Abstract
T helper type 17 (Th17) and regulatory T cells (Treg) play an important role in the pathogenesis of inflammation and autoimmune disorders. Recent studies have suggested that they also had an impact on tumour immunology. However, the relationship between Th17 and Treg cells in the pathogenesis of bladder carcinoma is still unclear. Flow cytometry was used to analyse the numbers, phenotype and cytokine production of Th17 cells in peripheral blood and tumour tissue from bladder carcinoma patients, in parallel with analysis of Treg cells. The suppressor capacity of Treg and the potential effects of interleukin (IL)-2 on the differentiation of Th17 and Treg cells in vitro were studied in a T cell stimulation and suppression assays. The results were as follows: Th17 cells were enriched in the tumours of patients with bladder carcinoma compared with the peripheral blood of patients and controls; patients with bladder carcinoma had a higher proportion of Treg cells in peripheral blood compared with healthy controls and nearly all patients examined showed a relative enrichment of tumour-infiltrating Treg with respect to peripheral blood; there appeared to be an inverse relationship between tumour-infiltrating Th17 and Treg cells; IL-2 could convert tumour-infiltrating Treg cells cultured in the presence of the autologous irradiated CD3– fraction into Th17 cells, down-regulate forkhead box P2 expression and suppressive capacity of Treg cells. This study is the first to define the frequency and characteristics of Th17 cells in bladder carcinoma. We suggest that the balance between Th17 and Treg cells may be involved in the development or progression of bladder carcinoma.
Keywords: bladder carcinoma, Th17, Treg
Introduction
CD4+ T helper (Th) cells play a central role in orchestrating host immune responses through their capacity to help other cells of the immune system. More recently, a novel CD4+ T cell subset termed Th17 cells has been identified, which expresses the transcription factor retinoid-related orphan receptor (ROR)-γt and produce the proinflammatory cytokine interleukin (IL)-17 [1,2]. Although Th17 cells play a critical role in the pathogenesis of many inflammatory and autoimmune diseases [3,4], their prevalence among tumour-infiltrating lymphocytes (TILs) and function in human tumour immunity remain largely unknown. The results from two studies in prostate and ovarian cancer patients have suggested both beneficial and harmful implications of Th17 cells in tumour development [5,6]. Apart from its proinflammatory role, IL-17 up-regulates the production of a variety of proangiogenic factors, thus contributing to tumour angiogenesis and development. The basis for this discrepancy is not yet understood, and the presence or absence of the adaptive immune system has been suggested to account for it [7].
CD4+CD25+ regulatory T cells (Treg), constitutively expressing high levels of CD25 (the IL-2Rα chain) and the transcription factor forkhead box P3 (FoxP3), are essential for maintaining peripheral tolerance, preventing autoimmune diseases and chronic inflammatory diseases [8–10]. However, they also limit beneficial responses by suppressing sterilizing immunity and limiting anti-tumour immunity. The outcome of this activity appears to promote the survival of cancer cells by affording protection from both the innate and adaptive immune systems. Several studies have shown that higher numbers of Treg were associated with progression in a variety of malignancies [11,12]. Antigen-specific Treg have also been demonstrated at the tumour site or in the draining lymph nodes, which suppress the proliferation of naive CD4+ T cells and inhibit IL-2 secretion by effector T cells upon activation by tumour-specific ligands [13,14]. In various animal models, depletion of Treg has been shown to induce immune responses and prevent the growth or trigger the regression of tumours when performed before or very early after tumour cell injection [15,16]. Depletion of immune cells before the adoptive transfer of tumour-reactive T cells has also been shown to be a promising result in human melanoma [17].
Apart from a functional antagonism between Treg and Th17 cells in autoimmunity [18], the differentiation of these two lineages is reciprocally regulated both in mice and human. It is now well established that although transforming growth factor (TGF)-β alone induces FoxP3+ regulatory T cells, TGF-β and IL-6 induce the differentiation of mouse naive T cells into Th17 cells by up-regulating the ROR-γt [19,20]. IL-1β and IL-6 have been identified recently as key factors for the differentiation of human Th17 cells, whereas the role of TGF-β remains to be elucidated completely [21]. In addition, studies have shown that IL-2 might play a central role in balancing Treg cells and IL-17+ T cells in multiple diseases [22].
There is increasing evidence that cell-mediated immunity plays a key role in tumour immunology of patients with bladder cancer. Recently, Loskog found that bladder carcinoma was a Tr1-dominated tumour and CD4+CD25+ T cells were increased in patient blood [23]. However, the identification and definition of regulatory and immunosuppressive cells in bladder cancer is still in its infancy. Little information is available on the involvement of Th17 cells in human bladder cancer. Here, we have examined the characteristic of Treg and Th17 cells, with the aim of further elucidation of the role of Treg and Th17 cells, and their balance, in patients with bladder cancer.
Materials and methods
Patients and specimens
Forty-five newly diagnosed patients with histologically confirmed bladder carcinoma and 20 healthy controls were included in this study. The characteristics of the study subjects are summarized in Table 1. None of the patients received radiotherapy, chemotherapy or other medical interventions within 4 weeks of blood donation. Both patients and donors signed a consent form before tumour or peripheral blood samples were obtained.
Table 1.
Patient characteristics.
| Superficial group | Invasive group | Healthy controls | |
|---|---|---|---|
| Patients (male/female) | 11/9 | 16/9 | 14/6 |
| Mean age (range) (year) | 52·3 (25–71) | 50·1 (29–74) | 49·2 (20–65) |
| Clinical stage (n, %) | |||
| Ta | 12 (60) | – | |
| T1 | 8 (40) | – | |
| T2 | – | 5 (20) | |
| T3a | – | 9 (36) | |
| T3b | – | 8 (32) | |
| T4, N+, M0 | – | 3 (12) | |
| Grade (n, %) | |||
| I | 5 (25) | 7 (28) | |
| II | 11 (55) | 10 (40) | |
| III | 4 (20) | 8 (32) |
Peripheral blood mononuclear cells and tumour-infiltrating lymphocytes preparations
Peripheral blood (PB) was diluted 1:1 in RPMI-1640 and layered onto Ficoll-Hypaque medium before centrifugation. Peripheral blood mononuclear cells (PBMCs) were then collected off the interface, washed twice in RPMI-1640 and resuspended in T cell media consisting of RPMI-1640 supplemented with 25 mmol/l HEPES, 50 µm mol/l β-mercaptoethanol, 2 mmol/l L-glutamine, 50 IU/ml penicillin, 50 µg/ml streptomycin (all from Sigma) and 5% human AB serum. Total cell numbers were quantified using trypan blue exclusion.
Freshly isolated bladder carcinoma specimens were dissected to remove necrotic material, fat, normal bladder and connective tissue. The remaining tumour was minced using a scalpel into cubes approximating 2 mm, washed in phosphate-buffered saline (PBS) and then immersed in RPMI-1640 containing 0·1% collagenase I, 0·01% hyaluronidase I and 0·002% deoxyribonuclease I (all from Sigma Chemical Co, St Louis, MO, USA). The samples were then agitated gently for 4–8 h at 37°C and the resulting digest was washed three times in PBS, layered on to Ficoll-Hypaque medium and centrifuged at 800 g for 20 min. The resulting tumour-infiltrating lymphocytes (TILs) suspension was washed twice in T cell medium and lymphocytes enumerated using trypan blue exclusion.
Flow cytometric analysis and purification of T cell subsets
For cytokine detection, the cells were stimulated with phorbol myristate acetate (50 ng/ml; Sigma) and ionomycin (1 µM; Sigma) for 4 h before staining. Cells were first stained extracellularly with combinations of CD3-phycoerythrin (PE) (UCHT1), CD4-allophycocyanin (APC) (RPA-T4), CD8-fluorescein isothiocyanate (FITC) (SK1), CD25-FITC (BC96), CD45RO-FITC (UCHL1), CTLA-4-PE (14D3), CD127-PE (M21), CD69-peridinin chlorophyll (PerCP) (CH-4), CCR4-PeCy7 (1G1) and CCR6-PE (11A9). Cells were fixed and permeabilized with Perm/Fix solution (eBioscience, San Diego, CA, USA), and intracellularly stained with anti-IL-17, anti-FoxP3, anti-tumour necrosis factor (TNF)-α and anti-interferon (IFN)-γ (all from BD Biosciences, San Jose, CA, USA, except anti-IL-17; eBioscience). Flow cytometric analysis was performed on a fluorescence activated cell sorter (FACS)Calibur cytometer. Data processing was performed with CellQuest software (Becton Dickinson, San Jose, CA, USA).
CD4+CD25- and CD4+CD25+ T cells were isolated from peripheral blood mononuclear cells and tumour-infiltrating lymphocytes by sorting with the FACSCalibur system after staining with anti-CD4 and anti-CD25 monoclonal antibodies (mAbs). The purity of the isolated CD4+CD25- and CD4+CD25+ T cells was greater than 97%.
Real-time quantitative polymerase chain reaction (PCR)
FoxP3 mRNA expression was quantified by real-time PCR using ABI PRISM 7700 Sequence Detector (Applied Biosystems, Foster City, CA, USA). The human housekeeping gene β-actin primers and probe set was used as a reference for sample normalization. Total RNA isolated from CD4+CD25high T cell was reverse-transcribed into cDNA using random hexamer primers. The primer set for FoxP3 was 5′-TTCGAAGAGCCAGAGGACTT-3′ and 5′-GCTGCTCCAGAGACTGTACC-3′. The probe for FoxP3 was 5′-FAM-CTCAAGCACTGCCAGGCGGACCATC-TAMRA-3′. The primer set for β-actin was 5′-ATCTGCTGGAAGGTGGACAGCGA-3′ and 5′-CCCAGCACAATGAAGATCAAGATCAT-3′. The probe for β-actin was 5′-FAM-TGAGCGCA AGTACTCCGTGTGGATCGGCG-TAMRA-3′. The primers and probes used in the real-time PCR were ordered from Sangon (Shanghai, China) and designed not to amplify genomic DNA. Standard curves were generated from serial dilutions of purified plasmid DNA encoding the respective genes with a linear regression R greater than 0·99 and used to quantify mRNA copy numbers for each sample. The amplification protocol used was described as follows: 1 µl of synthesized cDNA product was subsequently added into PCR mix containing 25 µl of TaqMan 2 × PCR master mix (Applied Biosystems), 30 pmol human FoxP3 primer with 10 pmol probe, 2·5 µl β-actin primer/probe set, and distilled water was added to make a total reaction volume of 50 µl. The PCR was programmed as an initial incubation for 10 min at 95°C followed by 40 thermal cycles of 15 s at 95°C and 1 min at 60°C. The normalized values in each sample were calculated as the relative quantity of FoxP3 mRNA expression divided by the relative quantity of β-actin mRNA expression. All reactions were confirmed by at least one additional independent run.
T cell stimulation and suppression assays
The suppressor capacity of Treg was studied in a co-culture suppression assay. A 96-well U-bottomed plate was treated by coating with 10 µg/ml anti-CD3 (UCHT1) and 10 µg/ml anti-CD28 (clone 28·2) monoclonal antibodies in sodium hydrogen carbonate buffer (pH = 9·2) for 2 h. The buffer was washed off with PBS and the plates blocked using T cell media. CD4+CD25- T cells (responders) and Treg cells (suppressor) (103 cells/well) were cultured in RPMI medium supplemented with 10% fetal calf serum in different responder/suppressor ratios (0:1, 1:1, 1:1/2, 1:1/4, 1:1/8 and 1:0). All cells were cultured in a final volume of 200 µl in the presence of 1 × 104 irradiated peripheral mononuclear cells as antigen-presenting cells. All tests were conducted in triplicate. Cell cultures were then incubated at 37°C for 4 days and supernatants were obtained for cytokine measurements before being pulsed with 1 µCi [3H]-thymidine per well for the final 18 h of incubation. Plates were harvested onto nylon filters using the Betaplate system and radioactivity was quantified using a Betaplate counter. Results are expressed in counts per minute (cpm) as the mean of triplicate cultures ± standard error of the mean (s.e.m.). Percentage suppression was calculated using the formula: (1−cpm in presence of Treg cells/cpm in the absence of Treg cells) × 100.
Conventional (CD4+CD25-) and Treg (CD4+CD25high) populations were isolated from tumour samples by flow cytometry cell sorting and stimulated with the irradiated autologous CD3- fraction, containing tumour cells and tumour-associated antigen-presenting cells (APCs), in the presence or absence of IL-2 (50 ng/ml) for 10 days. Cultures were then stimulated with phorbol myristate acetate (PMA)/ionomycin and stained with anti-CD4 and anti-IL-17 mAb.
Measurement of cytokine production
The supernatants were diluted for measurement of cytokine concentration by enzyme-linked immunosorbent assay (ELISA) (R&D kits, Minneapolis, MN, USA). Briefly, microtitre plates precoated with capturing mAbs were blocked with 2% bovine serum albumin (BSA)/PBS. After washing, samples and controls were added at 50 µl per well and incubated for 2 h with a biotinylated detecting antibody (50 µl per well) in 2% BSA/PBS/Tween-20. Plates were washed and incubated for 30 min with streptavidin-conjugated horseradish peroxidase. Next, 100 µl of 0·0125% tetramethylbenzidine and 0·008% H2O2 in citrate buffer was used as substrate. A standard curve was performed for each plate and used to calculate the absolute concentrations of cytokines.
Statistical analysis
Normally distributed data sets were analysed by Student's t-test, paired t-test, analysis of variance (anova) and linear regression and correlation analysis (using ‘Primer for Biostatistics’). The Wilcoxon two-sample test and Kruskall–Wallis test were used for data sets that were not normally distributed (using sas). P ≤ 0·05 was considered significant.
Results
Bladder carcinoma TILs contained high proportions of Th17 cells
Although the high frequency of Th17 cells has been shown to correlate with favourable outcome in patients with several types of cancer, their distribution is unclear as yet in human bladder tumours. Those prompted us to assess the presence of Th17 cells in the peripheral blood and tumours tissue of patients with bladder carcinoma.
PBMCs in patients with bladder carcinoma (n = 45) and in healthy controls (n = 20) were examined for the prevalence of Th17 cells. The population of Th17 cells as a percentage of total CD4+ cells was evaluated by flow cytometric analysis. Representative plots showed that healthy individual and bladder carcinoma patients had similar Th17 numbers in the PBMCs (Fig. 1a). As shown in Fig. 1b, the mean frequency of peripheral blood Th17 cells in bladder carcinoma patients was comparable with that in healthy individuals (1·2 ± 0·7% versus 1·3 ± 0·6%).
Fig. 1.
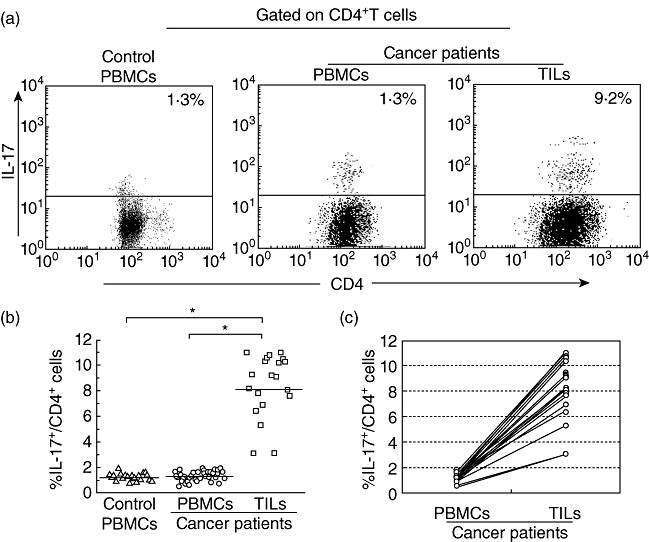
Increased population of T helper type 17 (Th17) cells in the tumour-infiltrating lymphocytes (TILs) from patients with bladder carcinoma. Results were expressed as the percentage of Th17 cells in total CD4+ cells in the peripheral blood mononuclear cells (PBMCs) or TILs. (a) Representative fluorescence activated cell sorter (FACS) pictures from a single bladder carcinoma patient and a healthy donor. Black dots in each plot represent Th17 cell. (b) The mean frequency of peripheral blood Th17 cells in bladder carcinoma patients (n = 45) was comparable with that in healthy individuals (n = 20), but the percentage of Th17 cells in the TILs (n = 20) was higher than that in the PBMCs. The horizontal lines represent the median values of the groups and asterisks show statistical significance (*P < 0·01). (c) The percentage of Th17 cells in the TILs was higher than that in the PBMCs in the same patient (n = 20).
The population of Th17 cells in the TILs isolated from resected tumour specimens of patients with bladder carcinoma (n = 20) was also evaluated. Strikingly, as representative data showed, the percentage of Th17 cells in the TILs was higher than that in the PBMCs in the same patient (Fig. 1a and c). The mean percentage of Th17 cells in the CD4+ population was significantly higher in TILs (8·2 ± 4·6%) compared with that in the PBMCs from bladder carcinoma patients (1·2 ± 0·7%, P < 0·01, Fig. 1b) or healthy individuals (1·3 ± 0·6%, P < 0·01, Fig. 1b). In some patients up to 11% of the CD4+ TILs secreted IL-17 upon brief stimulation, suggesting that IL-17+ T cells may be differentiated predominantly in the tumour microenvironment.
To characterize more effectively the CD4+ T cell population producing IL-17 ex vivo, we also analysed their phenotype and cytokine profile in the tumour microenvironment. Our data showed that the CCR6 surface expression on Th17 cells in TILs was similar to that in PBMCs from patients or healthy controls (Fig. 2a), whereas CCR4 expression on Th17 cells in TILs was significantly higher than that in PBMCs from patients or healthy controls (Fig. 2a). Our results showed that most of the tumour-infiltrating IL-17+ T cells expressed high levels of homing molecules, which might be involved in regulating lymphocyte migration.
Fig. 2.
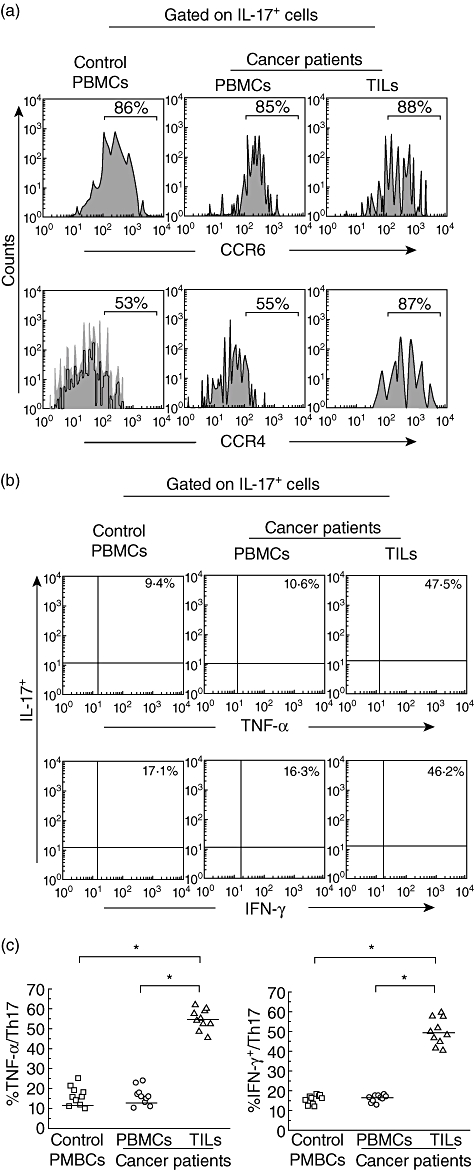
Phenotype and cytokine profile of T helper type 17 (Th17) cells. Single-cell suspensions were made from fresh peripheral blood and tumour specimens. The cells were subjected to membrane and intracellular staining and analysed by fluorescence activated cell sorter (FACS). (a) One representative FACS pictures from a single cancer patient and a healthy donor showed the expressions of CCR4 and CCR6 on tumour-infiltrating lymphocytes (TILs) and peripheral blood mononuclear cells (PBMCs). Results were representative of 10 separate experiments performed on patients with cancer and healthy subjects. (b) One representative FACS picture from a single cancer patient and a healthy donor showed the effector cytokine profile of Th17 cells. Results were representative of 10 separate experiments performed on patients with cancer and healthy subjects. (c) Circulating Th17 cells from healthy controls (n = 10) and bladder carcinoma patient (n = 10) barely expressed tumour necrosis factor (TNF)-α or interferon (IFN)-γ, whereas almost half the Th17 cells isolated from cancer tissues (n = 10) could produce TNF-α or IFN-γ. The horizontal lines represent the median values of the groups and asterisks show statistical significance (*P < 0·01).
We further analysed the cytokine profile of human Th17 cells in TILs and PBMCs. Representative plots showed that Th17 cells in PBMCs from a healthy individual and a bladder carcinoma patient had similar lower levels of polyfunctional effector cytokines, including TNF-α and IFN-γ (Fig. 2b). In contrast, Th17 cells in TILs expressed high levels of TNF-α and IFN-γ. Almost half of the tissue Th17 cells were able to produce TNF-α or IFN-γ (Fig. 2c), which implied the possible existence of a developmental and/or functional relationship between Th17 and Th1 cells in bladder tumours.
Defining the human Treg population
Treg were identified as CD4+CD25high T cells by selecting those CD4+ cells whose CD25 expression exceeded the level of CD25 positivity seen on the CD4 negative population [24] (Fig. 3a). The phenotypic characteristics of CD4+CD25–, CD4+CD25int and CD4+CD25high subsets from cancer patients and healthy donors were then analysed further by flow cytometry. The highest percentage of CD45RO+CTLA-4+ was detected in the CD4+CD25high subsets and the percentage, respectively, was 92% ± 2·5% (range: 89–94%) and 94% ± 3·6% (range: 85–99%), but CD127 and CD69 were not expressed in the CD4+CD25high subsets (Fig. 3b). In agreement with previous reports, the expression level of all of these markers was unchanged on the CD4+CD25high T cells from either healthy controls or patients with bladder carcinoma.
Fig. 3.
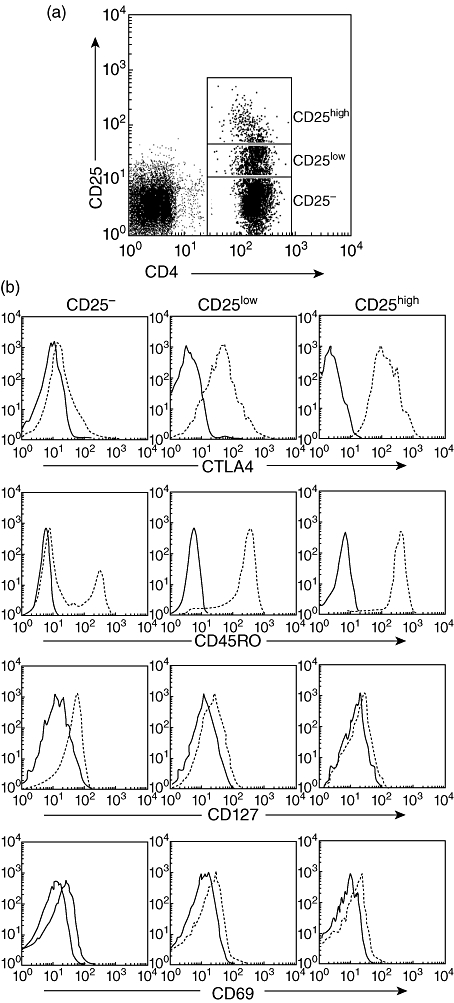
Defining the human regulatory T cell (Treg) population. (a) Based on CD25 expression, the CD4 population was subdivided into CD25-, CD25int and CD25high populations. Treg cells were identified as CD4+CD25high T cells by selecting those CD4-positive cells whose CD25 expression exceeded the level of CD25 positivity seen in the CD4 negative population. (b) CD4+CD25high T cells from either healthy subjects or cancer patients expressed the same high levels of CTLA4 and CD45RO, but CD127 and CD69 were not expressed. Isotype controls were depicted as solid lines and expression of various antigens were depicted as broken lines. Results were representative of 12 separate experiments performed on patients with bladder carcinoma and healthy subjects.
The transcription factor FoxP3 has been described as the most specific molecular marker for Treg[25,26]. We therefore analysed FoxP3 expression in CD4+CD25high T cells isolated from cancer patients and healthy donors using real-time PCR. As depicted in Fig. 4a, CD4+CD25high T lymphocytes from both cancer patients and healthy donors expressed similar high levels of FoxP3. Together, these results indicated that CD4+CD25high T cells isolated from patients demonstrated specific phenotypic features of immunosuppressive regulatory T cells. Furthermore, no phenotypic difference was observed on the CD4+CD25high T cells from cancer patients or healthy donors.
Fig. 4.
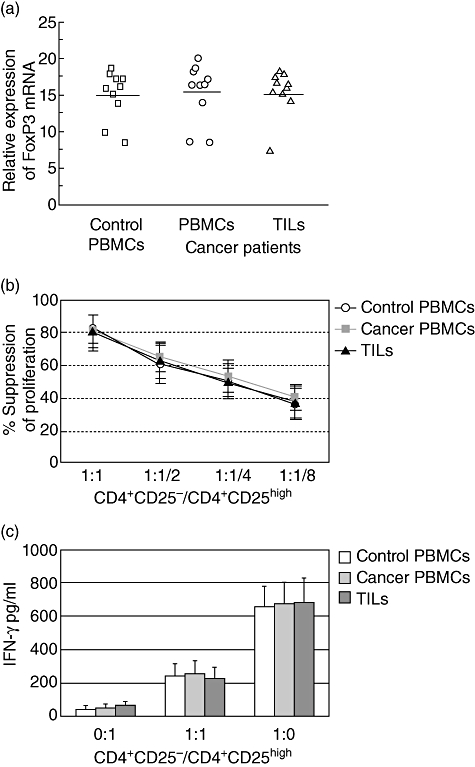
(a) CD4+CD25high T cells isolated from peripheral blood mononuclear cells (PBMCs) of healthy subjects (n = 10), patients with bladder carcinoma (n = 10) and from tumour-infiltrating lymphocytes (TILs) (n = 10) exhibited equal forkhead box P3 (FoxP3)-expression levels. The horizontal lines represent the median values of the groups. (b) CD4+CD25high T cells isolated from PBMCs in both healthy subjects (n = 10) and patients with bladder carcinoma (n = 10) or from TILs (n = 10) exhibited equal suppressor activity at different ratio of responder/suppressor T cells. The mean percentage inhibition of the proliferative response by CD4+CD25high T cells was calculated. (c) The CD4+CD25high T cells did not secrete cytokines, but could suppress the secretion of interferon (IFN)-γ by co-cultured CD4+CD25- T cells in a dose-dependent manner. Culture supernatants were removed from the proliferation cultures before the addition of [3H]-thymidine incorporation. Levels of IFN-γ were determined by enzyme-linked immunosorbent assay. The columns represent means, the bars represent standard deviation.
We sought to compare the functional status of sorted CD4+CD25high T cells from cancer patients and healthy controls. Quantitative analysis of the regulatory function of CD4+CD25high T cells was performed by co-culturing them with autologous T responder cells at different ratios. CD4+CD25high cells from the PBMCs or TILs were anergic to this stimulation and the proliferation of CD4+CD25– T cells induced by anti-CD3 and anti-CD28 was reduced in the presence of CD4+CD25high T lymphocytes. Increasing the ratio of CD4+CD25–/CD4+CD25high T cells resulted in less suppression. No significant differences were detected between cancer patients and healthy controls under the conditions we tested (Fig. 4b).
We also analysed the concentrations of cytokines in the supernatants obtained from the co-culture of CD4+CD25high T cells and CD4+CD25–T cells. As shown in Fig. 4c, CD4+CD25– T cells cultured alone produced large amounts of IFN-γ from both healthy controls and cancer patients. Supernatants from cultures of CD4+CD25high T cells alone with APCs contained few IFN-γ. Co-culture of CD4+CD25high T cells with CD4+CD25– T cells at a 1:1 ratio resulted in significant inhibition of IFN-γ secretion in the culture supernatants from healthy controls and cancer patients. This suppressive effect was not significantly different between CD4+CD25high from cancer patients and those from healthy donors.
The results indicated that CD4+CD25high T cells isolated from patients or healthy donors showed a conventional phenotype and equal ability to suppress the proliferation and cytokine secretion of CD4+ effector T cells, thereby allowing identification of these cells as Treg.
Bladder carcinoma patients with a high number of Treg cells
The percentage of Treg cells in the CD4+ population from the PBMCs in healthy controls or bladder carcinoma patients was evaluated. Our data showed that the patients with bladder carcinoma had a significantly higher Treg frequency in the PBMCs [8·7% ± 5·4% (range: 2·4–15·5%); n = 45] compared with healthy controls [2·4% ± 1·0% (range: 1·1–4·2%); n = 20] (Fig. 5a and b). The proportion of Treg cells in tumour tissue from patients with bladder carcinoma (n = 20) was also examined. As shown in Fig. 5c, most of the patients examined showed a relative enrichment of Treg with respect to peripheral blood. The percentage of Treg cells in the tumour tissue was 15·4%, with a standard deviation (s.d.) of 9·9% (range: 7·2–23·6%).
Fig. 5.
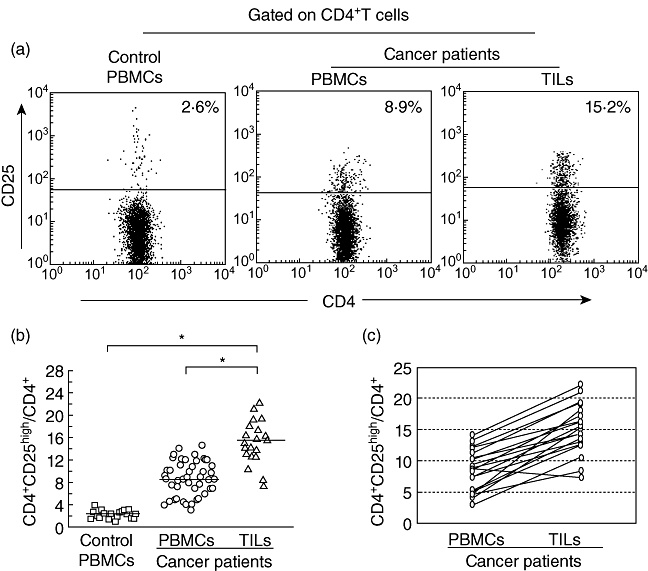
Increased frequencies of human regulatory T cell (Treg) cells in patients with bladder carcinoma compared with healthy donors. (a) One representative fluorescence activated cell sorter (FACS) picture from a normal control and a bladder carcinoma patient. (b) Statistical result of the proportion of Treg cells among CD4+ T cells in bladder carcinoma patients (n = 20) and normal donors (n = 45). The horizontal lines represent the median values of the groups and asterisks show statistical significance (*P < 0·01). (c) Nearly all patients (n = 20) examined showed a relative enrichment of Treg with respect to peripheral blood.
The relationship between Th17 cells and other immune cell subsets in the tumour microenvironment
There were multiple immune cell populations in the tumour microenvironment. The relationships were evaluated further between Th17 cells and other immune cell subsets, such as IFN-γ+ CD4+ T cells and Treg cells in the same tumours. Flow cytometry analysis revealed that the proportion of Th17 cells was correlated positively with that of IFN-γ+ CD4+ T cells, but correlated inversely with Treg cells in the same tumour microenvironment (Fig. 6a).
Fig. 6.
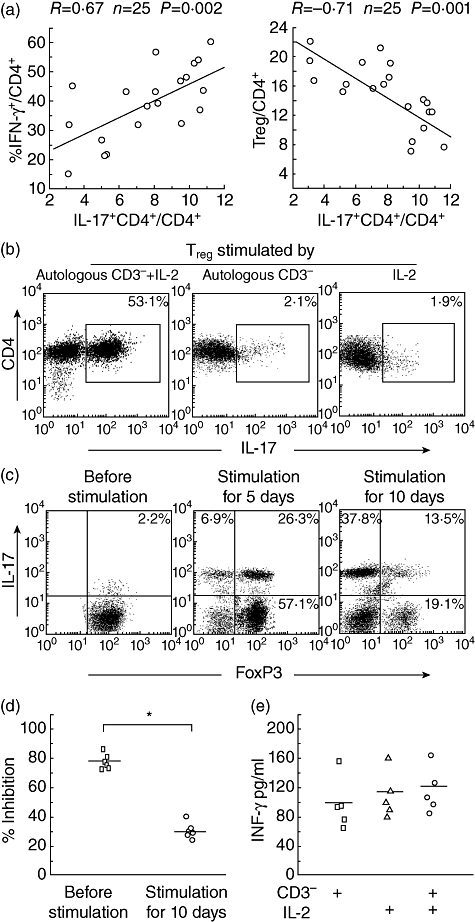
(a) Correlation coefficients were computed to evaluate the relationship between the percentages of Th17 cells and interferon (IFN)-γ+ CD4+ T cells and human regulatory T cell (Treg) cells in the same tumours (n = 20). (b) A single representative experiment showed flow cytometric analysis of interleukin (IL)-17 production in the sorted Treg populations that were cultured for 10 days with the autologous irradiated CD3– fraction plus IL-2, autologous irradiated CD3– fraction or IL-2, respectively, and then stimulated for 4 h with phorbol myristate acetate plus ionomycin. Numbers in the plots indicate the percentages of IL-17-producing CD4+ cells as assessed by intracellular cytokine staining. (c) A single representative experiment showed intracellular expression of IL-17 and forkhead box P3 (FoxP3) at days 0, 5 and 10 after culture of Treg with the autologous irradiated CD3– fraction plus IL-2, and then stimulated for 4 h with phorbol myristate acetate plus ionomycin. (d) Bladder tumour-associated Treg suppressive activity was evaluated before (n = 6) and after (n = 6) stimulation with the autologous irradiated CD3– fraction in the presence of IL-2 for 10 days. The suppressive activity of bladder tumour-associated Treg was decreased significantly after stimulation. The horizontal lines represent the median values of the groups and asterisks show statistical significance (*P < 0·01). (e) Sorted populations were stimulated with autologous irradiated CD3– fraction in the absence (n = 5) or in the presence of IL-2 (n = 5), or stimulated with IL-2 alone. Culture supernatants were removed from the proliferation cultures and levels of IFN-γ were determined by enzyme-linked immunosorbent assay. The horizontal lines represent the median values of the groups.
IL-2 converts bladder tumour-associated Treg into Th17 cells ex vivo
Several studies suggested that instillations of IL-2 into the urinary bladder might be effective for treatment of superficial bladder cancer, and recent data also indicated that IL-2 might play a role in regulating the TH17/Treg balance in the tumour microenvironment, so we investigated the potential effects of IL-2 on Th17 and Treg cell differentiation in vitro. A Treg subset from tumour samples was sorted ex vivo by flow cytometry cell sorting and the purity of the separated cells subset was confirmed to be >97%.
Next, we analysed IL-17 production of sorted Treg after stimulation with the autologous irradiated CD3– fraction in the presence of IL-2 for 10 days. As shown in Fig. 6b, Th17 cells were clearly detectable in populations from the purified Treg cell fractions. However, no proliferation or IL-17 production was observed after culture of tumour Treg stimulated by the autologous irradiated CD3– fraction in the absence of IL-2. We also failed to detect any significant proliferation or IL-17 production when the purified tumour Treg cells were cultured with IL-2 alone.
To characterize further the tumour Treg after in vitro expansion, we assessed IL-17 production and FoxP3 expression simultaneously by these cells stimulated by the autologous irradiated CD3– fraction in the presence of IL-2. As shown in Fig. 6c, the sorted Treg gradually expressed IL-17 and lost FoxP3 expression. The proportion of Treg co-expressing FoxP3 and IL-17 was increased gradually in the early days, but decreased as culture time went on.
Co-culture with responder CD4+CD25– cells and Treg was used to evaluate the function change of tumour Treg after conversion. As shown in Fig. 6d, compared with the tumour Treg before stimulation, the tumour Treg after conversion exhibited hampered inhibition of responder CD4+CD25– cell proliferation, which may be associated with down-regulated FoxP3 expression. Little IFN-γ production was found in the Treg cultures (Fig. 6e).
Discussion
Studies have shown that tumour is potentially immunogenic and that the host immune response influences survival [27]. It has been shown that tumour-infiltrating effector T cells correlates with improved prognoses of several types of cancer, whereas tumour-infiltrating Treg cells are associated negatively with patient outcome [28,29]. It has emerged that bladder carcinoma is a particularly good example of immune-associated cancer. Thus, it is important to investigate the presence and functional role of effector T cells and Treg cells in the patients with bladder carcinoma.
Accumulating data have suggested that Th17 cells play an important role in host defence against microbial infections and appear to be important mediators in the pathogenesis of inflammatory and autoimmune diseases [30]; however, the distribution, phenotype and cytokine profile of Th17 cells in human tumours still remain poorly defined. The results of studies from both experimental tumour models and cancer patients have shown that the role of Th17 cells in tumour immunity remain controversial [5,6]. In our current studies, T cell populations in PBMCs and TILs from bladder cancer patients were analysed and the results showed a prominence of Th17 cell populations in the TILs. Our data also indicated that tumour-infiltrating Th17 cells highly expressed polyfunctional effector cytokines, including TNF-α and IFN-γ. These data suggested that tumour-infiltrating Th17 cells might be functional effector T cells. Recent studies have suggested that some chemokine receptors are expressed selectively on Th17 cells from certain origins [31,32]. Our data indicated that tumour-infiltrating Th17 cells expressed high levels of homing molecules CCR4 and CCR6, which might be associated with Th17 cell migration and retention within the tumour. A significant correlation between Th17 cells in cancer patients and disease progression was not observed due to the small sample size in the present studies. Further studies will be needed to advance our understanding of the role of Th17 cells in the immunopathogenesis of bladder cancers.
Our data showed that bladder carcinoma patients had increased population of Treg in the peripheral blood, especially in the tumour environment, which further confirmed recent studies that Treg infiltrated the human bladder carcinoma [22]. The correlation of proportion of Treg with stage or grade of bladder carcinoma could not be performed because of the limited sample size and heterogeneous patient sample of the present study. There was a large amount of evidence illustrating the increase in the number of Treg cells in the tumour setting [33,34], but very little work has been conducted to compare directly Treg cells from bladder cancer patients with those from healthy controls. In the present study, Treg cells from peripheral blood and the tumour tissue were compared directly at both the molecular and functional levels. Our data indicated that the CD4+CD25high T cell population from peripheral blood and the tumour tissue of patients displayed the same functional and phenotypic characteristics as that from controls, thereby allowing identification of these cells as Treg. The accumulation of Treg cells in the tumour might be caused by multiple factors, including increased proliferation, decreased apoptosis, selective recruitment and altered expression of chemokines and so on. The presence of the Treg cells is likely to hinder the development of anti-tumour immune responses following the delivery of an immunotherapeutic agent, such as resistance to bacille Calmette–Guérin (BCG) therapy. For this reason, methods of abrogating the activity of Treg cells might be critical for the successful immunotherapeutic treatment of cancer.
Studies showed that Treg and Th17 cells co-existed in the microenvironment of different types of tumour, and the development of Th17 cells was described to be linked to that of Treg in a reciprocal fashion; however, information on human bladder cancer Th17/Treg development and differentiation is limited. Our data revealed that Th17 cells were correlated inversely with Treg cells and correlated positively with IFN-γ+ CD4+ T cells in the same tumour microenvironment. It has shown that recombinant IL-2 is a promising agent for the activation of immune response against tumour and plays a central role in balancing Treg cells and IL-17+ T cells in multiple diseases. Kryczek et al. reported that IL-2 regulated the balance between tumour Treg and Th17 cells by stimulating the differentiation of Treg and inhibiting that of Th17 cells [35]. However, Leveque et al. revealed that under some stimulated conditions, IL-2 rapidly converted epithelial ovarian cancer (EOC) Treg into Th17 cells, down-regulated FoxP3 expression, and lost their suppressive capacity [17]. Due to the above conflicting data, we sought to determine whether IL-2 would also play a role in balancing Treg cells and IL-17+ T cells in bladder cancers. Our results indicated that tumour-infiltrating Treg cells cultured in the presence of the autologous irradiated CD3– fraction and IL-2 could be converted into Th17 cells, which might be involved in the mechanism that instillations of IL-2 into the urinary bladder is effective in the treatment of superficial bladder cancer.
In conclusion, the present data suggest that Th17 cells, together with Treg cells, might contribute to the immunopathogenesis of bladder cancer, and inhibition of Th17 cell development might be a novel immune evasion mechanism. We further identified that IL-2 played a role in balancing Treg cells and IL-17+ T cells by converting bladder cancer Treg into Th17 cells, our results encouraged a deep in vivo exploration of its effects on in situ immune responses. Further studies are still needed to identify the mechanisms of underlying regulation and dynamic interaction among Th17 cells and Treg and Th1 cells in human pathological conditions such as bladder cancer.
Disclosure
The authors have no financial conflict of interest.
Acknowledgments
This study was supported by Heilongjiang Province Science Foundation for Youths (project number: QC2009C05), China Postdoctoral Science Foundation, Innovation of science and technology of Harbin youth (project number: 2008RFQXS008) and Foundation of Heilongjiang Educational Committee (project number: 11531160).
References
- 1.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–48. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 2.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–6. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Sarkar S, Cooney LA, Fox DA. The role of T helper type 17 cells in inflammatory arthritis. Clin Exp Immunol. 2010;159:225–37. doi: 10.1111/j.1365-2249.2009.04016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Behi M, Rostami A, Ciric B. Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol. 2010;5:189–97. doi: 10.1007/s11481-009-9188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–61. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kryczek I, Wei S, Zou L, et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–3. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Orozco N, Dong C. The IL-17/IL-23 axis of inflammation in cancer: friend or foe? Curr Opin Investig Drugs. 2009;10:543–9. [PubMed] [Google Scholar]
- 8.Viglietta V, Baecher–Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang B, Zhang X, Tang F, Zhu L, Liu Y. Reduction of forkhead box P3 levels in CD4+CD25high T cells in patients with new-onset systemic lupus erythematosus. Clin Exp Immunol. 2008;153:182–7. doi: 10.1111/j.1365-2249.2008.03686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi LJ, Wang HB, Wang WZ. Impairment of circulating CD4+CD25+ regulatory T cells in patients with chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2008;13:54–63. doi: 10.1111/j.1529-8027.2008.00158.x. [DOI] [PubMed] [Google Scholar]
- 11.Ichihara F, Kono K, Takahashi A, et al. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9:4404–8. [PubMed] [Google Scholar]
- 12.GriYths RW, Elkord E, Gilham DE, Kawaida H, Sugai H, Fujii H. Frequency of regulatory T cells in renal cell carcinoma patients and investigation of correlation with survival. Cancer Immunol Immunother. 2007;56:1743–53. doi: 10.1007/s00262-007-0318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French JD, Weber ZJ, Fretwell DL, Said S, Klopper JP, Haugen BR. Tumor-associated lymphocytes and increased FoxP3+ regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J Clin Endocrinol Metab. 2010;95:2325–33. doi: 10.1210/jc.2009-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang HY, Lee DA, Peng G, et al. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–18. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 15.Jones E, Dahm-Vicker M, Simon AK, et al. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun. 2002;2:1. [PubMed] [Google Scholar]
- 16.Hong H, Gu Y, Zhang H, et al. Depletion of CD4+CD25+ regulatory T cells enhances natural killer T cell-mediated anti-tumour immunity in a murine mammary breast cancer model. Clin Exp Immunol. 2010;159:93–9. doi: 10.1111/j.1365-2249.2009.04018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:23–46. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148:32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 20.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–9. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 22.Leveque L, Deknuydt F, Bioley G, et al. Interleukin 2-mediated conversion of ovarian cancer-associated CD4+ regulatory T cells into proinflammatory interleukin 17-producing helper T cells. J Immunother. 2009;32:101–8. doi: 10.1097/CJI.0b013e318195b59e. [DOI] [PubMed] [Google Scholar]
- 23.Loskog A, Ninalga C, Paul-Wetterberg G, de la Torre M, Malmström PU, Tötterman TH. Human bladder carcinomais dominated by T-regulatory cells and Th1 inhibitory cytokines. J Urol. 2007;177:353–8. doi: 10.1016/j.juro.2006.08.078. [DOI] [PubMed] [Google Scholar]
- 24.Zhu LY, Chi LJ, Wang X, Zhou H. Reduced circulating CD4+CD25+ cell populations in haemorrhagic fever with renal syndrome. Clin Exp Immunol. 2009;156:88–96. doi: 10.1111/j.1365-2249.2008.03858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 26.Chang X, Zheng P, Liu Y. FoxP3: a genetic link between immunodeficiency and autoimmune diseases. Autoimmun Rev. 2006;5:399–402. doi: 10.1016/j.autrev.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Banerjea A, Bustin S, Dorudi S. The immunogenicity of colorectal cancer with high-degree microsatellite instability. World J Surg Oncol. 2005;3:26. doi: 10.1186/1477-7819-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C, Wang S, Wang F, et al. Increased frequencies of T helper type 17 cells in the peripheral blood of patients with acute myeloid leukaemia. Clin Exp Immunol. 2009;158:199–204. doi: 10.1111/j.1365-2249.2009.04011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah K, Lee WW, Lee SH, et al. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Res Ther. 2010;12:R53. doi: 10.1186/ar2964. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–46. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Rohowsky-Kochan C. Regulation of IL-17 in human CCR6+ effector memory T cells. J Immunol. 2008;180:7948–57. doi: 10.4049/jimmunol.180.12.7948. [DOI] [PubMed] [Google Scholar]
- 33.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–12. [PubMed] [Google Scholar]
- 34.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089–99. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 35.Kryczek I, Wei S, Zou L, et al. Cutting edge: TH17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–3. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]


