Abstract
Chronic inflammation as a risk factor for cancer development is driven in part by monocyte/macrophages, which in many cancers exhibit pro-tumorigenic activity. In this study we identified elevation in CD14+CD16+, a minor blood monocyte subpopulation in cholangiocarcinoma (CCA) patients, compared to normal and biliary disease patient specimens. Tumour association was suggested by the observation that this elevated level decreased to normal after tumour resection. Moreover, the elevated level of CD14+CD16+ monocytes in CCA patient blood correlated with degree of MAC387-positive (recent blood-derived macrophage migrant-specific marker) tumour-associated macrophage infiltration as determined by immunohistochemistry. These CD14+CD16+ monocytes were suggested to enhance tumour progression as this subpopulation possesses (i) high expression of adhesion molecules (CD11c, CD49d, and CD54) and scavenger receptor (CD163), which enable them to adhere strongly to endothelial cells, and (ii) that peripheral blood monocytes from CCA patients express high levels of growth and angiogenic factor-related genes (epiregulin, VEGF-A and CXCL3). Elevation of peripheral CD14+CD16+ monocyte levels was associated with features associated with poor prognosis CCA parameters (non-papillary type and high number of tissue macrophages). These data indicate that the CD14+CD16+ monocytes from CCA patients with pro-tumorigenic characteristics may associate with rapid tumour progression and poor patient outcome. If confirmed in subsequent studies, the level of CD14+CD16+ monocytes may serve as a marker for disease activity in CCA patients and serve as a target for pathogenic macrophage specific drug development.
Keywords: CD14+CD16+ monocyte, cholangiocarcinoma, pro-tumorigenicity
Introduction
It is well documented that chronic inflammation contributes to cancer development and disease progression in different types of solid tumour. Macrophages represent the major class of immune cells within the tumour microenvironment [1,2], and have been shown to exhibit pro-tumoral function [3]. The associations of tumour-associated macrophages (TAMs) with poor patient outcome have been reported for patients with various cancers [4–6].
It is generally accepted that blood monocytes, a heterogeneous cell population, are precursors of tissue macrophages. Monocytes participate in pro- or anti-inflammatory conditions depending upon their state of differentiation and activation [7]. The majority of monocytes express cell surface CD14 (CD14+CD16-) and are thought to represent classical monocytes that mediate inflammatory responses (‘inflammatory’ monocytes). A minor subpopulation of monocytes also express the cell surface activation marker CD16, identifying them as more mature than the CD14+CD16– subpopulation. CD14+CD16+ monocytes are thought to be the precursors of tissue-resident macrophages and are referred to as ‘resident’ monocytes [8–13].
The functional significance of CD14+CD16+ monocytes is unclear. CD14+CD16+ monocytes have been documented to have proinflammatory activity, as they produce tumour necrosis factor (TNF)-α after lipopolysaccharide stimulation [14,15]. In addition, CD14+CD16+ cells have been shown to participate in anti-tumour responses as judged by the enhanced production of proinflammatory cytokines [TNF-α, interleukin (IL)-12p40 and IL-12p70], reactive nitrogen intermediate and increased cytotoxic and cytostatic activity [16]. Conversely, in certain disease states CD14+CD16+ monocytes have been shown to promote tumour growth and express the vascular endothelial growth factor receptor (VEGFR) Tie 2-associated angiogenic activity [17].
The CD14+CD16+ subset of monocytes in healthy individuals varies from 3% to 13% of all CD14+ cells [14,18–21], but this proportion has been shown to increase in a number of acute and chronic inflammatory syndromes, such as acquired immune deficiency syndrome (AIDS) [22], bacterial sepsis [23], solid cancer [24], AIDS dementia [25], acute and chronic infection undergoing haemodialysis [26] and sporadic amyotrophic lateral sclerosis [27].
Cholangiocarcinoma (CCA), a malignancy of bile duct epithelial cells lining the intrahepatic and extrahepatic bile duct, is now increasing both in worldwide incidence and mortality rate [28]. CCA is found more frequently in Southeast Asia, with the highest prevalence in the Northeast of Thailand. Both epidemiology and experimental evidence implicate chronic inflammation from the carcinogenic liver fluke Opisthorchis viverrini (OV), which is endemic in this region, as a major risk factor for CCA in Thailand [29]. High levels of infiltrating leucocytes within tumour tissues in the OV infection-associated CCA hamster model support the role of chronic inflammation in carcinogenesis and progression of CCA [30].
As TAMs derive from circulating monocytes that infiltrate tumour tissues and differentiate to macrophages, in the present study we have examined peripheral blood mononuclear cells from CCA patients to determine whether monocytes from CCA patients exhibited pro-tumorigenic activity and whether an activated monocyte subset, CD14+CD16+ monocytes, occurred more often in patients with CCA. In this study, we describe the correlation of CD14+CD16+ monocyte levels with clinicopathological features and survival of CCA patients.
Materials and methods
Blood samples
Heparinized blood was collected from three subject groups, namely healthy subjects (n = 46), patients with benign biliary tract disease (BBD, n = 18) and CCA patients (n = 44). Specimens from histologically confirmed CCA and BBD patients were obtained from Srinagarind Hospital, Faculty of Medicine, Khon Kaen University after appropriate human subjects approvals were in place. Pre- and two 6-month post-operative blood samples were collected from five CCA subjects after tumour resection without additional treatment. Specimens from healthy subjects were obtained from the Health Check-up Unit, Srinagarind Hospital, and were age- and sex-matched with CCA patients. These normal controls had normal complete blood counts and liver function tests, and had no apparent chronic inflammatory diseases such as diabetic mellitus or hepatitis. Informed consent was obtained from each subject and the Human Research Ethics Committee, Khon Kaen University approved our research protocol (HE471214 and HE480312).
The age, gender, tumour location, histological grading and pathological tumour–node–metastasis (pTNM) stage were evaluated by reviewing the medical charts and pathological records. Survival of each CCA patient was recorded from the date of surgery to the date of death or to 13 June 2008.
Monocyte preparation
Heparinized blood was diluted with an equal volume of Ca++ and Mg++ free phosphate-buffered saline (PBS). The diluted blood (2 vol) was overlayered on 1 vol of Ficoll Hypaque (Axis-Shield, Oslo, Norway). After centrifugation at 350 g, at room temperature for 20 min, cells at the plasma/Ficoll Hypaque interface were transferred and washed with PBS. After centrifugation (600 g for 5 min), the left-over red blood cells were lyzed with 5 ml red blood cell (RBC) lysis buffer for 3 min and the suspension was centrifuged at 600 g for 5 min. Peripheral blood mononuclear cells (PBMCs) were collected and suspended in RPMI-1640 with 10% fetal bovine serum (FBS). The PBMCs (5 × 106) were incubated at 37°C for 3 h in a 10-cm plastic tissue culture dish to obtain adherence of monocytes. The non-adhered lymphocytes were washed out by two successive rinses with warm medium. These preparations contained at least 90% monocytes as assessed by immunofluorescent staining with antibody to the CD14 antigen.
Measurement of mRNA expression by real-time reverse transcription–polymerase chain reaction (RT-PCR)
RNA extraction was performed using Trizol® Reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's protocol. Total RNA (500 ng) in 25 µl of sterile distilled water was heated at 65°C for 10 min and chilled on ice for 2 min. The RNA solution was then transferred into a tube containing first-strand reaction mixed beads (GE HealthyCare, Piscataway, NJ, USA), 0·5 µM random hexamer and sterile distilled water to a final volume of 33 µl. Reverse transcription was carried out in a 37°C water bath for 1 h.
Transcription copy number for a specific gene of interest (EREG, VEGFA, CXCL3 and CXCL10 and internal control; β-actin) was measured using an adaptation of a two-step real-time RT–PCR method. PCR was performed with ∼25 ng cDNA sample using SYBR® Green PCR Master Mix assay with the ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA). Amplifications included one cycle of template denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 30 s, 55°C for 30 s and 72°C for 45 s. The presence of a single amplified product was confirmed by DNA melting-point analysis. Threshold cycles (Ct) for each amplification reaction were determined using the 7500 System SDS software version 1·4 (Applied Biosystems, Foster City, CA, USA). The gene-specific primers for individual samples were normalized to signals obtained with β-actin from the same sample. Relative change in gene expression of CCA patients and healthy subjects was analysed using the 2–ΔCt method, where ΔCt = (average Cttarget – average Ctactin). Significant differences between groups were analysed by Student's t-test.
Flow cytometry for monocyte subset
Approximately 1 × 106 cells from heparinized blood were stained immediately using a stain-and-then-lyse direct immunofluorescence technique with CD14 fluorescein isothiocyanate (FITC) and CD16 phycoerythrin (PE) (Becton-Dickinson, San Jose, CA, USA), according to the manufacturer's specification. At least 5000 cells were counted per analysis for CD14+CD16+ monocytes using Coulter EPICS_MXL (Beckman Coulter, Fullerton, CA, USA) with CXP Software (Beckman Coulter).
The identification of surface adhesion molecules (CD11a, CD11c, CD18, CD29, CD49d and CD54 (Bioscience, San Diego, CA, USA) and scavenger receptors (CD163, and CD204; Bioscience) on CD14++ and CD14+CD16+ monocytes was performed using PBMCs separated via Ficoll Hypaque as described by the manufacturer (Axis-Shield, Oslo, Norway). For four-colour staining, approximately 1 × 106 PBMCs were stained with a CD14 Pacific Blue (Caltage, Little Balmer, Bucks, UK), CD16 allophycocyanin (APC) (Biolegend, San Diego, CA, USA) combination with a mix of either FITC or PE conjugates identifying specific cell surface markers. The expression levels of each molecule were measured and reported as mean fluorescence intensity (MFI) by LSR flow cytometer (Becton Dickinson). At least 20 000 events per sample were analysed. Fluorescence gating parameters were established with all antibodies using isotype-matched antibody controls; positive values represent those above the 99% negative staining threshold.
Immunohistochemistry of tissue macrophages
CCA tissues were fixed in 10% neutral formalin buffer and immunohistochemical staining was performed using an immunoperoxidase method. Briefly, tissue sections were incubated with 1:200 mouse monoclonal anti-human myeloid/histiocyte antigen (MAC387 clone; Dako, Glostrup, Denmark) for 30 min followed by the addition of Envision-labelled polymer peroxidase (Dakocytomation, Glostrup, Denmark) for 30 min. The MAC387 antibody was used to define recent blood monocyte derived migrants rather than using anti-CD68 antibodies, which recognize long-lived tissue macrophages. After washing, the sections were reacted with liquid 3,3′-diaminobenzidine tetrahydrochloride substrate chromogen system (Dakocytomation). All slides were counterstained with Mayer's haematoxylin.
The densities of MAC387 at the leading edge of invasive tumour were classified semi-quantitatively into four scoring categories: 0 = negative; 1+ = 1–25%; 2+ = 26–50%; and 3+ = >50% (supplementary data). For statistical analysis, the scores 0 and 1+ were categorized as low expression and score 2+ and 3+ as high expression.
Statistical analysis
The numbers of CD14+CD16+ monocytes among groups and expression levels of the adhesion molecules and scavenger receptors on CD14+CD16- and CD14+CD16+ monocytes were compared and analysed with Student's t-test. The associations between levels of CD14+CD16+ monocytes and clinicopathological features of CCA patients and density of MAC387-positive cells in CCA tissues were analysed using cross-tabulation with the χ2-test. Kaplan–Meier survival analysis was used to estimate the overall survival and comparison between-group analyses were conducted with a log-rank test. Statistic analyses were determined using SPSS statistical software version 16.0.1 (SPSS Inc., Chicago, IL, USA) and STATA version 8 (Statacorp, College Station, TX, USA). P < 0·05 was considered statistically significant.
Results
Peripheral blood monocytes from CCA patients exhibit pro-tumorigenic characteristics
Five CCA patients (three males, two females), average age 54·8 ± 5·9 years, and four healthy subjects sex- and age-matched with those of CCA patients, were asked to participate in this study. In order to investigate pro-tumorigenic gene expression characteristics in CCA blood monocyte populations from the peripheral blood of CCA patients, we performed real-time RT–PCR of four candidate genes including growth factor (EREG), angiogenic chemokines (VEGFA and CXCL3) and an angiostatic chemokine (CXCL10). The results demonstrated that the EREG and CXCL3 transcripts in monocytes from CCA patients were significantly higher, whereas CXCL10 transcripts were lower when compared with monocytes from healthy subjects (P < 0·05) (Fig. 1). The expression level of VEGFA in monocytes from CCA patients was higher than that of the healthy group; however, that difference was not statistically significant.
Fig. 1.
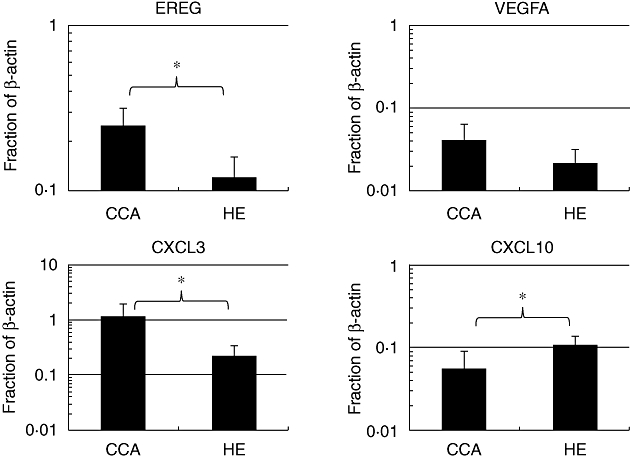
Expression levels of EREG, VEGFA, CXCL3 and CXCL10 of peripheral blood monocytes from cholangiocarcinoma (CCA) patients in comparison with those from healthy subjects. Monocytes from CCA patients expressed significantly higher levels of EREG and CXCL3 and lower levels of CXCL10 than those from healthy subjects. *P < 0·05; Student's t-test.
We analysed further whether the higher expression levels of these pro-tumorigenic genes (EREG, CXCL3 and CXCL10) observed in the monocytes from CCA patients were due to the minor subpopulation CD14+CD16+ monocytes. We first analysed the amount of CD14+CD16+ monocytes in the peripheral blood from CCA and healthy subjects used in the expression study and then analysed the possible association of these cells with the expression level of each gene using linear regression. The preliminary data indicated that peripheral blood from CCA patients contained higher number of CD14+CD16+ cells compared to those from healthy subjects and the amount of CD14+CD16+ cells associated strongly with expression levels of CXCL3 (r = 0·84; P < 0·03) and had a tendency to correlate with EREG, but without statistical significance (Fig. 2).
Fig. 2.
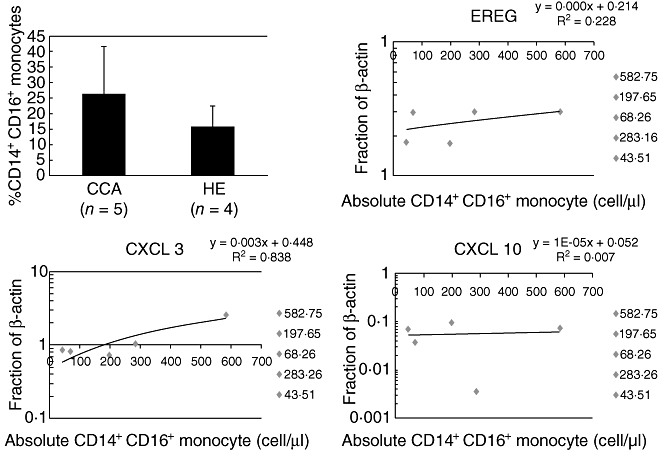
The association of CD14+CD16+ monocytes from cholangiocarcinoma (CCA) subjects and expression levels of pro-tumoringenic genes. (a) Amount of CD14+CD16+ cells in peripheral blood of CCA patients compared with healthy controls (P = 0·25). The data present as mean ± standard deviation. (b–d) The association between CD14+CD16+ monocyte subpopulation and the expression levels of EREG (P = 0·41), CXCL3 (P < 0·03) and CXCL10 (P = 0·88).
CCA patient blood specimens have high levels of CD14+CD16+ monocytes
Because previous studies have shown that CD14+CD16+ monocytes exhibit features related to tissue macrophage activation and anti-tumour response, in addition to our finding that these cells may exhibit pro-tumorigenic characteristics, we next investigated whether this specific monocyte subset was elevated significantly in the peripheral blood of CCA patients in a larger sample size. To quantitate the CD14+CD16+ monocyte subpopulation in peripheral blood, whole blood samples from CCA patients and healthy subjects were stained with CD14 and CD16 antibodies and analysed by flow cytometry. CD14+CD16+ cells from healthy donors (n = 46) accounted for 13 ± 6% of all CD14+ monocytes or 48·5 ± 26·6 cells/µl blood. A dramatic expansion of CD14+CD16+ monocytes was observed in the blood from both BBD (n = 18) and CCA patients (n = 44). The level of CD14+CD16+ monocytes in patients with CCA was 30 ± 12% of all monocytes or 171 ± 129 cells/µl, which was significantly higher than those of patients with BBD (23 ± 10% of all monocytes or 109 ± 89 cells/µl) and healthy subjects (P < 0·05; Fig. 3a,b).
Fig. 3.

Two-colour flow-cytometric analysis of circulating monocyte subpopulations in whole blood. Monocytes were gated according to their forward- and side-scatter characteristics. The expansions of CD14+CD16+ monocytes in peripheral blood of cholangiocarcinoma (CCA) patients, benign biliary disease patients (BBD) and healthy subjects were compared based on the numbers of CD14+CD16+ monocytes in percentage value (a) and absolute number (b). The expansions of CD14+CD16+ monocytes in peripheral blood of CCA patients between pre- and post-operations (n = 6) were compared based on the numbers of CD14+CD16+ monocytes in percentage value (c) and absolute number (d). *P < 0·05; **P < 0·001.
The association of CD14+CD16+ monocytes and tumour tissues was investigated further by quantifying the levels of CD14+CD16+ monocytes from peripheral blood of CCA patients’ pre- and post-tumour resection. Five CCA patients who did not obtain any treatment after tumour resection were included in this study. The elevated level of CD14+CD16+ monocytes observed in the CCA patients (n = 5) was 29 ± 9% of all monocytes or 111 ± 28 cells/µl, and decreased significantly to near the normal level (16 ± 5% of all monocytes or 68 ± 24 cells/µl) after tumour removal (P < 0·05; Fig. 3c,d).
Surface antigen expression patterns define unique features of CCA associated CD14+CD16+ monocytes
Adhesion molecules and scavenger receptors on monocytes may be involved in the localization of cells to a compartment such as the marginal pool and/or may represent define distinct subsets related to monocytic differentiation/maturation. To study whether the two monocyte subpopulations (CD14+CD16- and CD14+CD16+) differed in surface antigen expression, a four-colour flow cytometric analysis was performed. We found that several monocyte subset markers, CD11c, CD49d and CD54, were elevated significantly in CD14+CD16+ monocytes compared with those of CD14+CD16- subset (P < 0·05) (Table 1). The expression of two scavenger receptors (CD163 and CD204) showed trends towards higher levels on CD14+CD16+ monocytes than CD14+CD16- monocytes; however, these differences were not statistically significant. These data are consistent with blood CD14+CD16+ monocytes having properties of mature tissue macrophages and may be the blood form of macrophage responsible for supporting tumour growth and disease progression.
Table 1.
Phenotypes of CD14+CD16- and CD14+CD16+ monocyte subpopulations.
| CD14+CD16- | CD14+CD16+ | P-value | |
|---|---|---|---|
| Adhesion molecules | |||
| CD11a | 114·9 ± 56·4 | 137·3 ± 66·4 | 0·478 |
| CD11c | 276·6 ± 40·1 | 372·6 ± 54·11 | 0·001 |
| CD18 | 738·5 ± 108·4 | 779·1 ± 136·5 | 0·521 |
| CD29 | 601·2 ± 211·8 | 701·5 ± 267·2 | 0·420 |
| CD49d | 99·4 ± 25·9 | 176·6 ± 77·2 | 0·018 |
| CD54 | 139·8 ± 34·7 | 159·7 ± 47·6 | 0·035 |
| Scavenger receptors | |||
| CD163 | 35·3 ± 13·8 | 41·8 ± 15·0 | 0·399 |
| CD204 | 16·1 ± 4·4 | 24·7 ± 12·6 | 0·091 |
Elevated levels of blood CD14+CD16+ monocytes are associated with poor prognosis in patients with CCA
CD14+CD16+ monocyte levels were elevated in subsets of CCA patient blood specimens. To test whether these cells would be associated with CCA disease, pathogenesis, clinicopathological parameters of CCA patients and density of TAMs (MAC387 positive recent blood-derived macrophage migrants) in CCA tissues were analysed by univariate analysis. Only CCA patients with complete clinicopathological parameters (n = 37) were included in the analysis. CCA subjects were divided into two groups according to the mean ± standard deviation of the blood CD14+CD16+ monocyte levels in healthy subjects, into patients with low (<100 cells/µl) and patients with high levels (>100 cells/µl) of CD14+CD16+ monocytes. Immunohistochemistry of MAC387 staining demonstrated that tumour tissue from CCA patients with a high level of blood CD14+CD16+ monocytes exhibited a high density of tissue MAC387-positive cells (Table 2). These macrophages were observed at the leading edge of tumour tissues and perivascular areas (Fig. 4a). As shown in Table 2, a high level of CD14+CD16+ monocytes was associated significantly with non-papillary type CCA and the high density of TAMs with positive MAC387 staining in CCA tissues (P < 0·05). There was no correlation between levels of CD14+CD16+ monocytes with gender, age, tumour location, tumour staging and vascular invasion.
Table 2.
The correlation of CD14+CD16+ monocyte levels and clinicopathological parameters.
| CD14+CD16+ count (cells/ml) |
||||
|---|---|---|---|---|
| Variable | Number | Low (%) | High (%) | P-value |
| Age (years) | 0·588 | |||
| <57 | 20 | 5(25) | 15(75) | |
| ≥57 | 17 | 3(17·6) | 14(82·4) | |
| Sex | 0·321 | |||
| Female | 15 | 2(13·3) | 13(86·7) | |
| Male | 22 | 6(27·3) | 16(80) | |
| Tumour location | 0·795 | |||
| Intrahepatic CCA | 20 | 4(20) | 13(76·5) | |
| Extrahepatic CCA | 17 | 4(23·5) | 12(66·7) | |
| Tumour type | 0·767 | |||
| Mass-forming type | 9 | 2(22·2) | 7(77·8) | |
| Periductal infiltrating type | 20 | 5(25) | 15(75) | |
| Intraductal growth type | 8 | 1(12·5) | 7(87·5) | |
| Tumour stage | 0·752 | |||
| I–II | 10 | 3(30) | 7(70) | |
| III–IV | 27 | 5(18·5) | 22(81·5) | |
| Histology type | 0·040 | |||
| Non-papillary | 25 | 3(12) | 22(88) | |
| Papillary | 12 | 5(41·7) | 7(58·3) | |
| Vascular invasion | 0·379 | |||
| Absent | 28 | 7(25) | 21(75) | |
| Present | 9 | 1(11·1) | 8(88·9) | |
| Tissue macrophages(MAC387+ cells) | 0·040 | |||
| Low | 12 | 5(41·7) | 7(58·3) | |
| High | 25 | 3(12) | 22(88) | |
CCA: cholangiocarcinoma.
Fig. 4.

(a) The distribution and density of tissue MAC387 positive cells in tumour tissue. Immunostaining of MAC387-positive cells at leading edge of invasive tumour and perivascular areas (×40 HP); (b) The Kaplan–Meier survival curves of cholangiocarcinoma (CCA) patients who had high or low levels of CD14+CD16+ monocytes (median: 257 days and 365 days, respectively).
Overall survival was compared among CCA patients with low and high levels of CD14+CD16+ monocytes. Patients with survival under 30 days were labelled as ‘peri-operative death’ and were excluded from the analysis. There was no significant difference in the median overall post-resectional survival between CCA patients with low and those with high levels of CD14+CD16+ monocytes (log-rank, P = 0·597) (Fig. 4b). However, the higher frequency of patients who had post-resectional survival for 11 months in the CCA patients with low levels of CD14+CD16+ monocytes (six of eight, 75%) than those with high levels of CD14+CD16+ monocytes (13 of 29, 44·8%) was noticed.
Discussion
The roles of tumour-associated macrophages enhancing tumour growth by secreting various growth factors and pro-angiogenic cytokines are well documented. Monocytes are precursors of tissue macrophages and may exhibit special functions within a tumour environment. In the present study, we have shown that monocytes from peripheral blood of CCA patients had tumour-promoting characteristics, as these cells expressed higher levels of growth factor (EREG) and angiogenic chemokines (CXCL3) but lower levels of angiostatic chemokine (CXCL10) genes than monocytes obtained from healthy people. The expression pattern of these cytokines indicates that peripheral blood monocytes from CCA patients possess the M2 pro-tumorigenic phenotype. It has been shown in many studies that various tumour-derived molecules drive TAMs towards the tumour-promoting M2 phenotype [31,32]. Thus, peripheral blood monocytes from CCA patients may be influenced by tumour-derived molecules to express pro-tumorigenic factors, migrate to tumour-involved areas and differentiate to tissue macrophages to support growth and progression of the tumour.
The presence of elevated levels of CD14+CD16+ monocytes with tissue macrophage features and the association of this subpopulation with disease has been shown in various pathological conditions, including infection, inflammatory syndrome, sepsis and cancers. In the present study, we demonstrate for the first time that patients with CCA have increased levels of CD14+CD16+ monocytes in their blood. The direct association of this monocyte subpopulation with CCA tumour tissue is demonstrated by the fact that the elevated level of CD14+CD16+ monocytes was decreased to near the normal level found in healthy subjects after tumour resection. CD14+CD16+ monocytes evolve from CD14+CD16- monocytes [21]. Certain cytokines, e.g. macrophage colony-stimulating factor (M-CSF) [24], as well as IL-10 in the presence of M-CSF and IL-4 [33,34], have been shown to increase the proportional level of CD14+CD16+ monocytes from CD14+CD16- monocytes as precursors. CCA tumour tissues may produce these specific cytokines that possibly induce elevated levels of CD14+CD16+ monocytes in CCA patients. High expression of granulocyte colony-stimulating factor (G-CSF) and granulocyte–macrophage colony-stimulating factor (GM-CSF) in tumour epithelium of human intrahepatic CCA tissues reported by Sasaki [35] may support this postulation.
High expression of adhesion molecules CD11a, CD11c, CD18, CD29, CD49d and CD54 found in CD14+CD16+ monocytes implies that this monocyte subpopulation may adhere strongly to vascular endothelium and this may subsequently promote migration of blood monocytes into tumour-involved areas. There is substantial evidence that monocyte recruitment from the circulating bloodstream to sites of inflammation involves a complex sequence of adhesion and cytoskeletal events mediated by members of the integrin family. CD11b/CD18 and CD11c/CD18 regulate the effecter responses of leucocyte adhesion [36]. Elevated expression of CD11c also contributes to greater number of receptors present in clusters that are able to participate in ligand engagement and increased strength of adhesion [37,38]. The high density of MAC387-positive cells found in the leading edge of tumour and especially within perivascular areas as shown in this study may reflect recent blood-derived monocytes/macrophages recruited into the tumour vicinity. As MAC387 is not a general macrophage stain, but one that recognizes a tissue invasion molecular complex (S100A8/A9) required for transit from blood to tissues, it is likely that the MAC387+ cells in CCA represent recent migrants. The association of MAC387+ cells found in CCA tissues and the worst survival of patients has been reported recently [39]. As CCA cells rarely expressed matrix metalloproteinase-9 (MMP-9), while these tissue macrophages expressed MMP-9, it is therefore likely that the tissue macrophages are critical for degrading extracellular matrix and facilitating tumour metastasis.
High levels of CD14+CD16+ monocytes may influence tumour pathogenesis; however, it may also reflect the inflammation condition, as higher levels of these monocytes than those of healthy people were also observed in patients with benign biliary diseases. The observation that a higher frequency of patients surviving 11 months was found in the patients with low levels of CD14+CD16+ monocytes than those with high level of CD14+CD16+ monocytes. In addition, high levels of CD14+CD16+ monocytes were associated with poor prognostic types of CCA – namely non-papillary type CCA and high density of TAMs. Recent studies have shown that CD14+CD16+ but not CD14+CD16- monocytes expressed Tie 2 [17], which is an angiopoietin receptor (Tie 2/Tek) found in the human peripheral blood monocyte subpopulation with marked tumour-promoting proangiogenic activity. Angiopoietin 2 (Ang 2), a Tie 2 ligand, is found mainly in activated and angiogenic blood vessels as well as cancer cells [40], and may induce transmigration of Tie 2/CD14+CD16+ monocytes into the tissues [40,41]. These data support our hypothesis that CD14+CD16+ monocytes found in peripheral blood of CCA patients may be the subpopulation of TAMs with angiogenic properties and promote tumour progression.
Taken together, this study indicates that peripheral blood monocytes from CCA patients exhibit pro-tumorigenic features which may facilitate progression of CCA. An expansion of the CD14+CD16+ monocyte subpopulation found in peripheral blood of CCA patients was associated with tumour origin, high density of tumour-associated macrophages and poor prognosis of patients. It is likely that this monocyte subpopulation possibly promotes tumour progression and hence, if confirmed in subsequent studies, the therapy targeting to this monocyte subset may be an alternative treatment of CCA.
Acknowledgments
This works were co-supported by Basic Research Grant (BRG-4980015) and the Royal golden Jubilee-PhD Program, Thailand Research Fund for C. Subimerb and S. Wongkham; US National Cancer Institute AIDS and Cancer Specimen resource funding (U01CA 066529-14) for M. McGrath.
Disclosure
None.
References
- 1.Mantovani A, Allavena P, Sica A. Tumor-associated macrophage as a prototypic type II polarised phagocyte population: role in tumor progression. Eur J Cancer. 2004:1660–7. doi: 10.1016/j.ejca.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–15. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 3.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 4.Ishigami S, Natsugoe S, Tokuda K, et al. Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer Res. 2003;23:4079–83. [PubMed] [Google Scholar]
- 5.Li C, Shintani S, Terakado N, et al. Infiltration of tumor-associated macrophages in human oral squamous cell carcinoma. Oncol Rep. 2002;9:1219–23. [PubMed] [Google Scholar]
- 6.Orre M, Rogers PA. Macrophages and microvessel density in tumors of the ovary. Gynecol Oncol. 1999;73:47–50. doi: 10.1006/gyno.1998.5292. [DOI] [PubMed] [Google Scholar]
- 7.Rutherford MS, Witsell A, Schook LB. Mechanisms generating functionally heterogeneous macrophages: chaos revisited. J Leukoc Biol. 1993;53:602–18. doi: 10.1002/jlb.53.5.602. [DOI] [PubMed] [Google Scholar]
- 8.Allan DS, Colonna M, Lanier LL, et al. Tetrameric complexes of human histocompatibility leukocyte antigen (HLA)-G bind to peripheral blood myelomonocytic cells. J Exp Med. 1999;189:1149–56. doi: 10.1084/jem.189.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almeida J, Bueno C, Alguero MC, et al. Comparative analysis of the morphological, cytochemical, immunophenotypical, and functional characteristics of normal human peripheral blood lineage(–)/CD16(+)/HLA-DR(+)/CD14(–/lo) cells, CD14(+) monocytes, and CD16(–) dendritic cells. Clin Immunol. 2001;100:325–38. doi: 10.1006/clim.2001.5072. [DOI] [PubMed] [Google Scholar]
- 10.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 11.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 12.Kwakkenbos MJ, Chang GW, Lin HH, et al. The human EGF-TM7 family member EMR2 is a heterodimeric receptor expressed on myeloid cells. J Leukoc Biol. 2002;71:854–62. [PubMed] [Google Scholar]
- 13.Rothe G, Gabriel H, Kovacs E, et al. Peripheral blood mononuclear phagocyte subpopulations as cellular markers in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1996;16:1437–47. doi: 10.1161/01.atv.16.12.1437. [DOI] [PubMed] [Google Scholar]
- 14.Belge KU, Dayyani F, Horelt A, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–42. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 15.Frankenberger M, Sternsdorf T, Pechumer H, et al. Differential cytokine expression in human blood monocyte subpopulations: a polymerase chain reaction analysis. Blood. 1996;87:373–7. [PubMed] [Google Scholar]
- 16.Szaflarska A, Baj-Krzyworzeka M, Siedlar M, et al. Antitumor response of CD14+CD16+ monocyte subpopulation. Exp Hematol. 2004;32:748–55. doi: 10.1016/j.exphem.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 17.De Palma M, Venneri MA, Galli R, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–26. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Kreutz M, Krause SW, Hennemann B, et al. Macrophage heterogeneity and differentiation: defined serum-free culture conditions induce different types of macrophages in vitro. Res Immunol. 1992;143:107–15. doi: 10.1016/0923-2494(92)80087-2. [DOI] [PubMed] [Google Scholar]
- 19.Sadeghi HM, Schnelle JF, Thoma JK, et al. Phenotypic and functional characteristics of circulating monocytes of elderly persons. Exp Gerontol. 1999;34:959–70. doi: 10.1016/s0531-5565(99)00065-0. [DOI] [PubMed] [Google Scholar]
- 20.Schmid I, Baldwin GC, Jacobs EL, et al. Alterations in phenotype and cell-surface antigen expression levels of human monocytes: differential response to in vivo administration of rhM-CSF or rhGM-CSF. Cytometry. 1995;22:103–10. doi: 10.1002/cyto.990220205. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler-Heitbrock HW, Fingerle G, Strobel M, et al. The novel subset of CD14+CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol. 1993;23:2053–8. doi: 10.1002/eji.1830230902. [DOI] [PubMed] [Google Scholar]
- 22.Thieblemont N, Weiss L, Sadeghi HM, et al. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–24. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- 23.Fingerle G, Pforte A, Passlick B, et al. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–6. [PubMed] [Google Scholar]
- 24.Saleh MN, Goldman SJ, LoBuglio AF, et al. CD16+ monocytes in patients with cancer: spontaneous elevation and pharmacologic induction by recombinant human macrophage colony-stimulating factor. Blood. 1995;85:2910–17. [PubMed] [Google Scholar]
- 25.Pulliam L, Gascon R, Stubblebine M, et al. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–5. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 26.Nockher WA, Scherberich JE. Expanded CD14+ CD16+ monocyte subpopulation in patients with acute and chronic infections undergoing hemodialysis. Infect Immun. 1998;66:2782–90. doi: 10.1128/iai.66.6.2782-2790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang R, Gascon R, Miller RG, et al. Evidence for systemic immune system alterations in sporadic amyotrophic lateral sclerosis (sALS) J Neuroimmunol. 2005;159:215–24. doi: 10.1016/j.jneuroim.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Khan SA, Taylor-Robinson SD, Toledano MB, et al. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806–13. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 29.Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008;24:349–56. doi: 10.1097/MOG.0b013e3282fbf9b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinlaor S, Hiraku Y, Ma N, et al. Mechanism of NO-mediated oxidative and nitrative DNA damage in hamsters infected with Opisthorchis viverrini: a model of inflammation-mediated carcinogenesis. Nitric Oxide. 2004;11:175–83. doi: 10.1016/j.niox.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Allavena P, Sica A, Garlanda C, et al. The Yin–Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–61. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 32.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Hangoc G, Broxmeyer HE. Interleukin-10 in combination with M-CSF and IL-4 contributes to development of the rare population of CD14+CD16++ cells derived from human monocytes. Biochem Biophys Res Commun. 2004;322:637–43. doi: 10.1016/j.bbrc.2004.07.172. [DOI] [PubMed] [Google Scholar]
- 34.Wang ZQ, Bapat AS, Rayanade RJ, et al. Interleukin-10 induces macrophage apoptosis and expression of CD16 (FcgammaRIII) whose engagement blocks the cell death programme and facilitates differentiation. Immunology. 2001;102:331–7. doi: 10.1046/j.1365-2567.2001.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasaki M, Tsuneyama K, Ishikawa A, et al. Intrahepatic cholangiocarcinoma in cirrhosis presents granulocyte and granulocyte-macrophage colony-stimulating factor. Hum Pathol. 2003;34:1337–44. doi: 10.1016/j.humpath.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Arndt S, Melle C, Mondal K, et al. Interactions of TANGO and leukocyte integrin CD11c/CD18 regulate the migration of human monocytes. J Leukoc Biol. 2007;82:1466–72. doi: 10.1189/jlb.0407219. [DOI] [PubMed] [Google Scholar]
- 37.Georgakopoulos T, Moss ST, Kanagasundaram V. Integrin CD11c contributes to monocyte adhesion with CD11b in a differential manner and requires Src family kinase activity. Mol Immunol. 2008;45:3671–81. doi: 10.1016/j.molimm.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 38.Wu H, Gower RM, Wang H, et al. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subimerb C, Pinlaor S, Khuntikeo N, et al. Tissue invasive macrophage density is correlated with prognosis in cholangiocarcinoma. Mol Med Rep. 2010;3:597–605. doi: 10.3892/mmr_00000303. [DOI] [PubMed] [Google Scholar]
- 40.Venneri MA, De Palma M, Ponzoni M, et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109:5276–85. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 41.Murdoch C, Tazzyman S, Webster S, et al. Expression of Tie-2 by human monocytes and their responses to angiopoietin-2. J Immunol. 2007;178:7405–11. doi: 10.4049/jimmunol.178.11.7405. [DOI] [PubMed] [Google Scholar]


