Abstract
We studied the efficacy, safety and pharmacokinetic profiles of Intratect®, a recently developed polyvalent intravenous immunoglobulin (IVIG) preparation. Fifty-one patients (aged 6–48 years) with primary immunodeficiencies (PID) and established replacement therapy using a licensed IVIG were enrolled and treated for 12 months with Intratect®. Retrospective patient data served as prestudy controls. The primary efficacy variable was the annual rate of acute serious bacterial infection (ASBI) per patient. Secondary parameters were annual rate of acute relevant infection (ARI), days with antibiotic use, fever, absence from school/work and hospitalization. The average IVIG dose was 0·49 g/kg, with an average infusion rate of 2·4 ml/kg/h. The annual ASBI rate/patient was 0·02 and ARIs were detected 128 times during the 630 adverse events in 40 patients, specified mainly as bronchitis, sinusitis, respiratory tract infection, rhinitis and pharyngitis. The annual rate of respiratory ARIs/patient was 2·0 and the rates/patient for days with fever >38°C, school/work absence and hospitalization were 1·81, 3·99 and 0·36, respectively. A total of 630 adverse events (AEs) were observed in 50 of 51 (98·0%) of patients. In 46 of 51 patients the AEs were not related to infusion. Pharmacokinetic studies after the first infusion revealed a mean elimination half-life of 50·8 ± 30·3 days. During this study, 19 of 649 (2·9%) IgG trough levels were below 6 g/l, better than that of reference IVIGs during the 6 months before study start (10 of 201). These data suggest that Intratect® is a well tolerated, safe and effective IgG concentrate for the treatment of patients with PID.
Keywords: intravenous immunoglobulin, primary immunodeficiency
Introduction
Intravenous immunoglobulin (IVIG) replacement has been well established for prophylaxis of infections in patients with primary immunodeficiencies (PIDs), and IVIG has saved more lives in this patient population than any other medication over the past two decades [1–3]. Although subcutaneous administration of IgG has been proved a reasonable alternative, the vast majority of PID patients are still on IVIG worldwide. In addition to prophylaxis, IVIG may also be helpful for the treatment of active disease and in a variety of immunological and inflammatory conditions [3–8]. Maintaining the functional activity of IgG during the manufacturing process and assuring the safety of the preparations are critical determinants of IVIG concentrates. Intratect® (Biotest Pharma GmbH, Dreieich, Germany) is one of the most recently developed IgG preparations. During the manufacturing process, high-pressure liquid chromatography is used to assure high standards of purity and viral safety. In addition, the fractionated precipitation by Cohn was modified using filtration methods including filter aid instead of centrifugation for the separation of precipitates.
Based on efficacy and safety studies in a limited number of patients with PID and idiopathic thrombocytopenic purpura (ITP), Intratect® was first introduced for clinical use in Germany. According to the EU note for guidance these preliminary results were evaluated by German national and local authorities as part of a mutual recognition procedure, and the use of Intratect® was approved in Germany in 2006. To collect more data in PID patients and to allow the opportunity for established PID centres to gain experience with this novel preparation, a multi-centre clinical analysis was designed. We have conducted a 12-month observational study according to the European Medical Evaluation Agency (EMEA) guidelines with the principle aim of investigating the efficacy, safety and pharmacokinetic profile of Intratect®. Here we report that this novel IVIG preparation is well tolerated, safe, effective and has a slightly better pharmacokinetic profile compared to other IVIGs used currently for replacement therapy in patients with PID.
Materials and methods
Study design and patients
The aim of this multi-centre, open, prospective study was to investigate the efficacy, safety, tolerability and pharmacokinetic properties of Intratect® in patients with PID (common variable immunodeficiency, congenital agammaglobulinaemia, hypogammaglobulinaemia, severe combined immunodeficiency, ataxia telangiectasia, hyper-IgM syndrome, hyper-IgE syndrome and selective IgG subclass deficiency) predominated with antibody deficiency. The study was carried out according to the EMEA and Food and Drug Administration (FDA) guidelines for clinical investigation of human normal immunoglobulin for intravenous administration [9,10]. Approval was obtained from the respective national or institutional ethics committees of the five participating centres. It was planned to enrol at least 50 patients for a treatment period of 48 weeks to achieve adequate statistical power and to exclude an efficacy bias by seasonal influences. The main inclusion criteria were male and female patients of different ages, confirmed PID with antibody deficiency, established replacement therapy using a licensed IVIG and available written informed consent. Retrospective patient data served as prestudy controls.
Treatments
All patients were treated monthly with 5% solution of Intratect® containing 50 g/l human protein, 96% of which was IgG. Established dosage and infusion intervals of the previous reference IVIG therapy assured IgG trough levels of ≥6 g/l. The initial rate of infusions was 1·4 ml/kg/h for 30 min, which was increased to a maximum of 1·9 ml/kg/h during the first administration, and further during the following infusions in case of adequate tolerance. During the study Intratect® was the only IVIG preparation received by the patients, but other concomitant therapies were possible if required.
Evaluation criteria and data collection of acute serious bacterial infections (ASBI) were considered as primary variables, and acute and relevant infections (ARI), days with antibiotic use, fever >38·0°C, absence from school or work and hospitalization for treatment of infection as secondary parameters. For safety evaluation the incidence of adverse events, changes in vital signs and laboratory test results were recorded.
Pharmacokinetic properties [Cmax/tmax/t1/2/area under the curve (AUC)0–tz/AUC0–∞/clearance] were assessed by measurement of IgG serum levels from repeated blood sampling after the first infusion and after 24 weeks of treatment including trough levels of total IgG, serum concentration of IgG subclasses 1–4 and number of trough levels below 6 g/l compared to previously administered IVIG.
Results
Baseline characteristics
Fifty-one patients (14 females and 37 males) were treated for a mean duration of 47 weeks (48 patients with a 4-week and three patients with a 3-week treatment schedule) using a mean dose of 8·0 ml Intratect® per kg body weight (BW). Premature study discontinuation did not occur in any treated patient. Age ranged between 6 and 48 (median 15) years. The majority of patients suffered from common variable immunodeficiency (20), followed by congenital agammaglobulinaemia (12), hypogammaglobulinaemia (five), severe combined immunodeficiency (one) and other diagnoses in 13 cases, specified as ataxia telangiectasia (five), hyper-IgM syndrome (five), hyper-IgE syndrome (one) and selective IgG subclass deficiency (two). All patients received licensed IVIG therapies prior to the study, as specified in Table 1.
Table 1.
Intravenous immunoglobulin (IVIG) preparations used prior to this study.
| IVIG prior to this study†[number (%) of patients] | Total, n = 51 | Patients on 4-week regimen, n = 48 | Patients on 3-week regimen, n = 3 |
|---|---|---|---|
| Flebogamma | 17(33·3%) | 16(33·3%) | 1(33·3%) |
| Intraglobin | 16(31·4%) | 15(31·3%) | 1(33·3%) |
| Sandoglobulin | 9(17·6%) | 9(18·8%) | – |
| Endobulin | 6(11·8%) | 5(10·4%) | 1(33·3%) |
| Octagam | 5(9·8%) | 5(10·4%) | – |
| Endoglobulin | 1(2·0%) | 1(2·1%) | – |
| Gammagard | 1(2·0%) | 1(2·1%) | – |
| IG Vena | 1(2·0%) | 1(2·1%) | – |
Trademarks.
Treatment with Intratect®
A total of 642 infusions of Intratect® (598 in the 4-week and 44 in the 3-week schedules) were administered to 51 patients, of whom 27 received 12, 20 patients 13, two patients 14 and two patients 15 infusions. The mean number of visits was 19·8 ± 1·9 (4-week regimen) and 20·7 ± 0·6 (3-week regimen) and mean number of infusions was 12·5 (4-week) versus 14·7 (3-week). The mean duration for a single infusion was approximately 240 min (range 90–525). The corresponding infusion rates increased from 1·4 ml/kg/h (range 0·3–2·8) to 2·4 ml/kg/h (range 1·0–5·9) after 60 min. The calculated mean single dose was 8·0 ± 1·8 ml/kg BW (400 ± 91 mg/kg BW) for the first infusion and 7·7 ± 1·8 ml/kg BW (387 ± 88 mg/kg BW) for the remaining infusions, reflecting exactly the previously planned dosing requirements in terms of the administration of Intratect®.
Efficacy
During the entire study only one single ASBI was reported and specified as cellulitis in the connective tissue of the knee. Thus, the annual rate of ASBI (primary efficacy variable) was calculated as 0·02 per patient per year [one-sided 99% confidence interval (CI) = 0·00–0·11], based on a total observation period of 18 313 days and 50·17 patient-years, respectively. Furthermore, a total of 128 of 630 (20·3%) adverse events were rated as ARI in 40 of 51 patients (78·4%; 101 respiratory and 27 other), accounting for an annual rate of 2·0 respiratory ARIs per patient. The rate of days with respiratory ARIs corresponded well with the rate of days with use of antibiotics, excluding prophylaxis (40·38 versus 32·65).
The results for secondary efficacy parameters are summarized in Table 2 using total numbers, means ± standard deviations for days and proportions, and annual rates. Of the three cases with hospitalization due to infection, which were specified as acute cholecystitis, chronic sinusitis and acute cellulitis of the knee, the ASBI criteria were fulfilled appropriately only for the latter case according to the guidance's requirements [9,10].
Table 2.
Secondary efficacy parameters.
| Secondary parameter | Days/patients | Mean days per patient | Mean proportion |
|---|---|---|---|
| Antibiotic use (with prophylaxis) | 3792/46 | 4·35 ± 105·10 | 20·56 ± 29·05% |
| Antibiotic use (without prophylaxis) | 1638/44 | 32·12 ± 47·17 | 9·03 ± 13·25% |
| ARIs (respiratory) | 2026/40 | 39·73 ± 50·58 | 11·28 ± 14·71% |
| ARIs (other) | 344/17 | 6·75 ± 16·33 | 1·82 ± 4·24% |
| Fever (>38°C) | 91/21 | 1·78 ± 3·09 | 0·50 ± 0·88% |
| School/work absence | 200/23 | 3·92 ± 7·04 | 1·12 ± 2·02% |
| Hospitalization (days for treatment of infection) | 18/3 | 0·35 ± 1·47 | 0·10 ± 0·40% |
ARI, acute and relevant infection.
Safety
Infusions of Intratect® were generally well tolerated. A total of 630 adverse events (AEs) were observed in 50 of 51 (98·0%) patients. As expected, most frequently listed AEs were cough (31 patients), fever (23), rhinitis (23), bronchitis (20) and pharyngitis (17). Most of the events were rated as not related to the study drug (in 46 of 51 patients; 90·2%), including all 14 serious AEs in 11 patients (21·6%), which were characterized as hospitalizations due to lymphadenopathy in four patients, surgical interventions in four patients and infections in three patients. For the total of 642 infusions, 84 (13·1%) presented at least one infusional AE in 36 patients (two-sided 90% CI = 10·95–15·48%). Relationship to study drug was seen for only six infusions (0·9%), with infusional AEs in four patients. Deaths and premature termination due to AE did not occur during the study. Regarding the evaluation of newly occurred ARIs, a total of 101 respiratory events were selected in 40 of 51 patients, listed mainly as bronchitis, sinusitis, respiratory tract infection, rhinitis and pharyngitis, as displayed in Table 3.
Table 3.
Relevant acute respiratory infections.
| Respiratory infections [number (%) of patients] | Total n = 51 | Patients on 4-week regimen, n = 48 | Patients on 3-week regimen, n = 3 |
|---|---|---|---|
| Patients with an AE marked as respiratory | |||
| ARIs | 40(78·4%) | 38(79·2%) | 2(66·7%) |
| Bronchitis | 16(31·4%) | 14(29·2%) | 2(66·7%) |
| Sinusitis | 11(21·6%) | 10(20·8%) | 1(33·3%) |
| Respiratory tract infection | 11(21·6%) | 11(22·9%) | 0 |
| Rhinitis | 9(17·6%) | 8(16·7%) | 1(33·3%) |
| Pharyngitis | 9(17·6%) | 8(16·7%) | 1(33·3%) |
| Pneumonia | 4(7·8%) | 4(8·3%) | 0 |
| Tracheitis | 2(3·9%) | 2(4·2%) | 0 |
| Chronic bronchitis | 2(3·9%) | 2(4·2%) | 0 |
| Chronic obstructive pulmonary disease | 1(2·9%) | 1(2·1%) | 0 |
AE, adverse event; ARI, acute and relevant infection.
Changes in vital signs were not clinically relevant, and abnormal laboratory values observed for C-reactive protein (CRP), leucocytes, neutrophils, lymphocytes, fibrinogen and IgG and for blood and leucocytes in urine could be assigned predominantly to the observed infections (not shown).
Pharmacokinetics
Results for the standard pharmacokinetic parameters Cmax, tmax, t1/2, AUC0–tz, AUC 0–∞, clearance and duration of infusion (ti) are summarized in Table 4. One patient was excluded from analysis as no reliable calculation was possible, and in other patients missing values were replaced by linear interpolation. After the first infusion of Intratect® the mean elimination half-life was 50·8 ± 30·3 days. Maximum IgG concentrations (mean of 15·9 ± 3·1 g/l) occurred between 2·6 and 7·4 h after this infusion in the majority of patients (84·0%) and mean concentrations increased by about 7 g/l after the first infusion. The elimination half-life tended to be slightly longer under higher baseline IgG values, and maximum IgG concentrations had no influence on elimination half-life. Proportions of the IgG subclasses 1–4 remained almost unchanged after the infusions. Overall, pharmacokinetic results after week 24 infusion were comparable to the first infusion analysis, and the development of mean IgG values is displayed in Fig. 1.
Table 4.
Pharmacokinetic profile.
| Parameters | Item | After first infusion, n = 50 | After week 24 infusion, n = 17 |
|---|---|---|---|
| Cmax (g/l) | Arithmetic mean (s.d.) | 15·9(3·1) | 15·1(3·1) |
| Geometric mean(CV) | 15·6(19·1) | 14·9(20·2) | |
| Median(range) | 15·5(11·1–23·6) | 14·8(10·4–21·3) | |
| tmax†(h) | Arithmetic mean(s.d.) | 6·24(4·32) | 8·64(7·68) |
| Median(range) | 4·56(2·64–24·00) | 6·48(1·92–27·84) | |
| ti(h) | Arithmetic mean(s.d.) | 4·30(1·07) | 4·03(1·66) |
| Median(range) | 4·00(2·42–7·25) | 3·47(1·68–7·75) | |
| t1/2(days) | Arithmetic mean(s.d.) | 50·81(30·32) | 55·86(20·47) |
| Median(range) | 45·59(23·36–196·22) | 52·26(28·57–110·16) | |
| AUC(0–tz)(days × g/l) | Arithmetic mean(s.d.) | 251(97) | 234(114) |
| Geometric mean(CV) | 233(42) | 207(56) | |
| Median(range) | 261(75–520) | 179(71–467) | |
| AUC(0–∞)(days × g/l) | Arithmetic mean(s.d.) | 956(653) | 989(329) |
| Geometric mean(CV) | 840(49) | 941(34) | |
| Median(range) | 793(326–3830) | 950(564–1745) | |
| Clearance(ml/min) | Arithmetic mean(s.d.) | 28·9(16·9) | 19·9(13·3) |
| Geometric mean(CV) | 22·9(96·1) | 15·4(93·9) | |
| Median(range) | 26·7(1·3–76·6) | 16·5(3·6–46·6) |
tmax (d) was converted into tmax (h) for tabulation (i.e. factor 24). AUC, area under the curve; CV, geometric coefficient of variation; s.d., standard deviation.
Fig. 1.
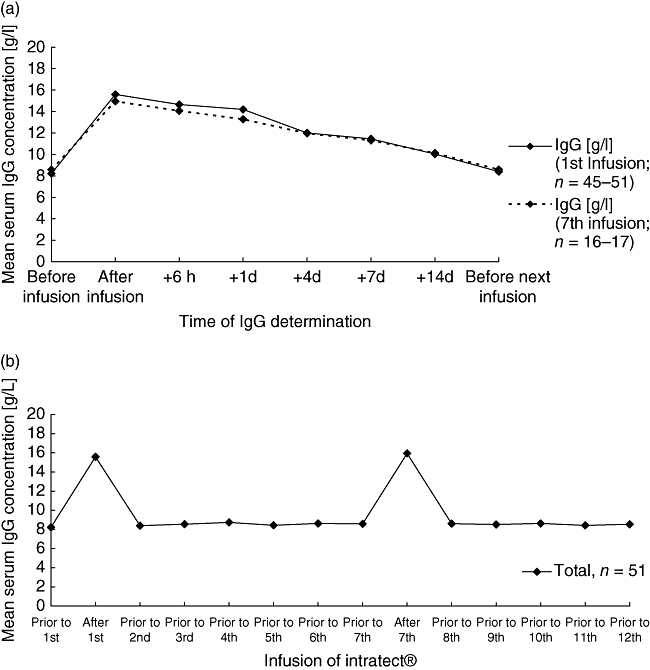
(a) Mean total immunoglobulin G (IgG) values measured after the first infusion (solid line) and the seventh infusion (interrupted line) were given. (b) Mean IgG concentrations measured during the whole period of the study.
Discussion
In this study, efficacy parameters were selected as recommended by the EMEA's ‘Note for Guidance on the Clinical Investigation of IVIG’ and the FDA's ‘Guidance for Industry’[9,10]. These included calculation of annual rates for ASBI, new ARI, use of antibiotics, days with fever >38·0°C, absence from school/work and hospitalizations due to infection. Fifty-one treated patients were included in efficacy analysis, and thus the sample size requirements were also met exactly (at least 40–50 available patients) [10]. Moreover, primary efficacy results were in complete adherence to the guidance's instructions [9,10] and to previous studies [11–13].
For the secondary efficacy, including rates of newly acquired acute and relevant respiratory infections, a consistent tendency towards lower annual rates than in other studies [11–13] appeared under Intratect®. However, comparisons with other studies are difficult due to the different severity of the immunodeficiency syndromes in the studied populations and due to differences in documentation and evaluation of efficacy data, showing a great variation, for example, in infection rates [14]. One potential limitation of this study was that the cohort of patients was heterogeneous. Therefore, further post-marketing vigilance is needed to monitor the immunological and clinical efficacy of this novel product. Overall, the outcome of this trial was at least within the range or even better compared to the majority of results published recently [15,16]. Trough levels below 6 g/l were detected less frequently under Intratect® treatment when compared with the reference IVIGs. Importantly, the mean elimination half-life of about 50 days was higher than that for native IgG in healthy subjects, indicating the functional integrity of the IgG contained in Intratect®.
Safety data did not reveal any potential unknown risks or unexpected or new signs and symptoms allocated to infusions with regard to the known range of IVIG-specific adverse reactions, as presented in the Summary of Product Characteristics (SPC) [17], such as pyrexia, headache, nausea or vomiting. Events timely associated with the infusions within 48 h occurred in 36 patients, but the relationship between these events and the study medication was evident in only four patients. None of the patients reported on any adverse event after the 48-h follow-up period that may, potentially, have been associated with the IVIG infusion. In addition, concomitant relevant infections such as sinusitis, bronchitis, rhinitis or pharyngitis are common in patients with PID and did not occur more frequently than reported previously [11–13,18,19]. Changes of vital signs were not clinically relevant, and abnormal laboratory values could be assigned predominantly to the observed infections.
In summary, this study provides details on safety, efficacy and pharmacokinetic profiles of Intratect®, a fourth-generation IVIG concentrate. We present here that Intratect® is a safe, well tolerated and effective preparation for the treatment of patients with PID.
Acknowledgments
This study was supported by Biotest AG.
Disclosure
Biotest Hungary provided support for the J Project Eastern- and Central European programme organized by LM.
References
- 1.Buckley RH, Schiff RI. The use of intravenous immune globulin in immunodeficiency diseases. N Engl J Med. 1991;325:110–17. doi: 10.1056/NEJM199107113250207. [DOI] [PubMed] [Google Scholar]
- 2.Chapel H. Consensus on diagnosis/management of primary antibody deficiencies. Br Med J. 1994;308:581–5. doi: 10.1136/bmj.308.6928.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orange JS, Hossny EM, Weiler CR, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117:S525–53. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Primary immunodeficiency diseases: report of a WHO sponsored meeting. Immunodefic Rev. 1989;1:173–205. [PubMed] [Google Scholar]
- 5.NIH Consensus Conference. Intravenous immunoglobulin. Prevention and treatment of the disease. JAMA. 1990;264:3189–93. [PubMed] [Google Scholar]
- 6.Jolles S, Sewell WA, Misbah SA. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol. 2005;142:1–11. doi: 10.1111/j.1365-2249.2005.02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sibéril S, Elluru S, Negi VS, et al. Intravenous immunoglobulin in autoimmune and inflammatory diseases: more than mere transfer of antibodies. Transfus Apher Sci. 2007;37:103–7. doi: 10.1016/j.transci.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Schiff RI, Williams LW, Nelson RP, Buckley RH, Burks W, Good RA. Multi-centre crossover comparison of safety and efficacy of Intraglobin-F with Gamimune-N, Sandoglobulin, and Gammagard in patients with primary immunodeficiency diseases. J Clin Immunol. 1997;17:21–8. doi: 10.1023/a:1027380210989. [DOI] [PubMed] [Google Scholar]
- 9.The European Evaluation Agency for Medicinal Products: Note for Guidance on the Clinical Investigation of human Normal Immunoglobulin for Intravenous Administration (IVIG) CPMP/BPWG/388/95; Rev. 1, 2000.
- 10.Guidance for Industry: safety, efficacy, and pharmacokinetic studies to support marketing of intravenous immune globulin as replacement therapy for primary humoral immunodeficiency. USA FDA Deptartment of Health and Human Services. Draft, November 2005.
- 11.Roifman CM, Schroeder H, Berger M, et al. Comparison of efficacy of IVIG-C, 10% (caprylate/chromatography) and IVIG-SD, 10% as replacement therapy in primary immune deficiency. A randomized double-blind trial. Int Immunopharmacol. 2003;3:1325–33. doi: 10.1016/s1567-5769(03)00134-6. [DOI] [PubMed] [Google Scholar]
- 12.Ochs HD, Pinciaro PJ the Octagam Study Group. Octagam® 5%, an intravenous IgG product, is efficacious and well-tolerated in subjects with primary immunodeficiency diseases. J Clin Immunol. 2004;24:309–14. doi: 10.1023/B:JOCI.0000025453.23817.3f. [DOI] [PubMed] [Google Scholar]
- 13.Berger M, Pinciaro PJ the Flebogamma® 5% investigators. Safety, efficacy, and pharmacokinetics of Flebo-gamma® 5% for replacement therapy in primary immunodeficiency diseases. J Clin Immunol. 2004;24:389–96. doi: 10.1023/B:JOCI.0000029108.18995.61. [DOI] [PubMed] [Google Scholar]
- 14.Nolte MT, Pirofsky B, Gerritz GA, Golding B. Intravenous immunoglobulin therapy for antibody deficiency. Clin Exp Immunol. 1979;36:237–43. [PMC free article] [PubMed] [Google Scholar]
- 15.Church JA, Leibl H, Stein MR, et al. Efficacy, safety, and tolerability of a new 10% liquid intravenous immunoglobulin [IGIV 10%] in patients with PID. J Clin Immunol. 2006;26:388–95. doi: 10.1007/s10875-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 16.Stein MR, Nelson RP, Church JA, et al. Safety and efficacy of Privigen (R), a novel 10% liquid immunoglobulin preparation for intravenous use in patients with PID. J Clin Immunol. 2009;29:137–44. doi: 10.1007/s10875-008-9231-2. [DOI] [PubMed] [Google Scholar]
- 17.The European Evaluation Agency for Medicinal Products: Core SPC for Human Normal Immunoglobulin for Intravenous Administration (IVIG) CPMP/BPWG/859/95; Rev. 2, 2000.
- 18.Webster AD. Clinical and immunological spectrum of common variable immunodeficiency. Iran J Allergy Asthma Immunol. 2004;3:103–13. [PubMed] [Google Scholar]
- 19.Knezevic-Maramica I, Kruskall MS. Intravenous immunoglobulins: an update for clinicians. Transfusion. 2003;43:1460–80. doi: 10.1046/j.1537-2995.2003.00519.x. [DOI] [PubMed] [Google Scholar]


