Abstract
As a result of age-associated thymic atrophy, T cell production declines with age. Some studies suggest that production undergoes an exponential decline starting at birth, while others consider the decline to be in a biphasic manner with a rapid reduction in output occurring before middle age followed by a phase in which output declines at a regular, albeit much slower, rate. Both approaches provide estimations of the time of termination of thymic output, but on the basis of limited amounts of data. We have analysed blood from more than 200 individuals between the ages of 58 and 104 years to determine changes in thymic output using signal-joint T cell receptor excision circles (sjTREC)/T cells as our measure. To reduce any potential geographical or nutritional bias we have obtained samples from five different European countries. Our results reveal that while the absolute number of T cells per microlitre of blood does not change significantly across the age range we tested, the values of sjTREC per microlitre show wide variation and reveal an age-associated decline in thymic output. In addition we show gender differences, with notably higher thymic output in females than males at each decade. More importantly, we noted a significant decline in sjTREC/T cell levels in those more than 90 years of age in both males and females. Our results provide information about the potential end-point for thymic output and suggest that sjTREC analysis may be a biomarker of effective ageing.
Keywords: elderly, mortality, thymic output, thymus TREC, TREC assay
Introduction
Epidemiological surveys, clinical observations and laboratory tests all reveal that the immune system declines with age. Indications of this decline include a poorer response to vaccination [1], a higher prevalence of certain cancers associated with viral infections [2], an increased susceptibility to infections [3] and a higher likelihood of being infected by emerging pathogens than younger individuals [4]. In addition, older individuals often show an increased difficulty in dealing with pathogens which they have overcome previously. Common problems include reactivation of persistent viruses such as herpes zoster [5] or cytomegalovirus [6] and also a disproportionate immune response to the latter [7]. The elderly also experience more problems than younger individuals following the yearly return of influenza and respiratory syncytial virus (RSV) [8]. Infection with influenza in younger individuals is followed normally by a disease limited in its duration to 1–2 weeks, but the consequences of infection in the elderly differ, being more likely to progress to chronic illness and an irreversible loss of physical condition [9].
Because of their central role in orchestrating the immune response, the age-associated changes noted in immune function have been linked to altered functional characteristics of individual T cells and also to modifications occurring in the peripheral T cell pool [10]. It has not been proved formally that changes in T cell function observed with advancing age are completely disconnected from the consequences of modification in the peripheral T cell pool, as events such as proliferation induced by the homeostatic milieu of the ageing organism may contribute to the reduced functional capacity of T cells [11].
A potential driver of age-related changes in the peripheral T cell pool is atrophy of the thymus. A reduction in thymic activity is a feature of ageing in mammals. In humans, fat accumulates in the thymus throughout life [12] reducing the active areas of thymopoiesis, and this contributes to a decline in the output of T cells. Measurement of this decline in previous studies has produced different views on the kinetics of this process. Some studies indicate an exponential decline [13] with T cell output beginning early in life and estimated to terminate at approximately 75 years of age [14]. Others suggest that the thymus atrophies in a biphasic manner [15] with the initial phase beginning early in life, at least as early as the first year and proceeding at a rate of 3% per year until middle age. Thereafter the rate changes to a constant rate of 1% per year, leading to the estimated total loss of thymic tissue by 105 years of age [16,17].
Recent work shows that the reversal of thymic atrophy is a viable option, but the timing of when such a procedure should begin would be critically dependent upon determining the period when thymic output ceases. In order to provide more information about the decrease in thymic output later in life we analysed samples collected from 215 healthy elderly individuals, with ages ranging from 60 to 100 years, and to reduce any bias related to environmental factors and/or lifestyle we obtained samples from participating centres across five European countries (France, Germany, Greece, Italy and Poland)) [18]. We quantified changes in thymic output using signal-joint T cell receptor excision circles (sjTRECs) per T cells measured by real-time polymerase chain reaction (PCR), as described previously [19].
Materials and methods
Sample collection and storage and analysis
Peripheral blood (PB) samples were collected from healthy elderly individuals from participating centres across five European countries (France, Germany, Greece, Italy and Poland) [18]. Informed consent was obtained from healthy adult volunteers, with ages ranging from 58–104 years. Peripheral blood mononuclear cells (PBMC) were isolated and the samples were stored at −140°C until required for analysis. Frozen PBMC were thawed and an aliquot containing 1 × 105 cells stained with phycoerythrin (PE)-conjugated anti-CD3 (BD Bioscience, Oxford, UK) according to the manufacturer's instructions. The T cell measurement was determined using a fluorescence activated cell sorter (FACS)Calibur (Becton Dickinson) flow cytometer. DNA was extracted from the remaining cells using the Puregene DNA purification kit (Flowgen, Ashby de la Zouch, UK). The DNA was stored at −20°C until required for analysis. When the DNA was thawed its concentration was determined by optical density readings using a spectrophotometer and aliquots of 50 ng was removed for use in real-time PCR experiments.
Real-time PCR assessment of sjTREC and albumin
Human sjTREC and albumin (ALB) levels were quantified using real-time PCR performed on the Roche Light Cycler (Roche Diagnostics, Lewes, UK). A PCR reaction mixture containing 50 ng of DNA, 0·5 µM of forward and reverse primers and 2× SYBR Green mix (Qiagen, Crawley, UK) in a final reaction volume of 10 µl, using sterile water. The primer sequences used were sjTREC forward: GGC AGA AAG AGG GCA GCC CTC TCC AAG and reverse: GCC AGC TGC AGG GTT TAG G or ALB forward: CTA TCC GTG GTC CTG AAC CAG TTA TG and reverse: CTC TCC TTC TCA GAA AGT GTG CAT AT, which produced amplicons of 195 base pairs (bp) and 206 bp, respectively. Real-time PCR conditions on the Light Cycler were 95°C for 15 min, followed by 45 cycles at 95°C for 15 s, 61°C for 30 s and 72°C for 20 s (fluorescent acquisition). The albumin reaction was performed as described above, except that the annealing temperature was changed to 60°C. The 195 bp and 206 bp PCR products were identified by melting-point analysis. A standard curve generated from a serial dilution of known concentration of sjTREC or albumin plasmid was used to enable calculation of the number of detectable molecules from the test samples. The copy number of sjTREC and ALB (x) was calculated using the following equations: ysjTREC = −3·468x + 42·09 and yALB = −3·374x + 40·593, where the cycle threshold (Ct) value is substituted as y. A standard concentration of 1 × 104 sjTREC or ALB molecules was included to determine variance between each run and comparability of the sample. All samples were run in duplicate and an average of the result used for statistical analysis. Where Ct values of the duplicates were greater than 1·5 cycles the samples were rerun.
From these readings we obtained a value of sjTREC per 50 ng of DNA. The amount of DNA obtained from the sample of PBMC was known, so we could calculate the number of sjTREC in the PBMC sample. Because sjTREC can be derived only from T cells and we had determined the number of CD3+ T cells by immunophenotyping in the sample, we could ascribe a definite value of sjTREC/T cell to the sample.
Statistical analysis
The results of the descriptive analysis are presented for numerical variables in the form of means ± standard deviation (s.d.) and median for age; sample sizes and percentages calculated for categorical outcomes. Subjects' characteristics and blood sample components were compared with respect to the age group. Statistical tests used for the comparative analysis were chosen according to the type of variable, the sample size under consideration and the number of group compared. Thus, numerical outcomes were tested using the Kruskal–Wallis test (when more than two groups) and the Mann–Whitney test (when two groups); Fisher's exact test was used for categorical outcomes. The level of significance was set at P = −0·05. In addition, linear Pearson correlation coefficients (r) were calculated using a linear regression model to measure the strengths and directions of the linear relationships between the number of TREC and the different age groups. These statistical analyses were performed using StatView (version 5·0; SAS Institute, Inc., Cary, NC, USA). Graph were drawn using Microsoft Office Excel© or GraphPad Prism (version 4·0 for Windows; GraphPad Software, San Diego, CA, USA; http://www.graphpad.com).
Results
Refining sample analysis according to DNA integrity
We were aware that any indication of relative changes in sjTREC values in the samples could be compromised through a loss of integrity of the DNA. In order to ensure equivalence we analysed in excess of 250 samples and selected those for further analysis on the basis of their DNA integrity as determined by the amplifiability of the albumin gene [20,21]. Any sample with a Ct value greater than 24·0 cycles, which approximates to fewer than 1 × 105 albumin molecules, was excluded from further analysis. Of the samples analysed approximately 17% were deemed unacceptable after albumin amplification, therefore we were able to identify 215 samples for further analysis. Surprisingly, a higher than expected proportion of unacceptable samples fell within the 80–89 age group, which is reflected as an apparent gap between 85 and 89 years.
sjTREC values, leucocytes and gender
Analysis of the sjTREC per 105 T cells in our population (Fig. 1) showed a slow decline in their numbers between the 6th and 9th decade of life, with the most pronounced decline seen in those individuals more than 90 years of age. Inter-decade comparison of the sjTREC levels revealed that individuals in their 10th decade had significantly lower levels (P < 0·05) than those obtained from individuals in the 7th, 8th and 9th decades (P-values of 0·0002, 0·0004 and < 0·0001, respectively). Moreover, samples from these earlier decades showed a wide range of values (see Table 1).
Fig. 1.
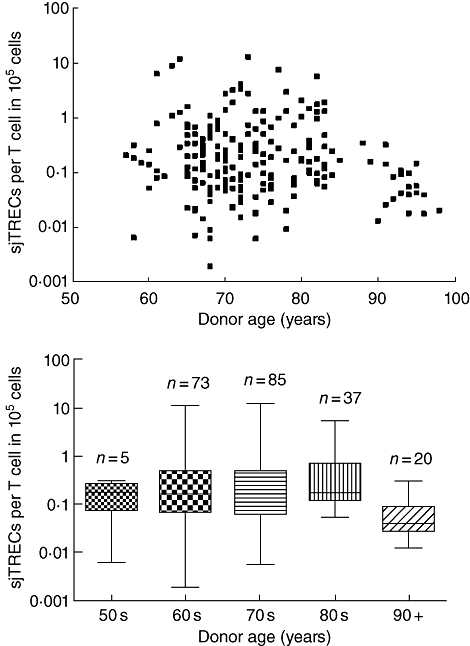
Age-related changes in T cell receptor excision circles (TRECs) per 105 T cells. Each dot represents an individual measurement.
Table 1.
Leucocytes, T cell and T cell receptor excision circle (TREC) values.
| 7th decade (age 60–69) n = 73 | 8th decade (age 70–79) n = 85 | 9th decade (age 80–89) n = 37 | 10th decade (age > 90) n = 20 | |
|---|---|---|---|---|
| Age (years) | ||||
| Mean ± s.d. | 66·18 ± 2·43 | 73·75 ± 2·80 | 82·22 ± 1·99 | 93·50 ± 2·01 |
| Median | 67 | 74 | 82 | 93·5 |
| Gender | ||||
| Female % (n) | 49·0% (36) | 48·2% (41) | 54·0% (20) | 60·0% (12) |
| Leucocytes (no./µl) | ||||
| Mean ± s.d. | 6 069 ± 1 572 | 6 319 ± 1 725 | 6 732 ± 1 527 | 6 338 ± 1 685 |
| T cells (no./µl) | ||||
| Mean ± s.d. | 1 320 ± 502 | 1 211 ± 471 | 1 383 ± 566 | 1 064 ± 407 |
| TRECs (no./ml) | ||||
| Mean ± s.d. | 8·34 ± 22·09* | 7·73 ± 16·37*§ | 9·38 ± 12·63* | 0·60 ± 0·44 |
| Total TRECs in blood† | 41 707 | 38 653 | 46 927 | 3 008 |
| Total TRECs in body+ | 2 085 364 | 1 932 671 | 2 346 388 | 150 400 |
Indicates a significant difference when compared to the 10th decade group (P < 0·05).
Extrapolation of total TRECs in the blood based on 5 litres circulation blood volume.
Extrapolation of TREC numbers in the body based on 2% of T cell pool in the circulating blood [26].
Indicates a significant difference in TREC numbers observed between males and females within this age range (P < 0·05). s.d.: standard deviation; no.; number.
Because of concerns that these results were due to changes either in the number of leucocytes or the number of CD3+ T cells in the blood of our donors [22] we analysed both of these parameters. Comparative analysis revealed no significant change across the age range (see Table 1), either in the number of leucocytes (P > 0·05) or in the absolute number of T cells (P > 0·05) as depicted in Fig. 2.
Fig. 2.
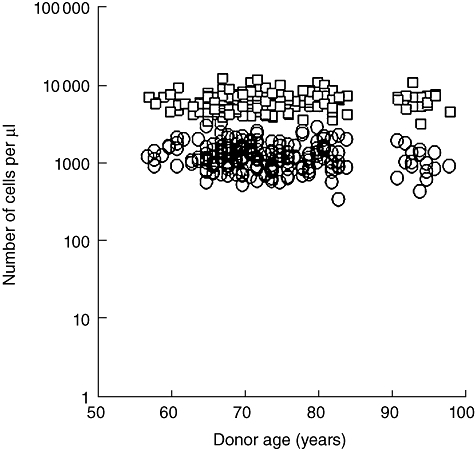
Number of leucocytes (□) and CD3+ T cells (○) per microlitre of blood at different ages.
Previous work has shown differences in sjTREC levels due to gender [23] The sex ratio measured in the present sample was near to 1, with approximately 52% (113 of 215) being females. In Fig. 3 the overall decline seen in both males and females highlights that females had higher levels of detectable sjTREC per 105 T cells compared to males at all age groups. However, this difference was not significant across the entire age range, but only between the males and females sampled in the 70–79 age range (P < 0·03).
Fig. 3.
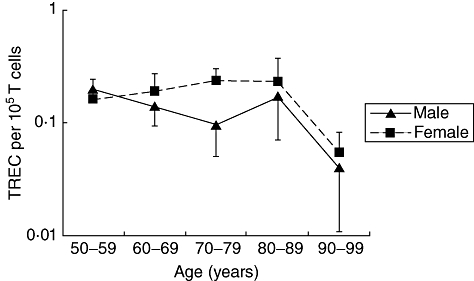
Age-related changes in T cell receptor excision circles (TRECs) per 105 T cells according to gender.
sjTREC distribution during separate decades
It is common to report T cell values as the number of T cells/ml and use this value to set defined ranges and differences between ages [24]. In order to normalize the values with respect to T cells numbers in the blood we calculated the values of the number of sjTREC+ cells per ml of blood and then compared this with the values we obtained for leucocyte numbers and T cell numbers in the 7th–10th decades (Table 1). The results confirm the maintenance of both leucocyte and T cell numbers in the blood over the entire age range and also provide further confirmation of the degree of variation in the numbers of sjTREC+ cells. Interestingly, there is an observable convergence of the overall spread of the sjTREC levels with advancing age in the decades analysed. The standard deviations of the values progress from almost three times the average value in the 7th decade to twice the value in the next decade to almost equal to the value in the 9th decade. During these decades the average sjTREC/ml remains fairly steady between the ages of 60 and 89 at about 0·0006% of the T cells.
The greatest change occurs in the 10th decade, and the degree of change is understood more easily if we calculate changes within the T cell pool as a whole. To do this we need to assume that each sjTREC is present as a single entity in a T cell (i.e. no doubles), that the blood volume in these individuals is close to average at 5 litres [25] and that approximately 2% of the total T cell pool resides within the blood [26]. From this we calculate that the average individual in their 9th decade has 2·35 × 106 sjTRECs in their T cell pool and that this value drops to 1·5 × 105 in the 10th decade. This is a dilution factor of almost fivefold without a comparative change in the overall T cell numbers.
Using a linear regression model, further analysis was performed of the observed decline in sjTREC level as a function of age. Table 2 highlights the relationship between sjTREC levels and increasing age. Across the entire age range no significant correlation was observed; however, as the transition is made from the 8th to 9th decades a significant correlation coefficient is seen of r = −0·285 (P = 0·05), progressing to −0·463 (P = 0·02) by the 10th decade.
Table 2.
Pearson correlation coefficients (r) calculated by regression linear model describing the relationship between the number of T cell receptor excision circles (TRECs)/105 T cells and subjects' age
| Age group (years) | Sample size (n) | Correlation coefficient (r)* | P-value† |
|---|---|---|---|
| 7th to 10th decades | 215 | −0·142 | n.s. |
| 7th decade | 73 | −0·109 | n.s. |
| 8th decade | 85 | −0·0554 | n.s. |
| 9th decade | 37 | −0·442 | 0·03 |
| 10th decade | 20 | −0·497 | 0·02 |
| 7th and 8th decades | 158 | −0·0989 | n.s. |
| 7th, 8th and 9th decades | 195 | −0·0946 | n.s. |
| 8th and 9th decades | 122 | −0·285 | 0·05 |
| 8th, 9th and 10th decades | 142 | −0·382 | 0·04 |
| 9th and 10th decades | 57 | −0·463 | 0·02 |
r: Expresses the degree that, on an average, two variables change correspondingly. The r-value is a number between −1 and 1; r-value: 0 indicating there is a random, non-linear relationship between the two variables. Positive and negative signs indicate the direction of a linear relationship between two variables.
P-value: the probability that r being not different from zero. If it is lower than < 0·05 the correlation coefficient calculated is statistically significant. n.s.: not statistically significant (P-value > 0·05).
Discussion
We have analysed thymic output through following the change in the sjTREC values in the peripheral blood CD3+ T cells of more than 200 individuals from five different European countries who were within the age range 60–100 years. Our results provide information about the potential end-point for thymic output and also provide a suggestion that sjTREC analysis may prove to be a biomarker of ageing. The observed convergence of the sample heterogeneity in the sjTREC levels with increasing age raises a number of interesting possibilities. First, are low sjTREC measurements reflective of an individuals immunosenescence status; if so, are the individuals in the lower left quadrant of Fig. 4 (low sjTREC level at younger age) at a more advanced stage of immunosenescence? The converse argument could also be inferred for individuals with the highest sjTREC levels (upper left quadrant). These individuals may therefore be more likely to progress to become the long-lived healthy individuals observed in the low right quadrant. This concept lends itself to the argument that immunosenescence is not merely a measurement of chronological age, but points towards immune exhaustion arising at different ages. The downward trajectory of an individual's thymic output profile over time has been demonstrated previously by Kilpatrick et al. [27] and could be considered as part of longitudinal studies similar to the Swedish OCTA and NONA studies [28,29] to investigate further the potential role of sjTREC as predictive markers of ageing.
Fig. 4.
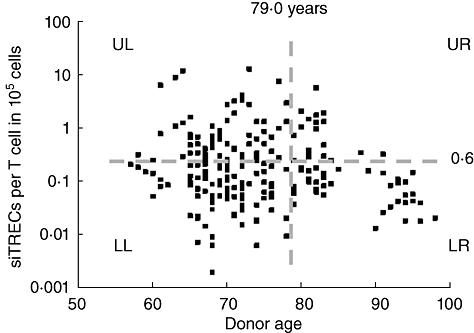
Signal-joint T cell receptor excision circles (sjTRECs) as a biomarker of immunosenescence. Annotated diagram of the age-related changes observed in sjTREC measurements. The dashed horizontal grey line indicates the median sjTREC per 105 T cells in the sample population. The dashed vertical grey line indicates the average life expectancy across the study population (79·0 years). UL, LL, UR, LR refer to the different quadrants formed by the bisection of the data horizontal and vertical lines.
Age-associated decline in immune function can be demonstrated clinically by changes in the prevalence of infectious disease within the elderly and can be evaluated in laboratory tests by the decreased functional capability of lymphocytes [30]. Some of this functional decline may be attributed to the accumulation of CD28- lymphocytes, a population which may contain senescent cells whose impact on immune function may not be benign [31–33]. Such changes are preceded by a measurable age-related decline in the output of αβ+ T cells from the thymus to the naive T cell pool which has been reported in chickens [34], rats [35], mice [36] primates [37] and man [13]. Recent thymic emigrants enter the naive T cell pool where they have a finite lifespan, and this combination of a limited lifespan, reduced thymic output and recruitment into activated and memory T cell pools, contribute to the reduction in the naive T cell pool seen with age.
Current estimations on the timing of cessation of thymic function are imprecise, because they have been derived previously using histological analysis of the thymus combined with phenotypic data on peripheral T cell populations [17,38] and the clear and unambiguous identification of naive T cells in older individuals is difficult [39]. Other means of resolving the issue have been to extrapolate from TREC data derived from studies where the age range was skewed towards younger individuals [14,40,41]. In our study we have looked at sjTREC values in the blood of more than 200 individuals from five different European countries, and our results suggest that between 55 and the mid-80s there appears to be a constant and relatively stable decline in thymic output, which is followed by a significant decline in the 10th decade. Because of the broad distribution area from which the samples were obtained we can discount localized influences, including diet and effects due to pockets of infection causing proliferation in the peripheral T cell pool and subsequent dilution of the sjTREC+ cells. Within the age range analysed there was an apparent gap between the ages 85 and 89; this may be reflective of a poorer quality and smaller proportion of samples collected in this 9th decade. In our study we have seen no significant decline in T cell numbers with age, discounting this as an influential factor, and we have further discounted the effects of gender differences.
Proliferation could contribute towards the differences seen in the 10th decade, although the derivation of the samples from several countries of origin should ameliorate the effects of infection, which may be geographically limited. However, age is also associated with greater proliferation within naive populations [42,43]. While this could also contribute to decline between the 9th and 10th decades it would seem unlikely to account for all of it, as the decline from a value of 2·35 × 106 to 1·5 × 105 would require all the T cells in the body undergoing more than four divisions. The decline could also be due to the loss of the sjTREC from the nucleus due to degradation of the DNA. However, if this occurs we would expect that it should occur at the same rate throughout life. While we cannot resolve whether the decline in thymic output over the entire lifespan is either exponential, biphasic or multiphasic, we have observed a dramatic and precipitous decline in TREC levels starting in the 9th decade. Comparison of the correlation coefficients obtained between the ages of 60–80, 80–90 and those greater than 90 years clearly shows a pronounced change in the rate of decline (Table 2). Despite the apparent discordance with the mean sjTREC levels in Table 1, which indicates an abrupt decline in the 10th decade, both results support the underlying argument that a significant decrease in sjTREC levels is evident by the 10th decade. The possible influences of limited data between the ages of 85–89 years, sample size and mean effects means the precise timing at which the rate declines cannot be calculated. However, it is suggestive that these findings are not attributable to outliers within the sample population. We consider that this may be due mainly to thymic output undergoing a severe decline in the mid-80s to the early 90s years. Such an explanation would also fit with the results from a recent study, which showed that 21 of 25 centenarians had undetectable sjTREC levels [44].
Acknowledgments
This project was funded by the EU (Zincage contract no. FOOD-CT-2003-506850). The authors would like to thank all the Zincage partners for providing samples and support throughout this project, in particular Dr George Dedousis from Greece, Professor Lothar Rink from Germany, Professors Tamas Fulop and George Herbein from Canada and France, Dr Jolanta Jajte from Poland and Professors Daniela Monti and Eugenio Mocchegiani from Italy. We would also like to extend our gratitude to all the healthy elderly volunteers from the different countries for agreeing to participate in this study.
Disclosure
None of the authors has any potential financial conflict of interest related to this manuscript.
References
- 1.Looney RJ, Hasan MS, Coffin D, et al. Hepatitis B immunization of healthy elderly adults: relationship between naive CD4+ T cells and primary immune response and evaluation of GM-CSF as an adjuvant. J Clin Immunol. 2001;21:30–6. doi: 10.1023/a:1006736931381. [DOI] [PubMed] [Google Scholar]
- 2.Shimoyama Y, Oyama T, Asano N, et al. Senile Epstein–Barr virus-associated B-cell lymphoproliferative disorders: a mini review. J Clin Exp Hematop. 2006;46:1–4. doi: 10.3960/jslrt.46.1. [DOI] [PubMed] [Google Scholar]
- 3.High KP, Bradley SF, Gravenstein S, et al. Clinical practice guideline for the evaluation of fever and infection in older adult residents of long-term care facilities: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:149–71. doi: 10.1086/595683. [DOI] [PubMed] [Google Scholar]
- 4.Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807–14. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 5.Schmader K. Herpes zoster in older adults. Clin Infect Dis. 2001;32:1481–6. doi: 10.1086/320169. [DOI] [PubMed] [Google Scholar]
- 6.Pawelec G, Akbar A, Caruso C, Effros R, Grubeck-Loebenstein B, Wikby A. Is immunosenescence infectious? Trends Immunol. 2004;25:406–10. doi: 10.1016/j.it.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Moss P, Khan N. CD8(+) T-cell immunity to cytomegalovirus. Hum Immunol. 2004;65:456–64. doi: 10.1016/j.humimm.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 9.McElhaney JE. The unmet need in the elderly: designing new influenza vaccines for older adults. Vaccine. 2005;23(Suppl 1):S10–25. doi: 10.1016/j.vaccine.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Aspinall R, Pitts D, Lapenna A, Mitchell W. Immunity in the elderly: the role of the thymus. J Comp Pathol. 2010;142(Suppl. 1):S111–15. doi: 10.1016/j.jcpa.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Musci M, Weng Y, Hubler M, et al. Predictors of early mortality in patients with active infective native or prosthetic aortic root endocarditis undergoing homograft aortic root replacement. Clin Res Cardiol. 2009;98:443–50. doi: 10.1007/s00392-009-0015-3. [DOI] [PubMed] [Google Scholar]
- 12.Kendall MD, Johnson HR, Singh J. The weight of the human thymus gland at necropsy. J Anat. 1980;131:483–97. [PMC free article] [PubMed] [Google Scholar]
- 13.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 14.Loeffler J, Bauer R, Hebart H, et al. Quantification of T-cell receptor excision circle DNA using fluorescence resonance energy transfer and the LightCycler system. J Immunol Methods. 2002;271:167–75. doi: 10.1016/s0022-1759(02)00337-x. [DOI] [PubMed] [Google Scholar]
- 15.Ritter MA, Palmer DB. The human thymic microenvironment: new approaches to functional analysis. Semin Immunol. 1999;11:13–21. doi: 10.1006/smim.1998.0148. [DOI] [PubMed] [Google Scholar]
- 16.Steinmann GG. Changes in the human thymus during aging. Curr Top Pathol. 1986;75:43–88. doi: 10.1007/978-3-642-82480-7_2. [DOI] [PubMed] [Google Scholar]
- 17.George AJ, Ritter MA. Thymic involution with ageing: obsolescence or good housekeeping? Immunol Today. 1996;17:267–72. doi: 10.1016/0167-5699(96)80543-3. [DOI] [PubMed] [Google Scholar]
- 18.Mocchegiani E, Burkle A, Fulop T. Zinc and ageing (ZINCAGE Project) Exp Gerontol. 2008;43:361–2. doi: 10.1016/j.exger.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Pido-Lopez J, Imami N, Andrew D, Aspinall R. Molecular quantitation of thymic output in mice and the effect of IL-7. Eur J Immunol. 2002;32:2827–36. doi: 10.1002/1521-4141(2002010)32:10<2827::AID-IMMU2827>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 20.Moppett J, van der Velden VH, Wijkhuijs AJ, Hancock J, van Dongen JJ, Goulden N, Inhibition affecting RQ–PCR-based assessment of minimal residual disease in acute lymphoblastic leukemia: reversal by addition of bovine serum albumin. Leukemia. 2003;17:268–70. doi: 10.1038/sj.leu.2402751. [DOI] [PubMed] [Google Scholar]
- 21.Dessars B, Heimann P, Swillens S, El Housni H. Limitations and practical procedure in BclII-Ig heavy chain gene rearrangement real-time quantitative polymerase chain reaction. J Mol Diagn. 2006;8:133–6. doi: 10.2353/jmoldx.2006.040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izaks GJ, Remarque EJ, Becker SV, Westendorp RG. Lymphocyte count and mortality risk in older persons. The Leiden 85-Plus Study. J Am Geriatr Soc. 2003;51:1461–5. doi: 10.1046/j.1532-5415.2003.51467.x. [DOI] [PubMed] [Google Scholar]
- 23.Pido-Lopez J, Imami N, Aspinall R. Both age and gender affect thymic output: more recent thymic migrants in females than males as they age. Clin Exp Immunol. 2001;125:409–13. doi: 10.1046/j.1365-2249.2001.01640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulstaert F, Hannet I, Deneys V, et al. Age-related changes in human blood lymphocyte subpopulations. II. Varying kinetics of percentage and absolute count measurements. Clin Immunol Immunopathol. 1994;70:152–8. doi: 10.1006/clin.1994.1023. [DOI] [PubMed] [Google Scholar]
- 25.Warrell DA, Cox TM, Firth JD, Benz EJ. Oxford textbook of medicine. Oxford: Oxford University Press; 2006. [Google Scholar]
- 26.Trepel F. Number and distribution of lymphocytes in man. A critical analysis. Klin Wochenschr. 1974;52:511–15. doi: 10.1007/BF01468720. [DOI] [PubMed] [Google Scholar]
- 27.Kilpatrick RD, Rickabaugh T, Hultin LE, et al. Homeostasis of the naive CD4+ T cell compartment during aging. J Immunol. 2008;180:1499–507. doi: 10.4049/jimmunol.180.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wikby A, Nilsson BO, Forsey R, et al. The immune risk phenotype is associated with IL-6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech Ageing Dev. 2006;127:695–704. doi: 10.1016/j.mad.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Strindhall J, Nilsson BO, Lofgren S, et al. No immune risk profile among individuals who reach 100 years of age: findings from the Swedish NONA immune longitudinal study. Exp Gerontol. 2007;42:753–61. doi: 10.1016/j.exger.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Pawelec G, Barnett Y, Forsey R, et al. T cells and aging, January 2002 update. Front Biosci. 2002;7:d1056–183. doi: 10.2741/a831. [DOI] [PubMed] [Google Scholar]
- 31.Saurwein-Teissl M, Lung TL, Marx F, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–9. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 32.Weng NP, Akbar AN, Goronzy J. CD28(–) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–12. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75:12182–7. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong F, Chen CH, Cooper MD. Thymic function can be accurately monitored by the level of recent T cell emigrants in the circulation. Immunity. 1998;8:97–104. doi: 10.1016/s1074-7613(00)80462-8. [DOI] [PubMed] [Google Scholar]
- 35.Hosseinzadeh H, Goldschneider I. Recent thymic emigrants in the rat express a unique antigenic phenotype and undergo post-thymic maturation in peripheral lymphoid tissues. J Immunol. 1993;150:1670–9. [PubMed] [Google Scholar]
- 36.Scollay RG, Butcher EC, Weissman IL. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210–18. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 37.Sodora DL, Douek DC, Silvestri G, et al. Quantification of thymic function by measuring T cell receptor excision circles within peripheral blood and lymphoid tissues in monkeys. Eur J Immunol. 2000;30:1145–53. doi: 10.1002/(SICI)1521-4141(200004)30:4<1145::AID-IMMU1145>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 38.Bertho JM, Demarquay C, Moulian N, Van Der Meeren A, Berrih-Aknin S, Gourmelon P. Phenotypic and immunohistological analyses of the human adult thymus: evidence for an active thymus during adult life. Cell Immunol. 1997;179:30–40. doi: 10.1006/cimm.1997.1148. [DOI] [PubMed] [Google Scholar]
- 39.Pfister G, Weiskopf D, Lazuardi L, et al. Naive T cells in the elderly: are they still there? Ann NY Acad Sci. 2006;1067:152–7. doi: 10.1196/annals.1354.018. [DOI] [PubMed] [Google Scholar]
- 40.Naylor K, Li G, Vallejo AN, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–52. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Lewin SR, Markowitz M, et al. Measuring recent thymic emigrants in blood of normal and HIV-1-infected individuals before and after effective therapy. J Exp Med. 1999;190:725–32. doi: 10.1084/jem.190.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cicin-Sain L, Messaoudi I, Park B, et al. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc Natl Acad Sci USA. 2007;104:19960–5. doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmed M, Lanzer KG, Yager EJ, Adams PS, Johnson LL, Blackman MA. Clonal expansions and loss of receptor diversity in the naive CD8 T cell repertoire of aged mice. J Immunol. 2009;182:784–92. doi: 10.4049/jimmunol.182.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nasi M, Troiano L, Lugli E, et al. Thymic output and functionality of the IL-7/IL-7 receptor system in centenarians: implications for the neolymphogenesis at the limit of human life. Aging Cell. 2006;5:167–75. doi: 10.1111/j.1474-9726.2006.00204.x. [DOI] [PubMed] [Google Scholar]


