoExtract Subcellular Proteome Extraction Kit (Calbiochem, San Diego, CA, USA). The protein solutions were mixed with an equal volume of 2× SDS-DTT buffer, boiled, and then centrifuged as described above. The supernatants obtained, which included 18 µg of the extracted proteins, were applied to 15·0% SDS-PAGE gel.
After the above SDS-PAGE procedures, separated proteins were transferred onto nitrocellulose membranes to conduct WB.
Preparation of anti-Prx2 antibodies
Polyclonal antibodies to Prx2 were generated (KAC Ltd, Kyoto, Japan) by immunization of rabbits with a keyhole limpet haemocyanin (KLH)-conjugated Prx2-derived peptide (N-GCKPNVDDSKEYFSKHN-C). The antibodies were affinity-purified from the rabbit sera using the Prx2 peptide without KLH. The purified antibodies were used in WB, immunocytochemistry and stimulation of HUVEC for cytokine and chemokine assay.
WB
For detection of AECA candidate autoantigens, the protein-transferred nitrocellulose membranes were incubated in 1% Block Ace (Yukijirushi, Sapporo, Japan) to block non-specific reaction, and then washed in PBS with 0·1% Tween 20 (PBS-T). Each of the serum samples from the patients with systemic vasculitis was diluted at 1:100 in 1% Block Ace. The membranes were reacted with serum samples for 1 h. After washing three times in PBS-T, the bound autoantibodies were reacted with horseradish peroxidase (HRP)-conjugated goat anti-human immunoglobulin G (IgG) antibodies (Invitrogen) diluted at 1:400 using 1% Block Ace. Finally, the bound IgG antibodies were visualized using diaminobenzidine (Wako, Osaka, Japan).
For detection of Prx2, the cellular protein-transferred nitrocellulose membranes were reacted with anti-Prx2 antibodies, then with HRP-conjugated goat anti-rabbit IgG (H+L) diluted at 1:2000 (Invitrogen). Finally, the bound anti-Prx2 antibodies were visualized using diaminobenzidine. Intensity of the obtained bands was measured using Typhoon 9400 (GE Healthcare).
Protein identification
Protein identification by mass spectrometry (MS) was conducted as described previously [23]. Briefly, 500 µg of the cell lysate was separated by 2-DE. Gel specimens of the target protein spots were recovered, and the proteins were subjected to in-gel digestion with trypsin. Next, the generated peptides were extracted from gel matrix using C18 hydrophobic carrier. Masses of the protein fragments were measured using a matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS). A list of the determined peptide masses was subjected to protein database search using the Mascot program (http://www.matrixscience.com), in which protein databases of the National Center for Biotechnology Information (NCBI, Bethesda, MD, USA) were searched. Finally, MS/MS spectra of peptides of interest were measured in collision-induced dissociation mode for identification of the peptides.
Preparation of recombinant fusion proteins
Recombinant fusion proteins were prepared as described previously [35]. Briefly, a DNA fragment for the entire protein-coding region of human Prx2 was amplified from HUVEC-derived cDNA library by reverse transcription–polymerase chain reaction (RT–PCR) using the following primers: sense 5′-TTTGAATTCATGGCCTCCGGTAACGCGCGC-3′ and anti-sense 5′-TTTGTCGACATTGTGTTTGGAGAAATATTCCTTGCT-3′. The obtained DNA fragment encoding Prx2 was digested by EcoRI and SalI, and cloned into a plasmid expression vector of pMAL-eHis, a derivative of pMAL-c2 (New England Biolabs, Beverly, MA, USA). A recombinant Prx2 protein with a tag of six histidines at the C-terminal was expressed in Escherichia coli as a fusion protein with MBP. Recombinant Prx2 protein (MBP-Prx2-His) as well as MBP alone (MBP-His) was purified using histidine-Ni+ affinity columns (His Trap; GE Healthcare, Piscataway, NJ, USA).
ELISA
Each well of a multi-titre plate for ELISA (Dynatech, Alexandra, VA, USA) was coated with 0·5 µg of MBP-Prx2-His or MBP-His solved in a carbonate buffer (50 mM sodium carbonate, pH 9·6). Non-specific reaction of the coated protein was blocked with 0·25% Block Ace for 1 h. To adsorb reactivity of the serum samples to MBP-His, the serum samples were diluted at 1:100 with 0·1% Block Ace containing 40 µg/ml MBP-His and then incubated for 2 h. The absorbed serum samples were reacted with the coated recombinant proteins for 1 h. The bound antibodies were detected with a HRP-conjugated goat anti-human IgG antibody (Invitrogen). Optical density at 492 nm was measured by a microplate reader (Bio-Rad, Hercules, CA, USA). The optical density value of the average ± 3 standard deviations (s.d.) in healthy donors was defined as 100 arbitrary binding units (AU), which was used as a cut-off point for determining reactivity to Prx2.
Immunocytochemistry
HUVEC were seeded in a chamber slide (LAB-TEK, Rochester, NY, USA) at a density of 6×105 cells/well. Adhered live HUVEC were washed in saline and incubated with rabbit anti-Prx2 antibodies (0·1 mg/ml) or with rabbit IgG (0·1 mg/ml; Dako, Glostrup, Denmark). After washing, the cells were incubated with phycoerythrin (PE)-conjugated goat F(ab′)2 anti-rabbit IgG (H+L) (Cosmo Bio, Tokyo, Japan). The cells were then washed and photographed using a fluorescence microscope (IX71-22TFL/PH; Olympus, Tokyo, Japan).
Detection of cytokines and chemokines secreted by HUVEC stimulated with the anti-Prx2 antibodies
HUVEC (1 × 106) were incubated with rabbit anti-Prx2 antibodies or rabbit IgG for 24 h. Secreted cytokines and chemokines in the cell culture supernatants were detected using the Proteome Profiler Array according to the manufacture's instructions (R&D Systems, Minneapolis, MN, USA). The resultant dot intensities were measured using Typhoon 9400 (GE Healthcare).
Statistics
Fisher's exact test and Student's t-test were conducted for comparison of frequency and average titres of anti-Prx2 autoantibodies between the subjected groups, respectively. Clinical parameters between the anti-Prx2 antibody-positive and -negative patients were compared by Mann–Whitney U-test and Fisher's exact test.
Results
Detection of AECA in patients with systemic vasculitis patients
We first examined whether AECA in sera from patients with systemic vasculitis were detected by WB. Proteins extracted from HUVEC were used as an antigen source. To select autoantigens expressed predominantly in endothelial cells, proteins extracted from HeLa cells were examined simultaneously as reference. Representative results from two patients with WG (WG1 and WG2), from a patient with PN, from a patient with AGA and from a healthy control (Healthy) are shown in Fig. 1. Multiple autoantigens were detected only in the HUVEC lanes, not in the HeLa cell lanes, in the results of two WG patients (arrows in Fig. 1a). Similarly, several autoantigens were detected only in the HUVEC lanes, not in the HeLa cell lanes, in the results of a PN patient and an AGA patient (arrows in Fig. 1b). Instead, no autoantigens were detected specifically in the HUVEC lanes in the results of healthy donors (a representative result is shown in Fig. 1b). Thus, presence of AECA in systemic vasculitis was confirmed in these experiments. Considering that the detected proteins were expressed predominantly in HUVEC rather than in HeLa cells, these autoantigens would be useful candidates for targets of AECA. This differential WB using HEVEC and HeLa cells would be an excellent method to identify novel antigens for AECA.
Fig. 1.
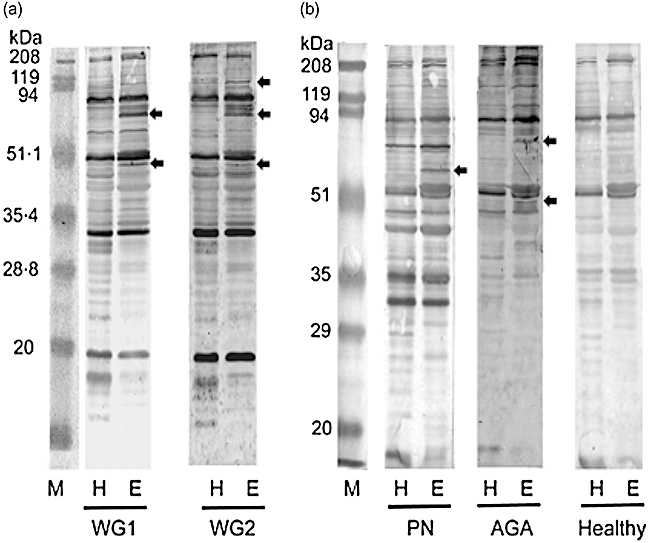
Detection of anti-endothelial cell antibodies (AECA) in systemic vasculitis. Protein extracted from HeLa cells (H) and human umbilical vein endothelial cells (HUVEC) (E) were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins reactive to serum samples from patients with systemic vasculitis were detected by Western blotting. Representative results of serum samples (a) from two patients with Wegener's granulomatosis (WG) (WG1 and WG2), (b) from a patient with polyarteritis nodosa (PN), from a patient with allergic granulomatous angitis (AGA) and from a healthy donor (Healthy) are shown. Arrows show candidate autoantigens for AECA in systemic vasculitis, which were detected specifically in HUVEC extracts. M, marker proteins.
Detection of candidate antigens for AECA in patients with systemic vasculitis by 2DE-WB
Next, we extended the screening method from 1DE-WB to 2DE-WB to make it more effective and to facilitate identification of detected antigens. To specify endothelium-specific proteins that react with sera from systemic vasculitis patients, the results of 2DE-WB using HUVEC (Fig. 2a, c, e and g) were compared with those using HeLa cells (Fig. 2b, d, f and h). Four sets of the serum mixtures were prepared to facilitate the WB. The first mixture was composed of sera from patients with PN, TA and SLE and used for the pair of WB (Fig. 2a and b). Similarly, the second mixture was composed of sera from patients with HS and WG (Fig. 2c and d), the third mixture was composed of sera from vBD (Fig. 2e and f) and the fourth mixture was composed of sera from RV (Fig. 2g and h).
Fig. 2.
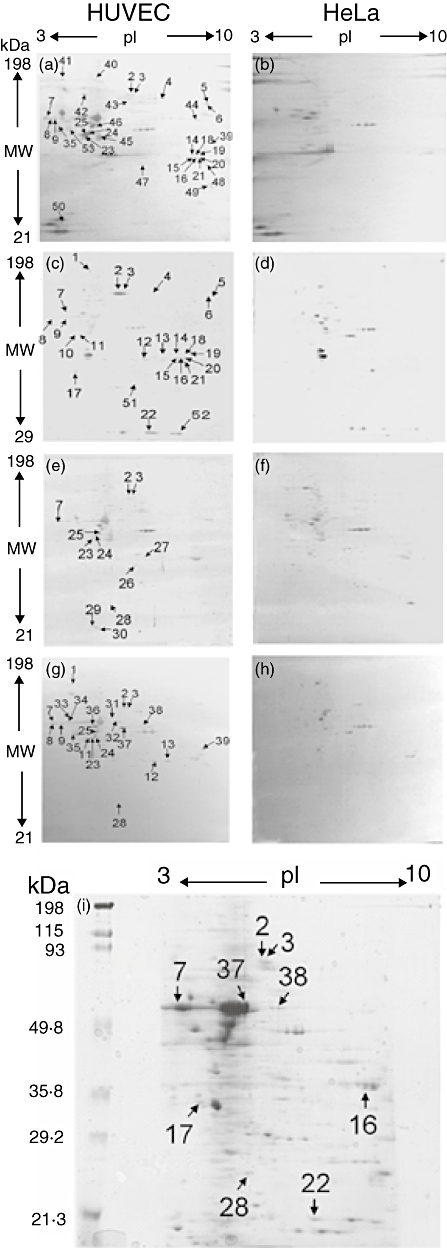
Candidate autoantigens for anti-endothelial cell antibodies (AECA) detected in systemic vasculitis by two-dimensional electrophoresis-Western blotting (2DE-WB). Protein extracted from human umbilical vein endothelial cells (HUVEC) and HeLa cells were separated by 2DE, and autoantigenic proteins were detected by WB using four sets of serum mixtures. In set 1, sera from a patient with polyarteritis nodosa (PN), a patient with Takayasu's arteritis (TA) and a systemic lupus erythematosus (SLE) patient with vasculitis were mixed and used (a,b). In set 2, sera from a patient with Henoch–Schonlein purpura (HS) and a patient with Wegener's granulomatosis (WG) were mixed and used (c,d). In set 3, sera from four patients with vasculo-Behçet's disease (vBD) were mixed and used (e,f). In set 4, sera from six patients with rheumatoid vasculitis (RV) were mixed and used (g,h). Protein spots detected predominantly in HUVEC results are indicated by arrows and spot ID numbers. Locations of the nine identified protein spots by mass spectrometric analysis are indicated in the Coomassie brilliant blue (CBB)-stained 2DE gel using HUVEC extracts (i).
As a result, 53 protein spots were detected specifically in 2DE-WB using HUVEC (arrows, Fig. 2a, c, e, g and i). Three protein spots (spot numbers 2, 3 and 7) were common in all the sets, five spots (spot numbers 8, 9, 23, 24 and 25) were common in three of the four sets, 16 spots were detected in two sets (spot numbers 4, 5, 6, 11, 12, 13, 14, 15, 16, 18, 19, 20, 21, 28, 35 and 39) and eight spots (apot numbers 26, 27, 29, 30, 34, 36, 37 and 38) were detected in only one of the four sets. These protein spots were speculated to be candidates of autoantigens for AECA in systemic vasculitis.
Identification of candidate autoantigens for AECA in systemic vasculitis
We next identified proteins of the 53 spots detected in the 2DE-WB results by MS spectrometry. Nine of the 53 protein spots recovered from 2DE gel were determined successfully (Table 1). Spot numbers 2, 3 and 7, detected in all the sets of 2DE-WB, were identified as zyxin, caldesmon 1 (isoform 3) and vimentin, respectively. Spot numbers 16 and 28, detected in the two sets, were identified as heterogeneous nuclear ribonucleoprotein A2/B1 (isoform B1) and Prx2, respectively. Spot numbers 17 and 22, detected in set 2, were identified as a protein highly similar to tropomyosin alpha chain (isoform F2) and cofilin 1 (non-muscle type), respectively. Spot numbers 37 and 38, detected in set 4, were identified as adenosine monophosphate deaminase 1 and hypothetical protein, respectively. From these results we decided to focus our study upon Prx2, because it is known that Prx2 modulates redox condition of proteins and that oxidative stress to endothelial cells is involved in the pathophysiology of systemic vasculitis [36,37].
Table 1.
Identification of candidate autoantigens for anti-endothelial cell antibodies (AECA) in systemic vasculitis patients.
| Spot number | Proteins | Accession number | MW(kDa)/pI (theoretical) | MW(kDa)/pI (observed) |
|---|---|---|---|---|
| 2 | Zyxin | gi|4508047 | 61·3/6·2 | 82/6·0 |
| 3 | Caldesmon 1 (isoform 3) | gi|15149467 | 65·7/6·4 | 80/6·2 |
| 7 | Vimentin | gi|62414289 | 53·7/5·1 | 60/3·4 |
| 16 | Heterogeneous nuclear ribonucleoprotein A2/B1 (isoform B1) | gi|14043072 | 37·5/9·0 | 37/9·3 |
| 17 | Highly similar to tropomyosin alpha chain (isoform F2) | gi|10435299 | 32·7/4·7 | 33/4·1 |
| 22 | Cofilin 1 (non-muscle type) | gi|5031635 | 18·5/8·2 | 21/7·5 |
| 28 | Peroxiredoxin 2 | gi|33875462 | 21·9/5·7 | 26/5·6 |
| 37 | Adenosine monophosphate deaminase 1 | gi|35505168 | 40·2/9·4 | 60/5·8 |
| 38 | Hypothetical protein | gi|31873302 | 47·1/7·6 | 54/6·9 |
The 53 protein spots indicated in Fig. 2a, c, e and g were recovered from two-dimensional electrophoresis gels. Proteins in the gel specimens were digested with trypsin and were subjected to mass spectrometry. Nine proteins indicated in Fig. 2i were identified by mass spectrometric analysis.
High frequency of the autoantibodies against Prx2 in systemic vasculitis
We evaluated titres of autoantibodies to recombinant Prx2 fusion protein (MBP-Prx2-His) by ELISA using 30 sera from patients with systemic vasculitis, 55 sera from patients with collagen diseases without clinical vasculitis and 25 sera from healthy donors. As a result, 60·0% (18 of 30) of the patients with systemic vasculitis were found to have autoantibodies to Prx2, whereas only 7·3% (four of 55) of the patients with collagen diseases without clinical vasculitis and 0% (none of 25) of the healthy donors were found to have autoantibodies to Prx2 (Fig. 3a). The frequency of anti-Prx2 autoantibodies in systemic vasculitis was significantly higher than those in collagen diseases without clinical vasculitis and healthy donors (systemic vasculitis versus collagen diseases without clinical vasculitis, P < 0·01; systemic vasculitis versus healthy donors, P < 0·01). The average titres of anti-Prx2 antibodies did not differ significantly between patients with systemic vasculitis and patients with collagen diseases without clinical vasculitis (144·8 ± 58·1 AU and 140·2 ± 15·3 AU, respectively, P = 0·78).
Fig. 3.
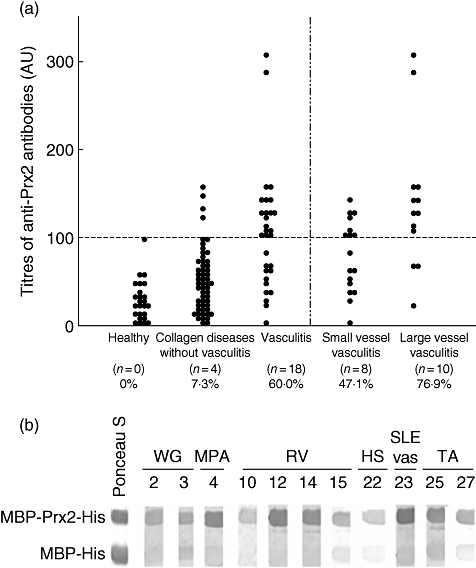
Titres of the autoantibodies to peroxiredoxin 2 (Prx2) in systemic vasculitis. (a) Serum samples from patients with systemic vasculitis, patients with collagen disease other than systemic vasculitis and healthy donors were examined for their autoantibody titres against Prx2 by enzyme-linked immunosorbent assay (ELISA). Optical density value of the average ± 3 standard deviations of the healthy donors was defined as 100 AU, and values more than 100 AU were regarded as positive. The patients with systemic vasculitis were divided into two groups of small-vessel vasculitis [Wegener's granulomatosis (WG), microscopic polyangitis (MPA) and allergic granulomatous angitis (AGA)] and large-vessel vasculitis [Takayasu's arteritis (TA), giant cell arteritis (GCA) and vasculo-Behçet's disease (vBD)] to compare the frequencies and titres of the anti-Prx2 antibodies. (b) Reactivity of serum samples against Prx2 were confirmed by Western blotting using maltose binding protein (MBP)-Prx2-His and MBP-His alone. Representative results of anti-Prx2 antibody-positive patients are shown.
The frequency of anti-Prx2 antibodies tended to be higher in the large-vessel vasculitis (TA, GCA and vBD; 76·9%) than in the small-vessel vasculitis (WG, MPA and AGA; 47·1%), although the difference did not achieve statistical significance (P = 0·14). In the antibody-positive patients, the titres were higher in the large-vessel vasculitis group (166·9 ± 70·5 AU) than in the small-vessel vasculitis group (111·7 ± 15·3 AU), even though the difference did not achieve statistical significance (P = 0·054).
We confirmed visually the presence of anti-Prx2 antibodies in the wide spectrum of systemic vasculitis patients by WB. Representative cases are shown in Fig. 3b. All the serum samples from the antibody-positive patients by ELISA showed stronger reactivity against MBP-Prx2-His compared to the reactivity against MBP-His. Taken together, autoantibodies to Prx2 were frequently present in systemic vasculitis compared to those in collagen diseases without clinical vasculitis. The antibodies were rarely found in healthy individuals.
Expression of Prx2 in vascular endothelial cells
We next examined whether Prx2 was expressed in various types of vascular endothelial cells. Whole cell extracts from HUVEC, HAEC, HPAEC, HMVEC and HMVEC-L were examined by WB. As a result, Prx2 was detected in all the cell types (Fig. 4a). To examine localization of Prx2, we specifically extracted proteins from membrane/organelle fractions of these cell lines. As a result of WB, considerable amounts of Prx2 were detected in the membrane/organelle fractions in all the examined endothelial cell lines (Fig. 4b). In immunocytochemistry, rabbit antibodies to Prx2 were found to bind to the cell surface of live HUVEC (Fig. 4c and d). Thus, a part of Prx2 is expressed on the surface of endothelial cells, and anti-Prx2 autoantibodies would be able to access Prx2 on vascular endothelial cells.
Fig. 4.
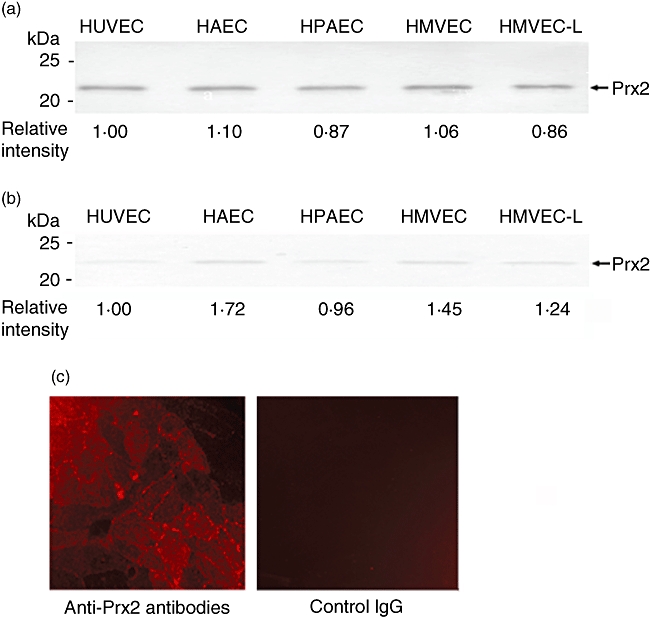
Expression of peroxiredoxin 2 (Prx2) in vascular endothelial cells. Prx2 was detected in whole cell extracts from human umbilical vein endothelial cells (HUVEC), human aortic endothelial cells (HAEC), human pulmonary artery endothelial cells (HPAEC), human microvascular endothelial cells (HMVEC) and human lung microvascular endothelial cells (HMVEC-L)] normal human lung microvascular endothelial cells by Western blotting (a). Prx2 was detected in membrane/organelle fractions separated from the above cell types (b). Relative intensities of the detected Prx2 bands are shown as intensities in HUVEC as 1·0. Expression of Prx2 on live HUVEC was examined by immunocytochemistry using rabbit anti-Prx2 antibodies (c, left) and rabbit immunoglobulin G as a negative control (c, right).
Clinical significance of anti-Prx2 antibodies
We next examined whether anti-Prx2 autoantibodies play a role in the pathophysiology of systemic vasculitis. Laboratory examinations were compared between the anti-Prx2 antibody-positive and -negative patients in an active phase of systemic vasculitis. Interestingly, levels of d-dimer of a marker of thrombus formation and thrombin–antithrombin complex (TAT) of ongoing coagulation were increased significantly in the anti-Prx2 antibody-positive group (Table 2). Levels of inflammatory markers of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and platelet counts (PLT) were not significantly different between the two groups. Similarly, the frequency of MPO-ANCA, that of anti-nuclear antibody and levels of IgG were not significantly different between the two groups. Thus, it is suggested that the anti-Prx2 antibodies would up-regulate coagulation activity in an active phase of systemic vasculitis.
Table 2.
Comparison of clinical laboratory data between anti-peroxiredoxin 2 (Prx2) antibody-positive and -negative patients.
| Anti-Prx2 | Antibodies | ||
|---|---|---|---|
| Clinical parameters | Positive | Negative | P-values |
| CRP (mg/dl) | 7·0 ± 5·2(n = 14) | 6·0 ± 5·9(n = 10) | n.s. |
| ESR(mm/h) | 64·7 ± 46·3(n = 13) | 59·7 ± 37·6(n = 10) | n.s. |
| PLT(×104 µl) | 38·4 ± 13·8(n = 14) | 39·2 ± 11·1(n = 10) | n.s. |
| D-dimer(µg/ml) | 6·8 ± 6·5(n = 11) | 2·2 ± 1·7(n = 5) | P < 0·05 |
| TAT(µg/l) | 4·3 ± 3·0(n = 7) | 2·4 ± 0·5(n = 5) | P < 0·05 |
| IgG(mg/dl) | 1903·6 ± 977·8(n = 11) | 1397·0 ± 337·0(n = 9) | n.s. |
| MPO-ANCA* | 20%(n = 15) | 17%(n = 12) | n.s. |
| ANA* | 23%(n = 13) | 50%(n = 8) | n.s. |
Ratios of the antibody-positive patients were shown.
Statistical analysis was conducted by Mann–Whitney U-test or Fisher's exact test (*). ANCA: anti-neutrophil cytoplasmic antibodies; ANA: anti-nuclear antibodies; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; IgG: immunoglobulin G; MPO: myeloperoxidase; TAT: thrombin–antithrombin complex; n.s.: not significant.
We further analysed the time–course of the anti-Prx2 antibody titres in patients with systemic vasculitis. In a patient with TA treated with 40 mg/day prednisolone, ratios of the anti-Prx2 antibody titre/total IgG were decreased gradually during the time–course in accordance with the decrease of CRP and ESR (Fig. 5a). As shown in Fig. 5b, the decrease of anti-Prx2 antibody titres were confirmed in the other five patients with systemic vasculitis treated with 40–60 mg/day prednisolone (Fig. 5b). Thus, the anti-Prx2 antibodies may be associated with the disease activity during the time–course of systemic vasculitis.
Fig. 5.
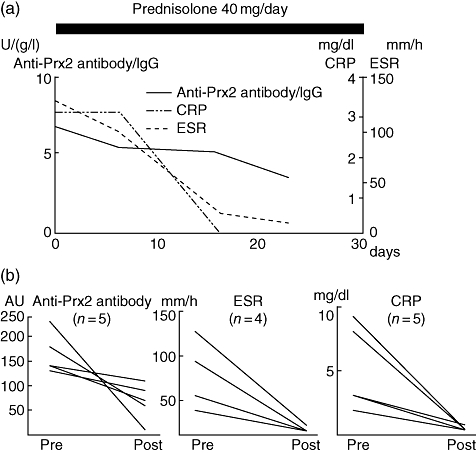
Time–course analysis of anti-peroxiredoxin 2 (Prx2) autoantibody titres. (a) Time–course of anti-Prx2 autoantibody titre was analysed in a patient with Takayasu's arteritis (TA). Titres of immunoglobulin G (IgG) class of the antibody were measured four times during the clinical course. (b) Titres of the anti-Prx2 antibodies, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were measured in five patients [four with TA and one with Henoch–Schonlein purpura (HS)] before and after the treatment. ESR was measured similarly in four patients (three with TA and one with HS).
Up-regulation of inflammatory cytokines and chemokines by anti-Prx2 antibodies
We investigated effects of the anti-Prx2 antibodies on endothelial cells, focusing upon the inflammation-related cytokines and chemokines. Specifically, we stimulated HUVEC with the rabbit anti-Prx2 antibodies or rabbit IgG, then measured concentrations of 19 types of cytokines and chemokines in the culture supernatant. As a result, concentrations of eight cytokines and chemokines in the anti-Prx2 antibody-stimulated culture supernatant were found to be more than twice as high as those in the control rabbit IgG-stimulated culture supernatant (Fig. 6a–c). The eight cytokines/chemokines consisted of IL-6 (6·4-fold), IL-1β (3·4-fold), IL-1ra (3·2-fold), growth regulated oncogene (GRO)-α (4·3-fold), G-CSF (2·8-fold), GM-CSF (2·7-fold), IL-8 (2·5-fold) and monocyte chemoattractant protein (MCP)-1 (2·4-fold). The other examined cytokines and chemokines of IL-1α, IL-10, IL-12p70, IL-13, IL-32α, IFN-γ-inducible protein (IP)-10, IFN-inducible T cell alpha chemoattractant (I-TAC), macrophage migration inhibitory factor (MIF), Serpin E1, regulated upon activation normal T cell expressed and secreted (RANTES) and stromal cell-derived factor 1 (SDF)-1 did not increase beyond twofold by the anti-Prx2 antibody stimulation. Thus, the anti-Prx2 antibodies would deteriorate vasculitis by up-regulation of the major inflammatory cytokines and chemokines secreted from endothelial cells.
Fig. 6.
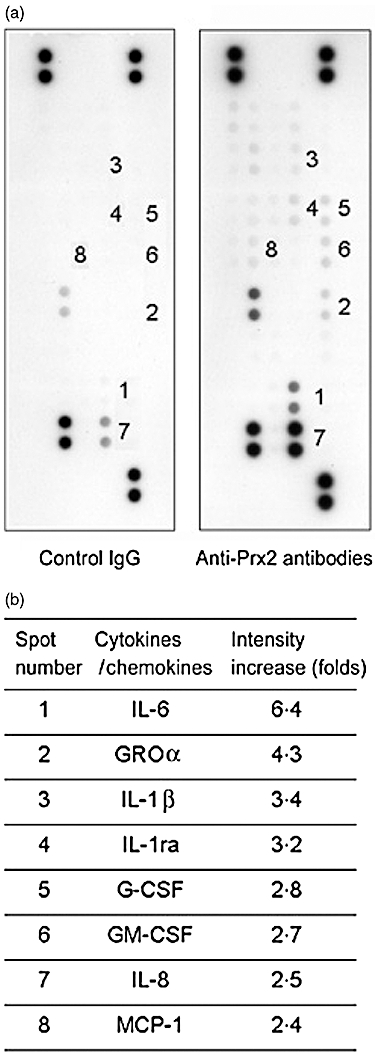
Up-regulated secretion of inflammatory cytokines and chemokines from endothelial cells by anti-peroxiredoxin 2 (Prx2) antibody stimulation. Human umbilical vein endothelial cells (HUVEC) was cultured in the presence of rabbit immunoglobulin G (IgG) or rabbit anti-Prx2 antibodies. Concentration of 19 cytokines and chemokines in the culture supernatants was measured using an array for the assay. Secretion of each of the cytokines and chemokines was examined in duplicate, and density of the resultant dots was measured. Results of rabbit IgG as a negative control (left) and of anti-Prx2 antibodies (right) are shown (a). In each cytokine or chemokine, the effect of anti-Prx2 antibody-stimulation was calculated by the following formula: [the average density of the two spots in the anti-Prx2 antibody stimulation]/[the average density of the two spots in the rabbit IgG treatment]. The numbered spots indicate cytokines and chemokines whose intensities were increased to more than twofold. The names of the increased cytokines and chemokines are shown (b).
Discussion
In this study, we have carried out comprehensive screening of target antigens of AECA by proteomics techniques to detect a disease marker for systemic vasculitis. We found a novel autoantigen, Prx2, autoantibodies to which were produced predominantly in systemic vasculitis. Anti-Prx2 antibodies up-regulated secretion of inflammatory cytokines and chemokines from vascular endothelial cells, suggesting their proinflammatory role in vascular injury.
Prx2, focused upon here, belongs to a family of anti-oxidant enzymes, which includes at least six Prxs in mammalian cells [36]. The enzymes exert an anti-oxidative role through their peroxidase activity, reducing and detoxifying hydrogen peroxide, peroxynitrite and hydroperoxidases. The oxidation status of Prx was found to reflect oxidative stress in the vasculature and to correlate with the extent of atherosclerotic lesions in an animal model [38]. Oxidative stress is known to cause inflammation, including vasculitis [37,39]. We have previously found presence of autoantibodies against Prx1 and Prx4 in autoimmune diseases such as SLE, RA and BD [35]. Thus, impaired function of Prx2 triggered by the autoantibodies may initiate vasculitis.
Anti-Prx2 autoantibodies were detected in 60% of patients with systemic vasculitis (Fig. 3a). This ratio was significantly higher compared to that in patients with collagen diseases other than systemic vasculitis (7%, P < 0·01). Thus, anti-Prx2 autoantibodies may be a useful diagnostic marker for systemic vasculitis. Further, as secondary vasculitis complicated with collagen diseases such as RA, SLE and BD is known to lead to poor prognosis, anti-Prx2 autoantibodies may be useful for early diagnosis of the secondary vasculitis. In addition, anti-Prx2 autoantibodies tended to be detected frequently in large-vessel vasculitis compared to small-vessel vasculitis. Because no useful diagnostic markers for large-vessel vasculitis have been established to date, anti-Prx2 autoantibodies may lead to a novel laboratory technique to detect large-vessel vasculitis by serum examination.
Specific roles of anti-Prx2 autoantibodies in systemic vasculitis were suggested further from the fluctuation of the antibody titres along with disease activity (Fig. 5), and from the correlation between the presence of anti-Prx2 autoantibodies and augmentation of d-dimer and TAT (Table 2). To reveal the molecular mechanisms, subcellular localization of Prx2, binding affinity of the anti-Prx2 antibodies to live endothelial cells and effect of the antibodies on cytokine and chemokine production from endothelial cells were examined.
Interestingly, Prx2 was detected in membrane and organelle fraction of all the endothelial cell lines examined, as well as in whole cell lysates (Fig. 4a and b). Anti-Prx2 antibodies bound to the surface of live HUVEC (Fig. 4c and d), in accordance with previous findings that AECA antigens are expressed on the surface of endothelial cells [10,20]. These data indicate that anti-Prx2 autoantibodies are capable to access Prx2 on the surface of endothelial cells, possibly giving rise to inflammatory or cytotoxic effects on endothelial cells. Stimulation of HUVEC with rabbit anti-Prx2 antibodies up-regulated secretion of IL-1, IL-6, IL-8, G-CSF, GM-CSF, MCP-1 and GRO-α more than twofold compared to the results of stimulation with rabbit IgG (Fig. 6). These cytokines and chemokines are known to be produced in endothelial cells, and are thus considered to be involved in the pathological process of vasculitis [40–42].
Hyperproduction of IL-6 in GCA and TA has been reported [43–45]. Further, IL-6 is known to up-regulate production of fibrinogens and to stimulate immature megakaryocytes to produce platelets [46]. The increased IL-6 detected here would lead to vascular inflammation and to up-regulation of coagulation activities in the anti-Prx2 antibody-positive patients (Table 2). Increased IL-1 has been reported in TA, WG and AGA [45,47]. The up-regulated secretion of IL-1β by anti-Prx2 antibodies would also contribute to vascular inflammation in a similar manner to IL-6.
The up-regulation of G-CSF and GM-CSF suggests enhanced differentiation and activation of neutrophils, whereas that of GRO-α, IL-8 and MCP-1 suggests enhanced migration of neutrophils and monocytes (Fig. 6). Up-regulation of IL-8 and MCP-1 has been reported in BD, KD and WG [47–50]. The use of G-CSF and GM-CSF in the treatment of neutropenia has been found to cause or flare-up vasculitis [51–53]. This indicates that these colony-stimulating factors have the potential to induce vascular inflammation. Anti-Prx2 autoantibodies may contribute to increasing these cytokines and chemokines in systemic vasculitis.
Secretion of IL-1ra, an antagonist of IL-1β, was also up-regulated by stimulation with anti-Prx2 antibodies (Fig. 6). In previous studies, serum IL-1ra levels were found to be elevated in patients with active ocular BD, and the levels tended to be increased in remission [54]. In IL-1ra knock-out mice, a PN-like arteritis developed spontaneously [55]. These indicate a potent role of IL-1ra in the suppression of vasculitis. Thus, the increased secretion of IL-1ra detected here may be triggered to compensate for increased IL-1β secretion, even though the level was not enough to antagonize it.
In conclusion, we have found a novel autoantigen, Prx2, as an AECA target. Anti-Prx2 autoantibodies may be a useful diagnostic marker and may be involved in the pathophysiology of systemic vasculitis. Further studies are needed to confirm the usefulness of the antibodies and to elucidate the mechanisms associated with systemic vasculitis.
Acknowledgments
The authors are grateful to Ms M. Tamaki, Ms H. Murakami, Ms A. Nozawa and Ms M. Kanke for their technical assistance.
Disclosure
None.
References
- 1.Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337:1512–23. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- 2.Kallenberg CG. Autoantibodies in vasculitis: current perspectives. Clin Exp Rheumatol. 1993;11:355–60. [PubMed] [Google Scholar]
- 3.Jayne D. The diagnosis of vasculitis. Best Pract Res Clin Rheumatol. 2009;23:445–53. doi: 10.1016/j.berh.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Scott DG, Bacon PA, Tribe CR. Systemic rheumatoid vasculitis: a clinical and laboratory study of 50 cases. Medicine (Balt) 1981;60:288–97. [PubMed] [Google Scholar]
- 5.Domiciano DS, Carvalho JF, Shoenfeld Y. Pathogenic role of anti-endothelial cell antibodies in autoimmune rheumatic diseases. Lupus. 2009;18:1233–8. doi: 10.1177/0961203309346654. [DOI] [PubMed] [Google Scholar]
- 6.Khasnis A, Langford CA. Update on vasculitis. J Allergy Clin Immunol. 2009;123:1226–36. doi: 10.1016/j.jaci.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Sneller MC, Fauci AS. Pathogenesis of vasculitis syndromes. Med Clin North Am. 1997;81:221–42. doi: 10.1016/s0025-7125(05)70512-5. [DOI] [PubMed] [Google Scholar]
- 8.Kallenberg CG. Pathogenesis of PR3-ANCA associated vasculitis. J Autoimmun. 2008;30:29–36. doi: 10.1016/j.jaut.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–7. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- 10.Brasile L, Kremer JM, Clarke JL, et al. Identification of an autoantibody to vascular endothelial cell-specific antigens in patients with systemic vasculitis. Am J Med. 1989;87:74–80. doi: 10.1016/s0002-9343(89)80486-3. [DOI] [PubMed] [Google Scholar]
- 11.Del Papa N, Conforti G, Gambini D, et al. Characterization of the endothelial surface proteins recognized by anti-endothelial antibodies in primary and secondary autoimmune vasculitis. Clin Immunol Immunopathol. 1994;70:211–16. doi: 10.1006/clin.1994.1031. [DOI] [PubMed] [Google Scholar]
- 12.Ferraro G, Meroni PL, Tincani A, et al. Anti-endothelial cell antibodies in patients with Wegener's granulomatosis and micropolyarteritis. Clin Exp Immunol. 1990;79:47–53. doi: 10.1111/j.1365-2249.1990.tb05125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sebastian JK, Mahr AD, Ahmed SS, et al. Antiendothelial cell antibodies in patients with Wegener's granulomatosis: prevalence and correlation with disease activity and manifestations. J Rheumatol. 2007;34:1027–31. [PubMed] [Google Scholar]
- 14.Varagunam M, Nwosu AC, Adu D, et al. Little evidence for anti-endothelial-cell antibodies in microscopic polyarteritis and Wegener's granulomatosis. Adv Exp Med Biol. 1993;336:419–22. doi: 10.1007/978-1-4757-9182-2_73. [DOI] [PubMed] [Google Scholar]
- 15.Navarro M, Cervera R, Font J, et al. Anti-endothelial cell antibodies in systemic autoimmune diseases: prevalence and clinical significance. Lupus. 1997;6:521–6. doi: 10.1177/096120339700600608. [DOI] [PubMed] [Google Scholar]
- 16.Aydìntug AO, Tokgöz G, D'Cruz DP, et al. Antibodies to endothelial cells in patients with Behçet's disease. Clin Immunol Immunopathol. 1993;67:157–62. doi: 10.1006/clin.1993.1059. [DOI] [PubMed] [Google Scholar]
- 17.Souza RC, Lage L, Goldenstein-Schainberg C, et al. Anti-endothelial cell antibodies and central nervous system involvement in Behçet's disease. Clinics (Sao Paulo) 2007;62:685–90. doi: 10.1590/s1807-59322007000600005. [DOI] [PubMed] [Google Scholar]
- 18.Savage CO, Pottinger BE, Gaskin G, et al. Vascular damage in Wegener's granulomatosis and microscopic polyarteritis: presence of anti-endothelial cell antibodies and their relation to anti-neutrophil cytoplasm antibodies. Clin Exp Immunol. 1991;85:14–19. doi: 10.1111/j.1365-2249.1991.tb05675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frampton G, Jayne DR, Perry GJ, et al. Autoantibodies to endothelial cells and neutrophil cytoplasmic antigens in systemic vasculitis. Clin Exp Immunol. 1990;82:227–32. doi: 10.1111/j.1365-2249.1990.tb05431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieudé M, Senécal JL, Raymond Y. Induction of endothelial cell apoptosis by heat-shock protein 60-reactive antibodies from anti-endothelial cell autoantibody-positive systemic lupus erythematosus patients. Arthritis Rheum. 2004;50:3221–31. doi: 10.1002/art.20564. [DOI] [PubMed] [Google Scholar]
- 21.Chauhan SK, Tripathy NK, Nityanand S. Antigenic targets and pathogenicity of anti-aortic endothelial cell antibodies in Takayasu arteritis. Arthritis Rheum. 2006;54:2326–33. doi: 10.1002/art.21921. [DOI] [PubMed] [Google Scholar]
- 22.Lee KH, Chung HS, Kim HS, et al. Human alpha-enolase from endothelial cells as a target antigen of anti-endothelial cell antibody in Behçet's disease. Arthritis Rheum. 2003;48:2025–35. doi: 10.1002/art.11074. [DOI] [PubMed] [Google Scholar]
- 23.Okunuki Y, Usui Y, Takeuchi M, et al. Proteomic surveillance of autoimmunity in Behcet's disease with uveitis: selenium binding protein is a novel autoantigen in Behcet's disease. Exp Eye Res. 2007;84:823–31. doi: 10.1016/j.exer.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Leavitt RY, Fauci AS, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum. 1990;33:1101–7. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 25.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 26.Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg–Strauss syndrome (allergic granulomatosis and angiitis) Arthritis Rheum. 1990;33:1094–100. doi: 10.1002/art.1780330806. [DOI] [PubMed] [Google Scholar]
- 27.Lightfoot RW, Jr, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum. 1990;33:1088–93. doi: 10.1002/art.1780330805. [DOI] [PubMed] [Google Scholar]
- 28.Mills JA, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Henoch–Schonlein purpura. Arthritis Rheum. 1990;33:1114–21. doi: 10.1002/art.1780330809. [DOI] [PubMed] [Google Scholar]
- 29.Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33:1129–34. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- 30.Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–8. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 31.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 32.Tunç R, Uluhan A, Melikoğlu M, et al. A reassessment of the International Study Group criteria for the diagnosis (classification) of Behçet's syndrome. Clin Exp Rheumatol. 2001;19:S45–7. [PubMed] [Google Scholar]
- 33.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 34.Xiang Y, Matsui T, Matsuo K, et al. Comprehensive investigation of disease-specific short peptides in sera from patients with systemic sclerosis: complement C3f-des-arginine, detected predominantly in systemic sclerosis sera, enhances proliferation of vascular endothelial cells. Arthritis Rheum. 2007;56:2018–30. doi: 10.1002/art.22645. [DOI] [PubMed] [Google Scholar]
- 35.Karasawa R, Ozaki S, Nishioka K, et al. Autoantibodies to peroxiredoxin I and IV in patients with systemic autoimmune diseases. Microbiol Immunol. 2005;49:57–65. doi: 10.1111/j.1348-0421.2005.tb03640.x. [DOI] [PubMed] [Google Scholar]
- 36.Wood ZA, Schroder E, Robin Harris J, et al. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 37.Cohen Tervaert JW. Translational mini-review series on immunology of vascular disease: accelerated atherosclerosis in vasculitis. Clin Exp Immunol. 2009;156:377–85. doi: 10.1111/j.1365-2249.2009.03885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayr M, Chung YL, Mayr U, et al. Proteomic and metabolomic analyses of atherosclerotic vessels from apolipoprotein E-deficient mice reveal alterations in inflammation, oxidative stress, and energy metabolism. Arterioscler Thromb Vasc Biol. 2005;25:2135–42. doi: 10.1161/01.ATV.0000183928.25844.f6. [DOI] [PubMed] [Google Scholar]
- 39.Bashir S, Harris G, Denman MA, et al. Oxidative DNA damage and cellular sensitivity to oxidative stress in human autoimmune diseases. Ann Rheum Dis. 1993;52:659–66. doi: 10.1136/ard.52.9.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krishnaswamy G, Kelley J, Yerra L, et al. Human endothelium as a source of multifunctional cytokines: molecular regulation and possible role in human disease. J Interferon Cytokine Res. 1999;19:91–104. doi: 10.1089/107999099314234. [DOI] [PubMed] [Google Scholar]
- 41.Swerlick RA, Lawley TJ. Role of microvascular endothelial cells in inflammation. J Invest Dermatol. 1993;100:S111–15. doi: 10.1111/1523-1747.ep12356595. [DOI] [PubMed] [Google Scholar]
- 42.Teofoli P, Lotti T. Cytokines, fibrinolysis and vasculitis. Int Angiol. 1995;14:125–9. [PubMed] [Google Scholar]
- 43.Martinez-Taboada VM, Alvarez L, RuizSoto M, et al. Giant cell arteritis and polymyalgia rheumatica: role of cytokines in the pathogenesis and implications for treatment. Cytokine. 2008;44:207–20. doi: 10.1016/j.cyto.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Goronzy JJ, Weyand CM. Cytokines in giant-cell arteritis. Cleve Clin J Med. 2002;69:SII91–4. doi: 10.3949/ccjm.69.suppl_2.sii91. [DOI] [PubMed] [Google Scholar]
- 45.Noris M. Pathogenesis of Takayasu's arteritis. J Nephrol. 2001;14:506–13. [PubMed] [Google Scholar]
- 46.Williams N, De Giorgio T, Banu N, et al. Recombinant interleukin 6 stimulates immature murine megakaryocytes. Exp Hematol. 1990;18:69–72. [PubMed] [Google Scholar]
- 47.Mukae H, Matsumoto N, Ashitani J, et al. Neutrophil-related cytokines and neutrophil products in bronchoalveolar lavage fluid of a patient with ANCA negative Wegener's granulomatosis. Eur Respir J. 1996;9:1950–4. doi: 10.1183/09031936.96.09091950. [DOI] [PubMed] [Google Scholar]
- 48.Kaneko F, Oyama N, Yanagihori H, et al. The role of streptococcal hypersensitivity in the pathogenesis of Behçet's Disease. Eur J Dermatol. 2008;18:489–98. doi: 10.1684/ejd.2008.0484. [DOI] [PubMed] [Google Scholar]
- 49.Ohlsson S, Bakoush O, Tencer J, et al. Monocyte chemoattractant protein 1 is a prognostic marker in ANCA-associated small vessel vasculitis. Mediat Inflamm. 2009;2009:584916. doi: 10.1155/2009/584916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung HS, Kim HY, Kim HS, et al. Production of chemokines in Kawasaki disease, Henoch-Schönlein purpura and acute febrile illness. J Korean Med Sci. 2004;19:800–4. doi: 10.3346/jkms.2004.19.6.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merkel PA. Drugs associated with vasculitis. Curr Opin Rheumatol. 1998;10:45–50. doi: 10.1097/00002281-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Vasiliu IM, Petri MA, Baer AN. Therapy with granulocyte colony-stimulating factor in systemic lupus erythematosus may be associated with severe flares. J Rheumatol. 2006;33:1878–80. [PubMed] [Google Scholar]
- 53.Iking-Konert C, Ostendorf B, Foede M, et al. Granulocyte colony-stimulating factor induces disease flare in patients with antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol. 2004;31:1655–8. [PubMed] [Google Scholar]
- 54.Kötter I, Koch S, Vonthein R, et al. Cytokines, cytokine antagonists and soluble adhesion molecules in patients with ocular Behçet's disease treated with human recombinant interferon-alpha2a. Results of an open study and review of the literature. Clin Exp Rheumatol. 2005;23:S20–6. [PubMed] [Google Scholar]
- 55.Nicklin MJ, Hughes DE, Barton JL, et al. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med. 2000;191:303–12. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]


