Abstract
The role of mast cells (MCs) in the generation of adaptive immune responses especially in the transplant immune responses is far from being resolved. It is reported that mast cells are essential intermediaries in regulatory T cell (Treg) transplant tolerance, but the mechanism has not been clarified. To investigate whether bone marrow-derived mast cells (BMMCs) can induce Tregs by expressing transforming growth factor beta 1 (TGF-β1) in vitro, bone marrow cells obtained from C57BL/6 (H-2b) mice were cultured with interleukin (IL)-3 (10 ng/ml) and stem cell factor (SCF) (10 ng/ml) for 4 weeks. The purity of BMMCs was measured by flow cytometry. The BMMCs were then co-cultured with C57BL/6 T cells at ratios of 1:2, 1:1 and 2:1. Anti-CD3, anti-CD28 and IL-2 were administered into the co-culture system with (experiment groups) or without (control groups) TGF-β1 neutralizing antibody. The percentages of CD4+CD25+forkhead box P3 (FoxP3)+ Tregs in the co-cultured system were analysed by flow cytometry on day 5. The Treg percentages were significantly higher in all the experiment groups compared to the control groups. These changes were deduced by applying TGF-β1 neutralizing antibody into the co-culture system. Our results indicated that the CD4+ T cells can be induced into CD4+CD25+FoxP3+ T cells by BMMCs via TGF-β1.
Keywords: CD4+CD25+Foxp3+ T cells, mast cells, mouse, TGF-β1
Introduction
Regulatory T cells (Tregs) can suppress immune responses to donor alloantigens, and have the potential to play an important role in both inducing and maintaining transplant tolerance in vivo[1]. The transcription factor forkhead box P3 (FoxP3) is the recognized master gene governing the development and function of both natural and induced Tregs, especially in mice [2–4].
Mast cells (MCs) have long been recognized as major players in allergy [5], but in recent years MCs have been identified as being responsible for a far more complex range of functions in the innate and adaptive immune responses [6–9]. However, the role of mast cells in the generation of adaptive immune responses, especially in transplant immune responses, is far from being resolved [10]. Recently, Lu et al. found that mast cells may be essential intermediaries in Treg-mediated transplant tolerance [11]. While the mechanisms involved are still not well understood, some previous studies have shown that MCs can serve as a source of transforming growth factor (TGF)-β1 [12], which is required for introduction and maintenance of Treg cells both in vitro and in vivo[13–16]. Therefore, this study was designed to test the hypothesis that bone marrow-derived mast cells (BMMCs) can induce CD4+ T cells to CD4+CD25+FoxP3+ Tregs via TGF-β1 in vitro.
Materials and methods
Preparation of cells
C57BL/6 (H-2b) mice were maintained and housed at the animal facilities of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. Bone marrow cells were obtained from C57BL/6 mice. The cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 10 mM Hepes, 50 µM 2-mercaptoethanol, penicillin/streptomycin/L-glutamine, 10 ng/ml mouse interleukin (IL)-3 (Peprotech, Rocky Hill, NJ, USA) and 10 ng/ml mouse stem cell factor (SCF) (Peprotech) at 37°C in a humidified atmosphere containing 5% CO2. Every 7 days, the non-adherent cells were transferred into fresh enriched medium. After 4 weeks, the purity of the mast cells was assessed by flow cytometry.
Spleen cells were obtained from C57BL/6 mice. T cells were isolated from the spleen cells with CD3 T cell isolation kit (Miltenyi, Bergisch Gladbach, Germany). Purity of CD3+ T cells typically exceeded 95%.
BMMC stained with toluidine blue
To determine the purity and the characteristic of BMMCs, BMMCs were collected after 4 weeks' culture. They were dropped onto a slide and stained with toluidine blue (1%, pH = 1) for 10–20 s. The slide was then washed with distilled water for about 2 min. The cells were observed under a microscope.
The expression of TGF-β1
Expression of TGF-β1 mRNA in BMMCs was assessed using reverse transcription–polymerase chain reaction (RT–PCR). Total RNA was extracted from BMMCs with Trizol reagent, then RT–PCR was performed following the instructions for the reverse transcription kit (Invitrogen, CA, USA) and PCR kit (Fermentas, Burlington, ON, Canada). Primer sequences were as follows: TGF-β1 forward: 5′-ACCGCAACAACGCCATCTA-3′, reverse: 5′-GCCCTGTATTCCGTCTCC-3′, β-actin forward: 5′-TGAGACCTTCAACACCCCAG-3′ and reverse: 5′-GCCATCTCTTGCTCGAAGTC-3′. The PCR programme was: 95°C for 10 min followed by 30 cycles of 95°C for 10 s, 56°C for 25 s and 72°C for 40 s.
TGF-β1 protein expression in BMMCs was determined by Western blot analysis. BMMCs were washed once in phosphate-buffered saline (PBS) and lysed in RIPA lysis buffer. Fifteen µg proteins were loaded and run on a sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), and then the proteins were transferred to a polyvinylidenefluoride membrane and blocked with 10% non-immune serum for 1 h. The membrane was incubated with primary antibody against TGF-β1 (R&D Systems, Minneapolis, MN, USA) or β-actin at 4°C overnight, then washed three times with PBS and 0·1% Tween 20, after being incubated with the secondary antibody [rabbit-derived anti-rat immunoglobulin G (IgG)] at room temperature for 1 h. Labelling was detected by chemiluminescence by addition of SuperSignal substrate solution.
In vitro assays
The carboxyfluorescein diacetate succinimidyl ester (CFSE) assay was used to determine T cell proliferation in response to mast cells. T cells were incubated with 2·5 µmol/l CFSE for 10 min at 37°C, and then washed with RPMI-1640 medium. BMMCs and CFSE-labelled T cells were co-cultured in 48-well plates at a ratio of 1:1 for 3 days with or without anti-CD3 (2 µg/ml) and anti-CD28 (2 µg/ml). The group of CFSE-labelled T cells only was used as the blank control.
In order to measure the ability of BMMCs to induce Tregs, BMMCs and T cells were co-cultured in 48-well plates at different ratios (1:1, 1:2, 2:1) with or without TGF-β1 neutralizing antibody (R&D; 1 µg/ml or 4 µg/ml) and IL-4 neutralizing antibody (R&D; 1 µg/ml); 1000 U/ml human IL-2 (Peprotech), 2 µg/ml anti-CD3 and 2 µg/ml anti-CD28 (eBioscience, San Diego, CA, USA) were added into the culture media, as described above. T cells in the culture media with IL-2, anti-CD3 and anti-CD28 served as the blank control. The cultures were analysed on day 5 by flow cytometry.
There was a total of 6 × 105 cells in each well. Experiments were performed in three duplicate wells and repeated at least three times.
Flow cytometry
FACSAria™ flow cytometer (Becton Dickinson) was used in the following assays.
Flow cytometry was used to determine the purity of BMMC suspensions. After being washed three times with PBS, phycoerythrin (PE)-anti-mouse-CD117 (eBioscience) and FITC-anti-mouse-FcεRIα (eBioscience) were added to BMMC suspensions. After incubation for 30 min at 4°C in the dark, the pellets were resuspended in 100 µl PBS and the percentage of double-positive cells were analysed.
Flow cytometry was used to determine the proliferation of CFSE-labelled T cells on day 3 co-culturing with or without BMMCs. The CFSE-labelled T cells and BMMCs were resuspended with 100 µl PBS after being washed with PBS. The T cell proliferation was analysed.
The percentage of CD4+CD25+FoxP3+ T cells was measured by flow cytometry on day 5 of co-culture with BMMCs. The cells obtained from the co-culture system were labelled with FITC-anti-mouse-CD4 (eBioscience), APC-anti-mouse-CD25 (eBioscience) and PE-anti-mouse FoxP3 (FJK-16s; eBioscience) after being washed three times with PBS. The pellets were resuspended in 500 µl cold staining buffer and the percentage of CD4+CD25+FoxP3+ T cells was analysed.
Statistical analysis
All experiments were performed at least three times. All data are presented as the mean ± standard deviation (s.d.). Data were analysed using one-way analysis of variance (anova) for differences among the multiple groups. An independent-samples t-test was used for analysing the differences between two groups by spss version 13·0 software. A P-value less than 0·05 was considered to indicate significant differences.
Results
The purity of BMMCs
After 4 weeks, cultured with 10 ng/ml IL-3 and SCF, the mouse bone marrow cells were converted to mast cells. The purity was judged by surface expression of CD117 (c-kit) and FcεRIα[17]. The percentage of double-positive (CD117+ FcεRIα+) cells was greater than 97% (Fig. 1a). Purple granules were found in the cells after staining with toluidine blue, which is the main characteristic of mast cells (Fig. 1b).
Fig. 1.
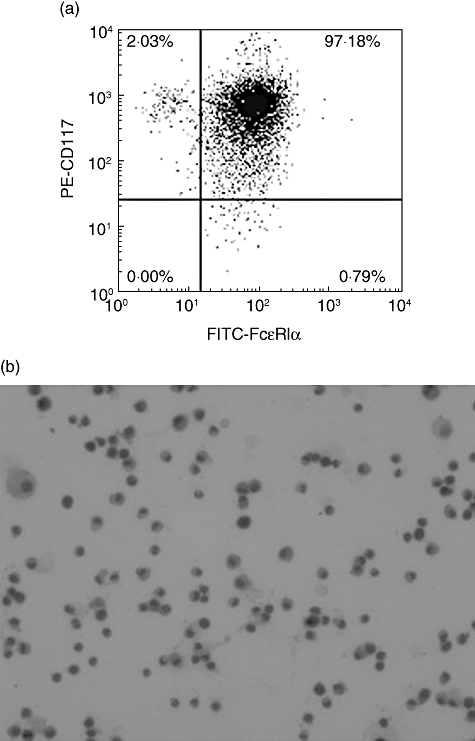
The purity of the bone marrow-derived mast cells (BMMCs). (a) After culture for 4 weeks, the BMMCs were labelled with phycoerythrin (PE)-anti-mouse-CD117 (c-kit) and fluorescein isothiocyanate (FITC)-anti-mouse-FcεRIα. The percentage of double-positive BMMCs was measured by flow cytometry. (b) BMMCs stained with toluidine blue (1%, pH = 1) were determined by microscopy (magnification ×400).
BMMCs cannot promote the proliferation of T cells
It is reported that activated MCs had the potential to recruit and activate T cells [6]. Whether the BMMCs could activate T cells and promote T cell proliferation in vitro was analysed. CFSE-labelled T cells were measured by flow cytometry after co-culture with BMMCs for 3 days. We found that the BMMCs could not promote the proliferation of T cells in the absence of anti-CD3 or anti-CD28. There was no significant difference (96·8 ± 1·10%) compared with controls (98·5 ± 0·93%) (Fig. 2a and b). When 2 µg/ml anti-CD3 and anti-CD28 were added, the T cells proliferated significantly (76·2 ± 0·81%) (Fig. 2c).
Fig. 2.
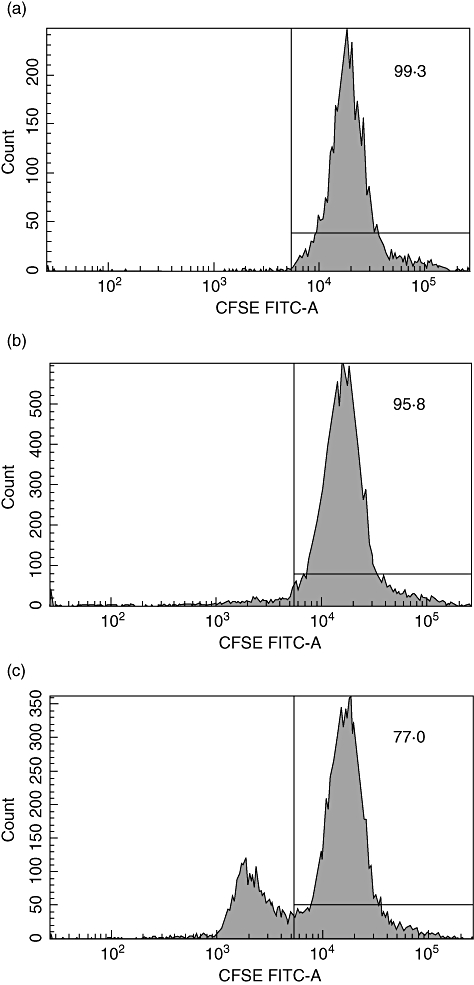
The proliferation of carboxyfluorescein diacetate succinimidyl ester (CFSE)-labelled T cells. The impact of bone marrow-derived mast cells (BMMCs) to the proliferation of CFSE- labelled T cells was determined by flow cytometry based on the CFSE signal after culturing. (a) The percentage of undivided CFSE-labelled T cells (1 × 106/ml) in the control group (T cells only). (b) The percentage of undivided CFSE-labelled T cells (1 × 106/ml) in the group of T cells co-cultured with BMMCs at a ratio of 1:1 in the absence of anti-CD3 and anti-CD28. (c) The percentage of undivided CFSE-labelled T cells (1 × 106/ml) in the group of T cells co-cultured with BMMCs at a ratio of 1:1 in the presence of anti-CD3 (2 µg/ml) and anti-CD28 (2 µg/ml).
Data shown are representative results of three independent experiments.
T cells were induced into Tregs by BMMCs
After in vitro co-culture of BMMCs and T cells with anti-CD3 and anti-CD28 for 5 days, the FoxP3 expression of T cells was measured by flow cytometry. The percentage of CD4+CD25+FoxP3+ T cells was higher in all the experimental groups than the control group (3·37 ± 0·40%) (Fig. 3). When the ratio of mast cells to T cells was 2:1, the highest percentage of CD4+CD25+FoxP3+ T cells was observed (13·63 ± 0·55%) (Fig. 3).
Fig. 3.
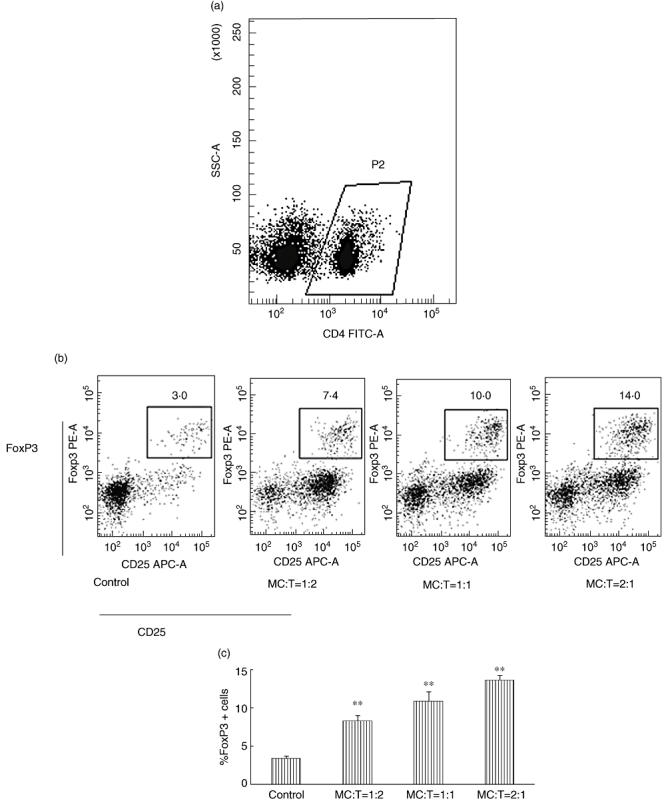
Bone marrow-derived mast cells (BMMCs) induced T cells to express forkhead box P3 (FoxP3). BMMCs and T cells were co-cultured in 48-well plates at different ratios (1:1, 1:2, 2:1) with anti-CD3, anti-CD28 and interleukin (IL)-2. The control group contained T cells with anti-CD3, anti-CD28 and IL-2. The total cells of each well were 6 × 105. Sorted cells were labelled with fluorescein isothiocyanate (FITC)-anti-mouse-CD4, APC-anti-mouse-CD25 and phycoerythrin (PE)-anti-mouse-FoxP3. (a) FITC-CD4+ T cells were chosen for analysis by flow cytometry. (b) Typical examples of the expression of CD4+CD25+FoxP3+ T cells in the different groups. (c) The percentage changes of the CD4+CD25+FoxP3+ T cells in the different groups. All experiments were performed in three duplicate wells and repeated at least three times. The data were reported as means ± standard deviation (**P < 0·001 compared with the control group).
BMMCs expression of TGF-β1
It has been reported that TGF-β1 is an important factor for the conversion of CD4+CD25– naive T cells to CD4+CD25+ Tregs by induction of transcription factor FoxP3 [13]. TGF-β1 expression of BMMCs was determined by RT–PCR assay and Western blot (Fig. 4).
Fig. 4.
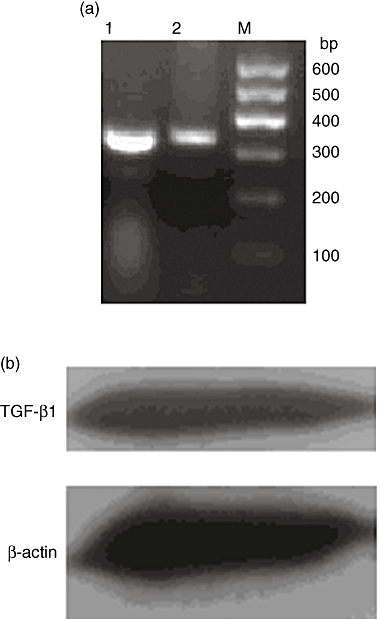
Bone marrow-derived mast cells (BMMCs) express transforming growth factor (TGF)-β1. (a) TGF-β1 mRNA in BMMCs was detected by reverse transcription–polymerase chain reaction. M: marker; 1: β-actin; 2: TGF-β1. (b) TGF-β1 protein in BMMCs was detected by Western blot.
The effects of TGF-β1 neutralizing antibody on Treg induction
To confirm further the role of TGF-β1 in the Treg-induced mediation by BMMCs, TGF-β1 neutralizing antibody (1 µg/ml) was applied to the co-culture system to block the function of TGF-β1. After application of the TGF-β1 neutralizing antibody, the BMMC Treg-mediated induction was reduced significantly in all experimental groups (P < 0·001) (Fig. 5a and b). In group 1:2, the percentage was decreased from 8·23 ± 0·80% to 4·47 ± 0·50%, and in groups 1:1 and 2:1, Tregs were decreased from 10·87 ± 1·25% to 6·13 ± 0·35% and 13·63 ± 0·55% to 6·40 ± 0·26%. However, the increase in Tregs due to BMMC induction was still significant in all the experimental groups compared to the control group (3·23 ± 0·25%) (P < 0·05) (Fig. 5a and b). Similar results were obtained with the TGF-β1 neutralizing antibody at a concentration of 4 µg/ml (data not shown).
Fig. 5.
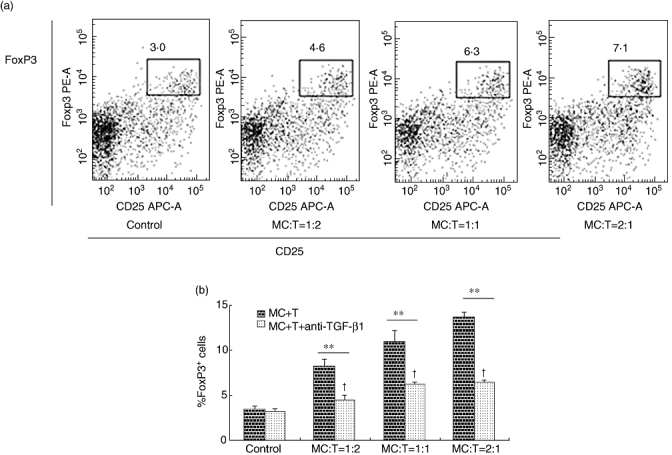
The induction of regulatory T cells (Tregs) was suppressed by transforming growth factor (TGF)-β1 neutralizing antibody. Bone marrow-derived mast cells (BMMCs) and T cells were co-cultured in 48-well plates at different ratios (1:1, 1:2, 2:1) with anti-CD3, anti-CD28, interleukin (IL)-2 and TGF-β1 neutralizing antibody. The control group contained T cells with anti-CD3, anti-CD28 and IL-2 and TGF-β1 neutralizing antibody. The total cells of each well were 6 × 105. Sorted cells were labelled with fluorescein isothiocyanate (FITC)-anti-mouse-CD4, allophycocyanin (APC)-anti-mouse-CD25 and phycoerythrin (PE)-anti-mouse-forkhead box P3 (FoxP3). (a) Typical examples of the expression of CD4+CD25+FoxP3+ T cells in the different groups with TGF-β1 neutralizing antibody. (b) The effects of TGF-β1 neutralizing antibody on Treg induction. **P < 0·001 compared the groups between with and without TGF-β1 neutralizing antibody by independent-samples t-test. †P < 0·05 compared the experimental groups with TGF-β1 neutralizing antibody versus the control group by one-way analysis of variance.
All the experiments were performed in duplicate wells and repeated at least three times. The data were reported as means ± s.d. An independent-samples t-test and one-way anova were performed to obtain a P-value.
IL-4 neutralizing antibody did not change the Treg induction
Metz et al. suggested that IL-4 may be related to the suppression function of MC in the immune response [6]. Therefore, to investigate whether IL-4 was related to the induction of Tregs, IL-4 neutralizing antibody was used to block the IL-4 function. FoxP3 expression was measured by flow cytometry on day 5. In the groups with ratios of 1:2, 1:1, 2:1, the percentages were 8·50 ± 0·65%, 10·30 ± 0·98% and 14·35 ± 1·12%, respectively. There were no significant differences between the groups with and without IL-4 neutralizing antibody (by independent-samples t-test, P > 0·05).
Discussion
Lu et al. have found that mast cells are essential intermediaries in Treg-mediated transplant tolerance [11], but the exact role that mast cells play in tolerance is still unclear. Our study was aimed at clarifying the relationship between the Treg and mast cells in vitro. We found that addition of BMMCs to the system of T cells with anti-CD3, anti-CD28 and IL-2 resulted in a significant increase in FoxP3 expression. In addition, FoxP3 expression was reduced in the presence of TGF-β1 neutralizing antibody but not IL-4 neutralizing antibody. However, the TGF-β1 neutralizing antibody did not reverse the induction completely. Therefore, T cells can be induced to Tregs by BMMCs partly through a process involving BMMC derived TGF-β1.
MCs are best known for their prominent role in allergic diseases and ‘allergic activation’ through IgE bound to high-affinity IgE-receptor (FceRI) expressed on the MC surface; this is the best-studied mechanism of MC activation [18]. One report showed that activated MCs had the potential to recruit and activate T cells [6]. However, it is unknown whether BMMCs, which have not been activated by IgE, can promote T cells proliferation directly. This study showed that BMMCs cannot promote T cell proliferation, meaning that stimulation signals are needed to activate T cells in the co-culture system.
The role of mast cells in transplant immunity has been debated [19]. Boerma et al. showed that donor heart survival was reduced significantly in mast cell-deficient rats [20]. Lu et al. have suggested that recipient-derived MCs are crucial for Treg-mediated peripheral tolerance [11], indicating that the function of mast cells in suppressing immune responses was related to Tregs. Our study showed that CD4+CD25+ FoxP3+ cells could be induced by BMMCs. This finding may supply a new mechanism suggesting that MCs are crucial for Treg-mediated transplant tolerance [11]. This method may also become a new method for the induction of Tregsin vitro.
Our results showed that the highest percentage of Tregs was found in the highest ratio (2:1) of BMMCs to T cells. TGF-β1 expression in BMMCs was determined in our experimental groups. Jahanyar et al. concluded that mast cell-derived TGF-β may serve as important mediators for Treg activation in allografts [21], and other studies reported that the percentage of Tregs increased with the higher level of added TGF-β1 [22]. Therefore, it seems that the increase of Tregs with a higher ratio of BMMCs may be related to more BMMCs-derived TGF-β1.
Consistent with previous studies, and in order to test whether BMMC-derived TGF-β1 is involved in the generation of Tregs, TGF-β1 neutralizing antibody was added to the co-culture system [4]. The conversion of Tregs was reduced significantly by the TGF-β1 neutralizing antibody, but the TGF-β1 neutralizing antibody could not reverse Treg induction completely. The percentages of Tregs were still higher than control, even with the application of TGF-β1 neutralizing antibody. Whether there were some other mediators derived from BMMCs which also had the potential to induce Tregs is debatable. Metz considered that IL-4 may be related to the suppression function of MC in the immune response [6]. Therefore, IL-4 neutralizing antibody was applied to block the function of IL-4, but there were no significant differences after the application of IL-4 neutralizing antibody. Although this study did not provide direct evidence for BMMCs as the main source of TGF-β1, it suggests that BMMC-derived TGF-β1 is involved in the regulation of Treg cell generation in vitro.
Our experiment concerned mainly the relationship between mast cells and Tregsin vitro. Huang et al. showed that tumour-infiltrating mast cells may promote tumour growth through one way of increasing Treg cells in vivo[23]. This leads us to conclude that perhaps Tregs can be induced by mast cells in vivo. More studies will be conducted to clarify this phenomenon.
In conclusion, our experiments demonstrate that Tregs can be induced by BMMCs in vitro, and secreting TGF-β1 by BMMCs is one of the principal factors for the effect. This finding may provide new evidence that mast cells have the ability to suppress immune responses by way of Treg induction. Furthermore, the study may supply new data for identifying clearly the role of mast cells in immune systems.
Acknowledgments
This work was funded by National Natural Science Foundation of China (no. 30772038, 30671989) and the ‘973’ Program of China (no. 2009CB522407).
Disclosure
The authors have no financial conflict of interest.
References
- 1.Sakaguchi S. Naturally arising CD4+regulatory t cells for immunologic selftolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 2.Long E, Wood KJ. Understanding FOXP3: progress towards achieving transplantation tolerance. Transplantation. 2007;84:459–61. doi: 10.1097/01.tp.0000275424.52998.ad. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4 +CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 4.English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156:149–60. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malbec O, Daëron M. The mast cell IgG receptors and their roles in tissue inflammation. Immunol Rev. 2007;217:206–21. doi: 10.1111/j.1600-065X.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 6.Metz M, Maurer M. Mast cells – key effector cells in immune responses. Trends Immunol. 2007;28:234–41. doi: 10.1016/j.it.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–42. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 8.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–99. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 9.Marone G, Triggiani M, de Paulis AL. Mast cells and basophils: friends as well as foes in bronchial asthma? Trends Immunol. 2005;26:25–31. doi: 10.1016/j.it.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Wasiuk A, de Vries VC, Hartmann K, Roers A, Noelle RJ. Mast cells as regulators of adaptive immunity to tumours. Clin Exp Immunol. 2009;155:140–6. doi: 10.1111/j.1365-2249.2008.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu LF, Lind EF, Gondek DC, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 12.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as ‘tunable’ effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–86. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JM. Rudensky A The role of the transcription factor Foxp3 in the development of regulatory T cells. Immunol Rev. 2006;212:86–98. doi: 10.1111/j.0105-2896.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 15.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced Treg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–74. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cobbold SP, Castejon R, Adams E, et al. Induction of foxp3–regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–10. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 17.Norozian F, Kashyap M, Ramirez CD, et al. TGFbeta1 induces mast cell apoptosis. Exp Hematol. 2006;34:579–87. doi: 10.1016/j.exphem.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. Allergy Clin Immunol. 2006;117:1214–25. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Colvin RB, Dvorak HF. Basophils and mast cells in renal allograft rejection (Letter) Lancet. 1974;1:212. doi: 10.1016/s0140-6736(74)92512-4. [DOI] [PubMed] [Google Scholar]
- 20.Boerma M, Fiser WP, Hoyt G, et al. Influence of mast cells on outcome after heterotopic cardiac transplantation in rats. Transpl Int. 2007;20:256–65. doi: 10.1111/j.1432-2277.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 21.Jahanyar J, Koerner MM, Loebe M, Youker KA, Torre-Amione G, Noon GP. The role of mast cells after solid organ transplantation. Transplantation. 2008;85:1365–71. doi: 10.1097/TP.0b013e31816fc0a3. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105:9331–6. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang B, Lei Z, Zhang GM, et al. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112:1269–79. doi: 10.1182/blood-2008-03-147033. [DOI] [PMC free article] [PubMed] [Google Scholar]


