Abstract
The immune receptor expressed on myeloid cells 1 (IREM-1) has been known to regulate the activities of myeloid cells through its immunoreceptor tyrosine-based inhibition motifs (ITIMs) in its intracellular region. In order to investigate its effect on macrophage activation, a human macrophage cell line (THP-1) was tested after stimulation of its membrane-bound form of B cell activation factor (BAFF), which has been shown to modulate inflammatory activities through induction of proinflammatory mediator expression and suppression of phagocytosis. IREM-1-specific monoclonal antibodies detected the expression of high levels of IREM-1 in THP-1 cells. Cross-linking of IREM-1 with these antibodies resulted in the blockage of the BAFF-mediated expression of interleukin (IL)-8 and matrix metalloproteinase (MMP)-9 through inhibition of the activation of extracellular regulated kinase (ERK) and phosphorylation/degradation of IκB. Furthermore, cross-linking of IREM-1 also reversed the BAFF-mediated inhibition of phagocytosis. In order to demonstrate the role of ITIM in the IREM-1-mediated suppression of BAFF signalling, a decapeptide containing YADL (an ITIM in IREM-1) was fused with HIV–TAT48–57 which was required for the internalization of the synthetic polypeptide (TAT–YADL). TAT–YADL, but not control peptides, recapitulated the effect of the anti-IREM-1 monoclonal antibody. These observations indicate that IREM-1 exerted its inhibitory effect on BAFF-medicated signalling through ITIM-mediated regulation of ERK activities in THP-1 cells.
Keywords: BAFF, inflammation, IREM-1, macrophage, signalling transduction
Introduction
B cell activation factor (BAFF) (TALl-1, THANK, BlyS, TNFSF13b, zTNF-4) is a member of the tumour necrosis factor superfamily (TNF SF) and is required for the regulation of immune responses such as B cell survival and the regulation of inflammatory responses [1]. The expression of both the membrane-bound and the soluble form of BAFF has been detected in various cells lineages, including myeloid cells and stromal cells [2–4]. BAFF is known to interact with three counterparts, transmembrane activator (TACI) and calcium-modulating cyclophilin ligand (CAML) interactor, BCMA (B cell maturation antigen) and BAFF-R (BAFF receptor, BR3), which can be found in lymphoid and myeloid cells [3,5]. BAFF-R is known to be able to recognize only BAFF, while TACI and BCMA can recognize both BAFF and its close relative, a proliferation-inducing ligand (APRIL). Recently, a membrane-bound form of BAFF has been demonstrated to be stimulated by its interaction with TACI or treatment with BAFF-specific monoclonal antibody (mAb) [6]. Activated BAFF initiated two signalling pathways in THP-1 cells, one of which was mediated through extracellular regulated kinase (ERK) and nuclear factor (NF)-κB leading to the induction of proinflammatory responses, while another pathway leads to the inhibition of PI3K activity, resulting in the suppression of phagocytic activities [6].
The immune receptor expressed on myeloid cells 1 (IREM-1, CD300F, IgSF13, CMRF-35A5), a member of the CD300 family of receptors, contains immunoreceptor tyrosine-based inhibition motifs (ITIMs) in its intracellular region. Cross-linking of IREM-1 inhibited FcεR-induced activation of mast cells through interaction between phosphotyrosine residue 205 and Src homology 2 (SH2) domains of SHP-1 [7]. Substitution of the tyrosine 205 residue with phenylalanine abolished its inhibitory activities [8]. Similarly, CMRF-35-like molecule-1 (CLM-1, LMIR3, MAIR-V, CD300LF), the mouse orthologue of IREM-1, has also been shown to be involved in the regulation of apoptosis [9] and the inhibition of inflammatory activation in myeloid cells [10]. In contrast, Izawa et al. reported that cross-linking CLM-1 augmented lipopolysaccharide (LPS) response in mast cells through its association with FcRγ[11]. These results demonstrate the complex nature of regulations that are mediated by IREM-1 and emphasize the requirement for detailed analysis of its function. Currently, the natural ligand for IREM-1 is not known.
Two IREM-1-specific monoclonal antibodies were generated though the immunization of mice with a fusion protein containing glutathione-S-transferase (GST) and the extracellular region of human IREM-1. Interestingly, the stimulation of IREM-1 with these antibodies resulted in the inhibition of both the BAFF-induced production of proinflammatory mediators and BAFF-mediated suppression of phagocytosis in THP-1 cells. Analysis utilizing a fusion protein containing HIV–TAT48–57[12] and the YADL sequence of the IREM-1 ITIM domain (TAT–YADL) and its control peptide containing Tyr to Phe substitution (TAT–FADL) revealed that the ITIM motif was responsible for the inhibitory effect of IREM-1 in the BAFF-mediated regulation of inflammatory processes.
Materials and methods
Reagents
Monoclonal antibody (mAb) against BAFF (clone 148725) was purchased from R&D Systems (Minneapolis, MN, USA); fusion protein containing an extracellular domain of hTACI and the Fc portion of human IgG (TACI:Fc) came from Alexis (San Diego, CA, USA); polyclonal antibodies for ERK, phospho-ERK were obtained from Calbiochem International Inc. (La Jolla, CA, USA); rabbit polyclonal antibody to IκB and mAb to phospho-IκB (Ser32/36) (5A5) were from Cell Signaling (Danvers, MA, USA); mouse IgG2a was from BD Pharmingen (San Jose, CA, USA); and mAb against IREM-1 (clones UP-D2) was purchased from Biolegend (San Diego, CA, USA). The human monocytic leukaemia cell lines, THP-1, and the human embryonic kidney cell line, 293T, were obtained from the American Type Culture Collection (Rockville, MD, USA). Fusion proteins containing YADL (or FADL) and HIV–TAT48–57, and its negative control containing only HIV–TAT was custom-designed and synthesized by Peptron Inc. (Daejeon, Korea). Inhibitor of SHP-1 [protein tyrosine phosphatase (PTP) inhibitor III] was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). The sequences for TAT and TAT–YADL are GRKKRRQRRR and GRKKRRQRRRGDLCYADLTL, respectively.
Cloning of IREM-1 and the generation of anti-IREM-1 mAb
For the cloning of full-length human IREM-1, reverse transcription–polymerase chain reaction (RT–PCR) analysis was performed with mRNA isolated from THP-1 cells using forward primer (5-GAGAAGATGCCCCTGCTG-3′) and reverse primer (5′-TGGAGTGCAGGCTAAGGC-3′) to obtain the 890 base pairs (bp) product which was cloned into pcDNA3·1 expression vector and transformed into the DH5α strain of Escherichia coli for mass production. For the generation of GST–IREM-1 fusion protein, the 353 bp extracellular region of IREM-1 was PCR amplified using a forward primer containing BamH1 linker (5′-ATGGGATCCGGTCCAACAACAGTGAATGG-3′) and a reverse primer containing EcoRI linker (5′-CGGAATTCGTTTCTTCTTGGGTGACTGG-3′). PCR products were then inserted into the BamH1/EcoRI site of the pGEX-6P-1 vector in such a way that the gene encoding GST was followed by the DNA fragment representing the IREM-1 extracellular region. The expression vector was transformed into the BL21(DE3)pLyS strain of E. coli (BioDynamics Laboratory Inc., Tokyo, Japan), which was then treated with isopropyl β-D-thiogalactopranoside (IPTG) for the induction of GST-IREM-1 fusion protein. The fusion protein was isolated from bacterial culture lysate using glutathione sepharose 4 Fast Flow (Amersham, Uppsala, Sweden), following the protocol provided by the manufacturer. For immunization, GST–IREM-1 fusion proteins (100 µg/mouse) were mixed with 100 µl of Freund's complete adjuvant and injected into six female BALB/c mice. The mice were booster injected three times at 2-week intervals using the same amount of antigen with incomplete Freund's adjuvant. A final intravenous injection of 100 µg antigen per mouse without adjuvant was performed 3 days before killing. Spleen cells isolated from the mice were fused with SP2/o–Ag-14 cells and hybridomas were selected in hypoxanthine aminopterin thymidine (HAT) medium under limiting dilution cloning conditions.
Transient transfection and flow cytometry
The 293T cells were seeded (5 × 106 cells/well) in six-well plates and incubated overnight before transfection with 2 µg of IREM-1 expression construct or pcDNA3·1 (empty vector control) that had been mixed with 8 µl of Superfect transfect reagent (Qiagen, Valencia, CA, USA). Twenty-four h after transfection, flow cytometry analysis was performed using fluorescence activated cell sorter (FACS)Calibur (Becton-Dickinson, Mountain View, CA, USA). The cells (5 × 105) were pelleted and incubated with 0·3 µg of anti-IREM-1 mAb in 30 µl of FACS solution [phosphate-buffered saline (PBS) containing 0·5% bovine serum albumin (BSA) and 0·1% sodium azide] for 20 min on ice. For background fluorescence, the cells were stained with an isotype-matching control antibody. The cells were then washed and incubated with 0·3 µg of fluorescein isothiocyanate (FITC)-labelled goat anti-mouse immunoglobulin (Ig)G in 30 µl of FACS solution. The fluorescence profiles of 2 × 104 cells were collected and analysed.
Phagocytosis
Zymosan opsonization and the measurement of phagocytic activity were performed as described previously. Briefly, zymosan tagged with Alexa Fluor 594 (Invitrogen, Carlsbad, CA, USA) was incubated with one-tenth volume of zymosan A opsonizing reagent (Invitrogen, Eugene, OR, USA) at 37°C for 1 h. THP-1 cells were pretreated for 30 min with 1 µg/ml of anti-IREM-1 mAb or 5 µM of TAT peptides and then incubated with 30 µg/ml of opsonized-zymosan-594 for 3 h. The percentage of cells that had phagocytozed zymosan was measured using flow cytometry analysis as described above.
Gelatin zymogram, enzyme-linked immunosorbent assay (ELISA) and Western blot analysis
The cells were activated by adding antibodies and fusion proteins to the medium containing 1 × 106/ml THP-1 cells in RPMI-1640 supplemented with 0·1% fetal bovine serum (FBS). The levels of interleukin (IL)-8 in the supernatants were measured by a sandwich ELISA (R&D Systems, Inc., MN, USA). The detection limit was <10 pg/ml. The matrix metalloproteases (MMP) activity in the culture supernatant was determined via substrate gel electrophoresis, as described previously [13]. For the Western blot analysis, cell lysates were obtained at various time-points after activation and analysis was performed as described previously [13,14].
Immunofluorescence
For the detection of TAT or TAT–YADL, THP-1 cells (2 × 105) were incubated with 10 µM of peptides for 30 min and then washed in PBS and resuspended in 10 µl of 4% formaldehyde in distilled water for fixation. The cells were then placed onto a slide glass and covered with a cover glass, in order to spread the cells. The cells were then permeabilized with 1% Triton in PBS for 10 min, incubated with 1 µg/ml anti-TAT mAb (clone ab63957; Abcam, Cambridge, UK) in PBS containing 3% BSA at 37°C for 45 min, washed, and incubated with Alexa Fluor 488-labelled goat anti-mouse antibody (A-21121, Molecular Probes) (10 µg/ml) at 37°C for 45 min, washed again, and then mounted in a 1:1 ratio mixture of Xylene and Malinol (Muto Pure Chemicals, Tokyo, Japan).
Results and discussion
New anti-IREM-1 mAbs detected high expression levels of IREM-1 in THP-1 cells
In order to generate IREM-1-specific mAb, a DNA fragment representing the extracellular part was cloned to generate GST fusion protein which was immunized into C57BL mice. Two hybridoma clones (2-3·6D and 2-1·7B) were screened to have IREM-1 specificity. Both isotypes of these mAbs were found to be IgG2a (data not shown). Flow cytometry analysis of 293T cells that had been transfected with IREM-1 expression vector showed that both 2-3·6D and 2-1·7B stained IREM-1-transfected cells, but not the empty vector-transfected cells (Fig. 1a). THP-1 cells, which had been shown to be expressing IREM-1 through RT–PCR [15], were stained by both 2-3·6D and 2-1·7B (Fig. 1b). The expression of IREM-1 by THP-1 is in agreement with previous reports which showed that myeloid cells express IREM-1 on the cell surface [7,16].
Fig. 1.
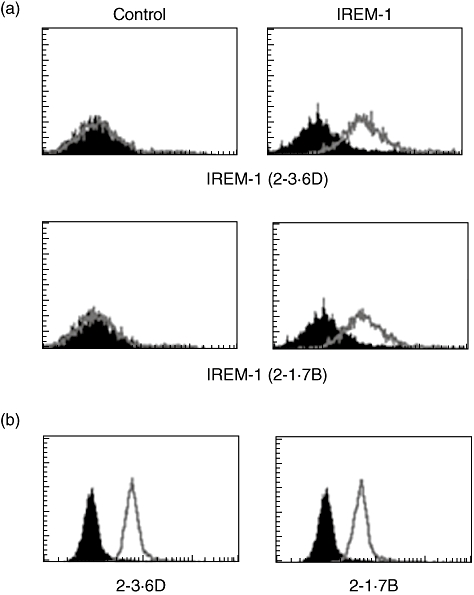
Immune receptor expressed on myeloid cells 1 (IREM-1)-specific monoclonal antibody (mAb) detected the expression of IREM-1 in human monocytic cell line (THP)-1 cells. (a) 293T cells were transiently transfected with empty vector (control) or IREM-1 expression vector. These cells were stained with 2-3·6D or 2-1·7B for flow cytometry. (b) THP-1 cells were stained with 2-3·6D or 2-1·7B for flow cytometry. Lines represent the fluorescence profiles of anti-IREM-1 staining and black areas represent the fluorescence profiles of background staining, which were stained with isotype-matching mouse immunoglobulin (Ig)G. These experiments were repeated more than three times with essentially the same results.
Cross-linking IREM-1 with mAb resulted in the inhibition of BAFF-mediated expression of IL-8 and MMP-9
Previous analysis of THP-1 cells demonstrated that stimulation of the membrane-bound form of BAFF using either BAFF-specific mAb or TACI:Fc fusion protein generates an activation signal through ERK and NF-κB, which results in the production of proinflammatory molecules such as IL-8 and MMP-9 [6]. Pretreatment of THP-1 cells with anti-IREM-1 mAb blocked TACI:Fc-induced expression of both IL-8 and MMP-9 (Fig. 2a). The activity of MMP-9 produced in the culture supernatant was analysed using gelatin zymogram and IL-8 concentrations were measured using ELISA. Because TACI has been known to interact with both BAFF and APRIL, a molecule with the highest homology to BAFF, it is not clear whether treatment with TACI:Fc stimulated BAFF or APRIL. In order to ensure that the signalling was through BAFF, anti-BAFF mAb was used to stimulate the BAFF on the surface of THP-1 cells. As shown in Fig. 2b, treatment with anti-BAFF mAb resulted in the induction of IL-8 and MMP-9 which was blocked by pretreatment with anti-IREM-1 mAb.
Fig. 2.
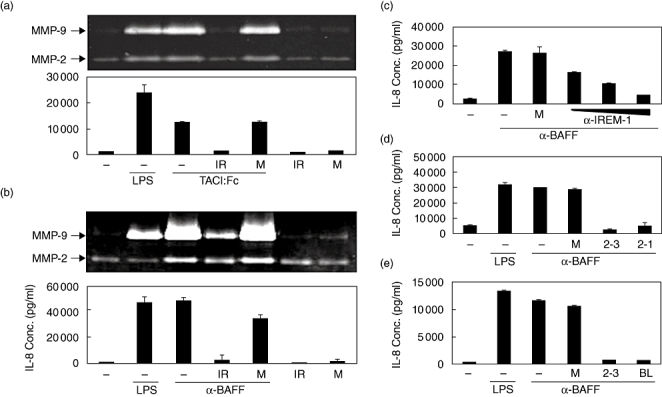
Stimulation of immune receptor expressed on myeloid cells 1 (IREM-1) blocks B cell activation factor (BAFF)-induced expression of interleukin (IL)-8 and matrix metalloproteinase (MMP)-9. Human monocytic cell line (THP)-1 cells were pretreated with either 1 µg/ml of 2-3·6D (IR) or mouse IgG (M) for 20 min and then stimulated with 1 µg/ml of TACI:Fc fusion protein (a) or 1 µg/ml of anti-BAFF monoclonal antibody (mAb) (b). Culture supernatants were collected 24 h after activation and the concentration of IL-8 was measured using enzyme-linked immunosorbent assay and MMP-9 activity was measure using gelatin zymogram. THP-1 cells were pretreated for 20 min with 1, 10 or 100 ng/ml of 2-3·6D (c), 1 µg/ml of 2-3·6D(2-3) or of 2-1·7B(2-1) (d) or 1 µg/ml of 2-3·6D(2-3) or commercially available anti-IREM-1 mAb (BL) (e). Mouse immunoglobulin G (M) was used for the pretreatment as an antibody control. The cells were then stimulated with 1 µg/ml of anti-BAFF mAb. Culture supernatants were collected 24 h after activation and the concentration of IL-8 was measured using ELISA and MMP-9 activity was measure using gelatin zymogram. These experiments were repeated more than three times, with essentially the same results.
Dose–response in anti-IREM-1 treatment was then tested at a concentration ranging from 1 to 100 ng/ml. Anti-IREM-1 mAb blocked BAFF-mediated expression of IL-8 (Fig. 2c) and MMP-9 (data not shown) at concentrations as low as 1 ng/ml and the extent of inhibition was dose-dependent. Both 2-3·6D and 2-1·7B were effective in the blocking of BAFF-induced IL-8 expression (Fig. 2d). A commercially available anti-IREM-1 mAb was also tested to determine whether the inhibitory effects are restricted to certain types of anti-IREM-1 mAb. Both 2-3·6D and the commercially available anti-IREM-1 mAb blocked the BAFF-induced expression of IL-8 (Fig. 2e) and MMP-9 (data not shown), indicating that the inhibitory effects were not restricted.
Synthetic peptide containing ITIM domains of IREM-1 also inhibited BAFF-mediated expression of IL-8 and MMP-9
The ITIM domains present in the intracellular region of IREM-1 were believed to be responsible for the generation of the inhibitory signal. Five potential ITIMs have been identified in the IREM-1 intracellular region. Previous analysis of these ITIM domains revealed that the YADL sequence encompassing the tyrosine 205 residue is essential for inhibitory activity and that the substitution of tyrosine into phenylalanine abolished the inhibitory activity [7,8]. In order to demonstrate that the inhibitory activities of anti-IREM-1 mAb were mediated through the ITIM domain, a hybrid polypeptide (TAT–YADL) was synthesized. TAT–YADL contains HIV–TAT48–57 and IREM-1 residues from 201 to 210, which encompass the YADL motif. The TAT sequence has been shown to have the ability to internalize peptides into cells without disrupting the cellular membrane [12]. As a control, TAT and a mutant polypeptide (TAT–FADL) which had Tyr to Phe substitution were also synthesized. Incubation of these peptides with cells resulted in internalization within 30 min (Fig. 3a). Treatment of TAT–YADL, but not TAT or TAT–FADL, blocked BAFF-induced expression of both IL-8 and MMP-9 (Fig. 3b).
Fig. 3.
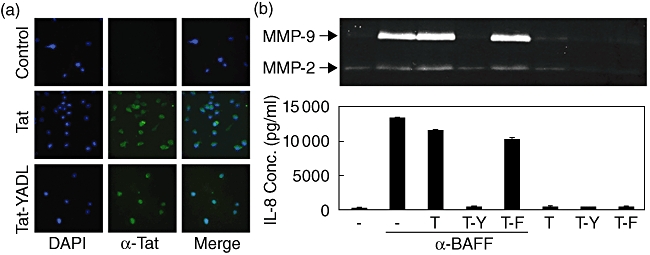
Immunoreceptor tyrosine-based inhibition motifs (ITIM) domain of immune receptor expressed on myeloid cells 1 (IREM-1) mediates the inhibitory effect. (a) Human monocytic cell line (THP)-1 cells were treated with 10 µM of either TAT or TAT–YADL fusion protein. After 30 min, internalization of the peptide was then analysed using immunofluorescence. (b) THP-1 cells were pretreated with 10 µM of either TAT (T), TAT–YADL (T-Y) or TAT–FADL (T-F) for 20 min and then stimulated with 1 µg/ml of anti-B cell activation factor (BAFF) mAb. Culture supernatants were collected 24 h after activation for the measurement of interleukin (IL)-8 concentrations and matrix metalloproteinase (MMP)-9 activity. These experiments were repeated more than three times, with essentially the same results.
Anti-IREM mAb or TAT–YADL inhibited the effect of BAFF on phagocytic activity of THP-1 cells
Stimulation of BAFF modulated the inflammatory activities of macrophages through the induction of proinflammatory mediator expression [6]. Conversely, BAFF stimulation blocked phagocytosis of opsonized zymosan. It has been demonstrated that signals generated from BAFF somehow block the activation of PI3K, which is known to be involved in the pseudopod extension and closure of the phagosome [17]. Anti-IREM-1 mAb was then tested in order to determine whether it can also block the BAFF-mediated inhibition of phagocytosis. As shown in Fig. 4a, anti-IREM-1 mAb, but not the isotype matching mouse IgG, blocked the BAFF-mediated inhibition of phagocytosis. Furthermore, TAT–YADL specifically recapitulated the blocking effect (Fig. 4b).
Fig. 4.
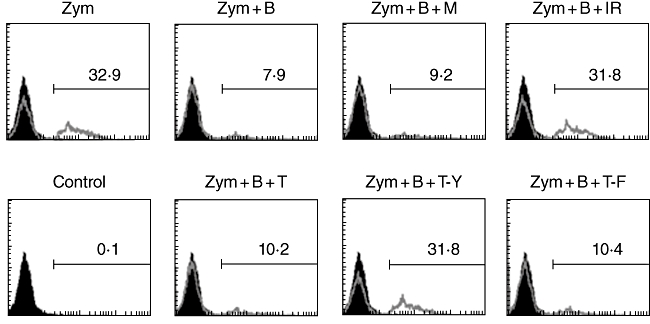
Stimulation of immune receptor expressed on myeloid cells 1 (IREM-1) or addition of TAT–YADL inhibits the effect of B cell activation factor (BAFF) in the phagocytosis of opsonized zymosan. Human monocytic cell line (THP)-1 cells were pretreated with 1 µg/ml of 2-3·6D (IR), 1 µg/ml of mouse IgG (M) or 10 µM of TAT (T), TAT-YADL (T-Y) or TAT-FADL (T-F) for 20 min and then treated with 1 µg/ml of anti-BAFF monoclonal antibody (b) for another 30 min. Opsonized zymosan was then added at 30 µg/ml concentration for 3 h, after which flow cytometry analysis was performed. Lines represent the fluorescence profiles of cells obtained after zymosan treatment and black areas represent the fluorescence profiles of background staining which was obtained in cells incubated without zymosan treatment. Numbers represent the percentage of cells that had uptaken zymosan particles. These experiments were repeated more than three times, with essentially the same results.
IREM-1 exerts its inhibitory activity through inhibition of BAFF-mediated activation of ERK and subsequent phosphorylation of IκB
Stimulation of BAFF induced phosphorylation of ERK within 2 min and the nuclear translocation of NF-κB within 30 min, and inhibitors specific for either ERK or NF-κB blocked the BAFF-induced expression of IL-8 and MMP-9 [6]. As treatment with anti-IREM-1 mAb or TAT–YADL blocked the BAFF-induced expression of IL-8 and MMP-9, it was expected that these agents would block the activation of ERK and/or NF-κB. When BAFF-mediated phosphorylation of ERK was analysed in the presence of anti-IREM-1 mAb or TAT–YADL, these agents blocked the phosphorylation of ERK. The action of these agents was specific, as the isotype matching mouse IgG or TAT failed to affect the BAFF-mediated phosphorylation of ERK (Fig. 5). Activation and nuclear translocation of NF-κB require the phosphorylation and subsequent degradation of IκB. The addition of anti-IREM-1 mAb or TAT–YADL specifically blocked the phosphorylation and degradation of IκB (Fig. 5).
Fig. 5.
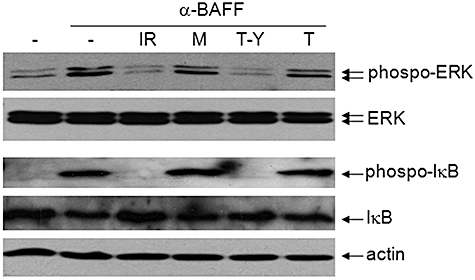
Stimulation of immune receptor expressed on myeloid cells 1 (IREM-1) blocks B cell activation factor (BAFF)-mediated cell signalling. Human monocytic cell line (THP)-1 cells were pretreated with 1 µg/ml of 2-3·6D (IR), 1 µg/ml of mouse (M) or 10 µM of TAT–YADL (T-Y) or TAT (T) for 20 min and then stimulated with 1 µg/ml of anti-BAFF monoclonal antibody. The levels of extracellular regulated kinase (ERK)/phospho-ERK and IκB/phospho-IκB/actin were analysed 2 min and 30 min after activation, respectively.
Activation of SHP-1 is involved in the inhibitory effect of IREM-1
Previous reports have demonstrated that the ITIM of IREM-1 can interact with SHP-1. SHP-1, along with SHP-2, has PTP activity which is known to have roles in cellular functions such as development, growth, inflammation and chemotaxis. Both SHP-1 and SHP-2 are composed of two Src homology 2 (SH2) N-terminal domains and a C-terminal protein–tyrosine phosphatase domain. In lymphocytes and myeloid cells, SHP-1 and SHP-2 have been demonstrated to modulate cellular signals that involve PI3K, Janus kinase 2, signal transducer and activator of transcription (STAT), mitogen-activated protein kinase (MAPK), ERK and NF-κB [18,19]. Furthermore, SHP-1 deficiency resulted in an enhanced macrophage activities in both human multiple sclerosis patients and mouse models [20–22] and overexpression of SHP-1 in murine macrophage inhibited LPS-induced TNF and inducible nitric oxide synthase (iNOS) production through the inhibition of LPS-induced phosphorylation of vav1 [23]. Pathogens including leishmania were found to use specialized mechanism to activate SHP-1 in order to inhibit the inflammatory activation of macrophages [24]. The ITIM of IREM-1 has been demonstrated to interact with the SH2 domain of SHP-1 through Tyr 205 residue [7,8].
The inhibitory activities of anti-IREM-1 mAb or TAT–YADL in BAFF-stimulated cells are likely to be mediated by SHP-1. Furthermore, the presence of inhibitory activity in TAT–YADL which encompasses Tyr 205 residue, but not in TAT–FADL which have Tyr to Phe substitution, also supports that the interaction with SHP-1 is required for the inhibitory activity. In order to confirm that SHP-1 activity is required for the inhibitory activities of anti-IREM-1 mAb and TAT–YADL in cells stimulated through BAFF, THP-1 cells were treated with anti-BAFF and anti-IREM-1 mAbs in the presence of PTP inhibitor III, which has been shown to inactivate SHP-1 through binding to its catalytic domain [25]. As shown in Fig. 6, addition of PTP inhibitor III abolished the inhibitory ability of both anti-IREM-1 mAb and TAT–YADL. Based on these data, it is highly likely that IREM-1 exerts its activity through SHP-1, which then inhibits the BAFF-mediated modulation of PI3K, ERK and NF-κB activities.
Fig. 6.
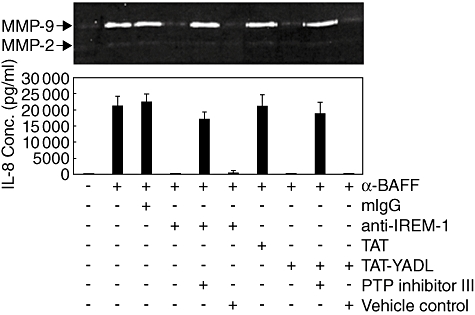
Inhibitory effects of both anti-immune receptor expressed on myeloid cells 1 (IREM-1) monoclonal antibody and TAT–YADL require protein tyrosine phosphatase (PTP) activity. Human monocytic cell line (THP)-1 cells were pretreated for 30 min with either 30 µM of PTP inhibitor III or 0·1% dimethylsulphoxide (DMSO) as a vehicle control and then treated for another 30 min with 1 µg/ml of mouse IgG [or anti-IREM-1 monoclonal antibody (mAb)] or 10 µM of TAT (or TAT–YADL). Finally, the cells were stimulated with 1 µg/ml of anti-B cell activation factor (BAFF) mAb. Culture supernatants were collected 24 h after activation and the concentration of interleukin-8 was measured using enzyme-linked immunosorbent assay and matrix metalloproteinase (MMP)-9 activity was measure using gelatin zymogram. These experiments were repeated twice, with essentially the same results.
IREM-1 has inhibitory effect on BAFF-mediated inflammatory activation in U937 cells
In order to test whether the inhibitory activity of IREM-1 is effective in other cell types, similar experiments were performed in a human monocytic cell line (U937) which expresses high levels of BAFF on the cell surface [26]. Simulation of U937 cells with anti-BAFF mAb induced the expression of both MMP-9 and IL-8. Pretreatment of U937 cells with anti-IREM-1 mAb or TAT–YADL significantly reduced the responsiveness to BAFF stimulation, while mouse IgG or TAT did not (Fig. 7). These data indicate clearly that the inhibitory activity of IREM-1 is not restricted to THP-1 cells, but more likely to be general in BAFF-mediated signalling.
Fig. 7.
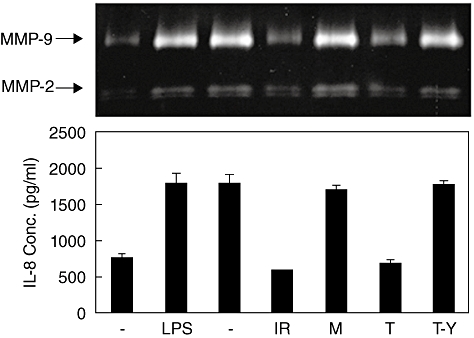
The inhibitory effect of immune receptor expressed on myeloid cells 1 (IREM-1) on B cell activation factor (BAFF)-mediated signalling is effective in U937 cells. U937 cells were pretreated for 30 min with 1 µg/ml of mouse IgG (M)/anti-IREM-1 mAb (IR) or 5 µM of TAT (T)/TAT–YADL (T-Y). The cells were then stimulated with 1 µg/ml of anti-BAFF monoclonal antibody. Culture supernatants were collected 24 h after activation and the concentration of interleukin-8 was measured using enzyme-linked immunosorbent assay and matrix metalloproteinase (MMP)-9 activity was measure using gelatin zymogram. These experiments were repeated twice, with essentially the same results.
The inflammatory activation of macrophages plays an important role in the pathogenesis of diseases where inflammation is involved. Examples of these diseases include chronic inflammatory diseases such as atherosclerosis and autoimmune diseases such as rheumatoid arthritis. Because BAFF expression has been detected in macrophage-rich areas of atherosclerotic plaques and synovial tissues of rheumatoid arthritis patients [6], it is likely that BAFF may enhance the production of proinflammatory mediators. As macrophages in tissue samples also express high levels of both TACI and BAFFR [26], the natural counterparts of BAFF, cell-to-cell interaction between macrophages will be sufficient for the inflammatory activation of these cells. Immunohistochemical and in situ hybridization analysis of human atherosclerotic plaques detected the expression of IREM-1 in macrophage-rich areas (our unpublished observation). It is likely that the presence or absence of IREM-1 or regulation of its activity may modulate the function of macrophages through regulation of BAFF-induced cell signalling.
Disclosure
The authors have no conflicts of interest or any relevant financial interest in any company or institutions that might benefit from this publication.
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2008-313-C00647).
References
- 1.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–64. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 2.Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med. 2003;198:937–45. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng LG, Mackay CR, Mackay F. The BAFF/APRIL system: life beyond B lymphocytes. Mol Immunol. 2005;42:763–72. doi: 10.1016/j.molimm.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 4.Dillon SR, Gross JA, Ansell SM, Novak AJ. An APRIL to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov. 2006;5:235–46. doi: 10.1038/nrd1982. [DOI] [PubMed] [Google Scholar]
- 5.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282–9. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Jeon ST, Kim WJ, Lee SM, et al. Reverse signaling through BAFF differentially regulates the expression of inflammatory mediators and cytoskeletal movements in THP-1 cells. Immunol Cell Biol. 2009;88:148–56. doi: 10.1038/icb.2009.75. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Errico D, Aguilar H, Kitzig F, Brckalo T, Sayos J, Lopez-Botet M. IREM-1 is a novel inhibitory receptor expressed by myeloid cells. Eur J Immunol. 2004;34:3690–701. doi: 10.1002/eji.200425433. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez-Errico D, Sayos J, Lopez-Botet M. The IREM-1 (CD300f) inhibitory receptor associates with the p85alpha subunit of phosphoinositide 3-kinase. J Immunol. 2007;178:808–16. doi: 10.4049/jimmunol.178.2.808. [DOI] [PubMed] [Google Scholar]
- 9.Can I, Tahara-Hanaoka S, Hitomi K, et al. Caspase-independent cell death by CD300LF (MAIR-V), an inhibitory immunoglobulin-like receptor on myeloid cells. J Immunol. 2008;180:207–13. doi: 10.4049/jimmunol.180.1.207. [DOI] [PubMed] [Google Scholar]
- 10.Xi H, Katschke KJ, Jr, Helmy KY, et al. Negative regulation of autoimmune demyelination by the inhibitory receptor CLM-1. J Exp Med. 2010;207:7–16. S1–5. doi: 10.1084/jem.20091508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izawa K, Kitaura J, Yamanishi Y, et al. An activating and inhibitory signal from an inhibitory receptor LMIR3/CLM-1: LMIR3 augments lipopolysaccharide response through association with FcRgamma in mast cells. J Immunol. 2009;183:925–36. doi: 10.4049/jimmunol.0900552. [DOI] [PubMed] [Google Scholar]
- 12.Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF. Cell-permeable peptide inhibitors of JNK: novel blockers of beta-cell death. Diabetes. 2001;50:77–82. doi: 10.2337/diabetes.50.1.77. [DOI] [PubMed] [Google Scholar]
- 13.Lee WH, Kim SH, Lee Y, et al. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2001;21:2004–10. doi: 10.1161/hq1201.098945. [DOI] [PubMed] [Google Scholar]
- 14.Kim WJ, Lee WH. LIGHT is expressed in foam cells and involved in destabilization of atherosclerotic plaques through induction of matrix metalloproteinase-9 and IL-8. Immune Netw. 2004;4:116–22. [Google Scholar]
- 15.Sui L, Li N, Liu Q, et al. IgSF13, a novel human inhibitory receptor of the immunoglobulin superfamily, is preferentially expressed in dendritic cells and monocytes. Biochem Biophys Res Commun. 2004;319:920–8. doi: 10.1016/j.bbrc.2004.05.065. [DOI] [PubMed] [Google Scholar]
- 16.Korver W, Zhao X, Singh S, et al. Monoclonal antibodies against IREM-1: potential for targeted therapy of AML. Leukemia. 2009;23:1587–97. doi: 10.1038/leu.2009.99. [DOI] [PubMed] [Google Scholar]
- 17.May RC, Machesky LM. Phagocytosis and the actin cytoskeleton. J Cell Sci. 2001;114:1061–77. doi: 10.1242/jcs.114.6.1061. [DOI] [PubMed] [Google Scholar]
- 18.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007;22:1251–67. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev. 2009;228:342–59. doi: 10.1111/j.1600-065X.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christophi GP, Hudson CA, Panos M, Gruber RC, Massa PT. Modulation of macrophage infiltration and inflammatory activity by the phosphatase SHP-1 in virus-induced demyelinating disease. J Virol. 2009;83:522–39. doi: 10.1128/JVI.01210-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christophi GP, Massa PT. Central neuroinvasion and demyelination by inflammatory macrophages after peripheral virus infection is controlled by SHP-1. Viral Immunol. 2009;22:371–87. doi: 10.1089/vim.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christophi GP, Panos M, Hudson CA, et al. Macrophages of multiple sclerosis patients display deficient SHP-1 expression and enhanced inflammatory phenotype. Lab Invest. 2009;89:742–59. doi: 10.1038/labinvest.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardin AO, Meals EA, Yi T, Knapp KM, English BK. SHP-1 inhibits LPS-mediated TNF and iNOS production in murine macrophages. Biochem Biophys Res Commun. 2006;342:547–55. doi: 10.1016/j.bbrc.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Nandan D, Tran T, Trinh E, Silverman JM, Lopez M. Identification of leishmania fructose-1,6-bisphosphate aldolase as a novel activator of host macrophage Src homology 2 domain containing protein tyrosine phosphatase SHP-1. Biochem Biophys Res Commun. 2007;364:601–7. doi: 10.1016/j.bbrc.2007.10.065. [DOI] [PubMed] [Google Scholar]
- 25.Arabaci G, Guo XC, Beebe KD, Coggeshall KM, Pei D. A-haloacetophenone derivatives as photoreversible covalent inhibitors of protein tyrosine phosphatases. J Am Chem Soc. 1999;121:5085–6. [Google Scholar]
- 26.Jeon ST, Kim WJ, Lee SM, et al. Reverse signaling through BAFF differentially regulates the expression of inflammatory mediators and cytoskeletal movements in THP-1 cells. Immunol Cell Biol. 2010;88:148–56. doi: 10.1038/icb.2009.75. [DOI] [PubMed] [Google Scholar]


