Abstract
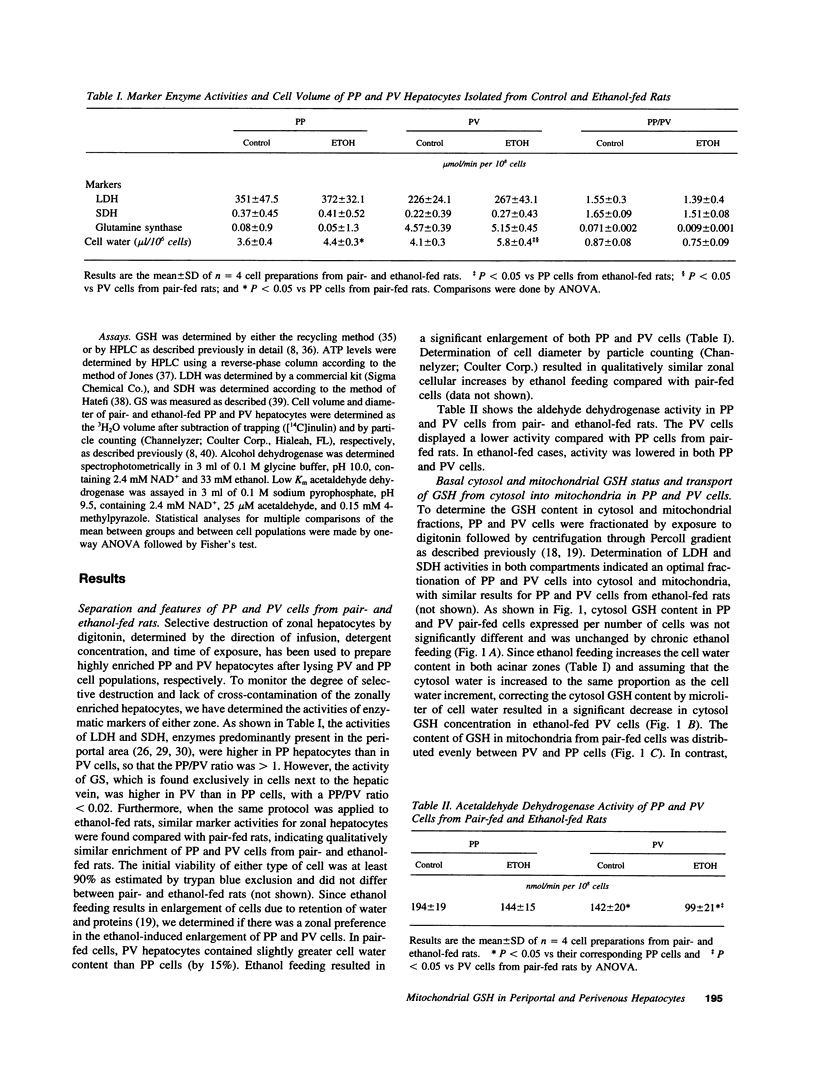
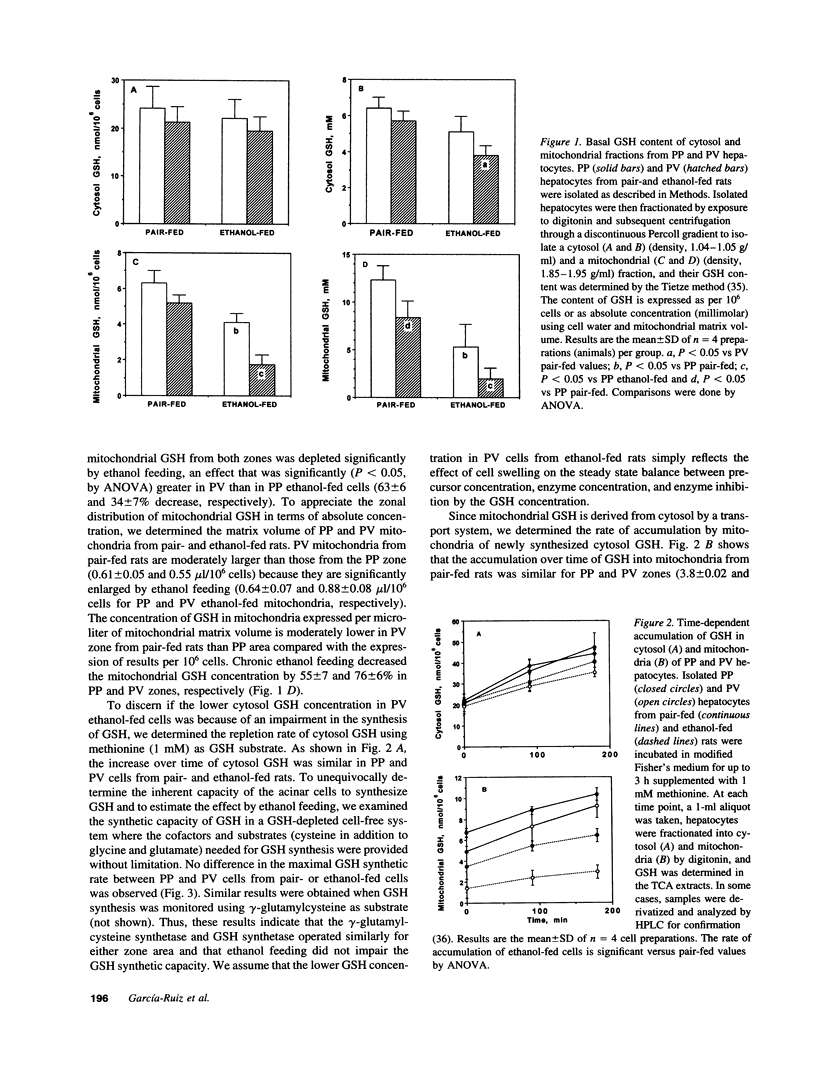
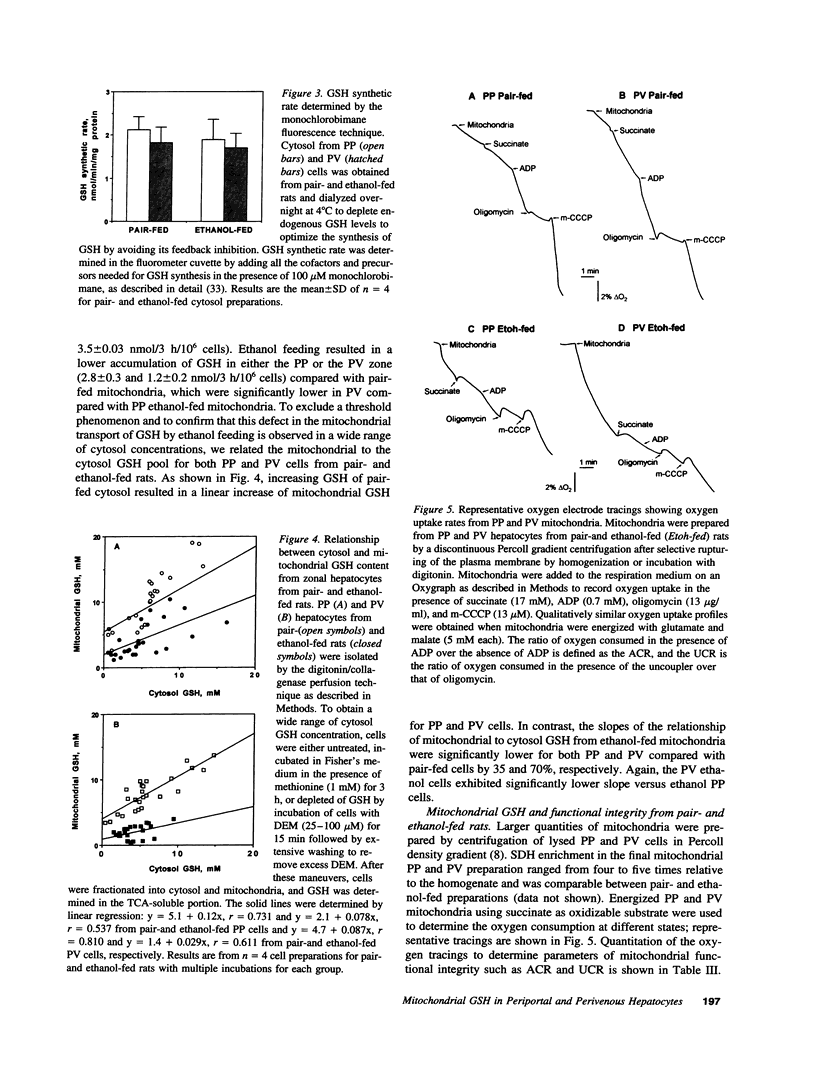
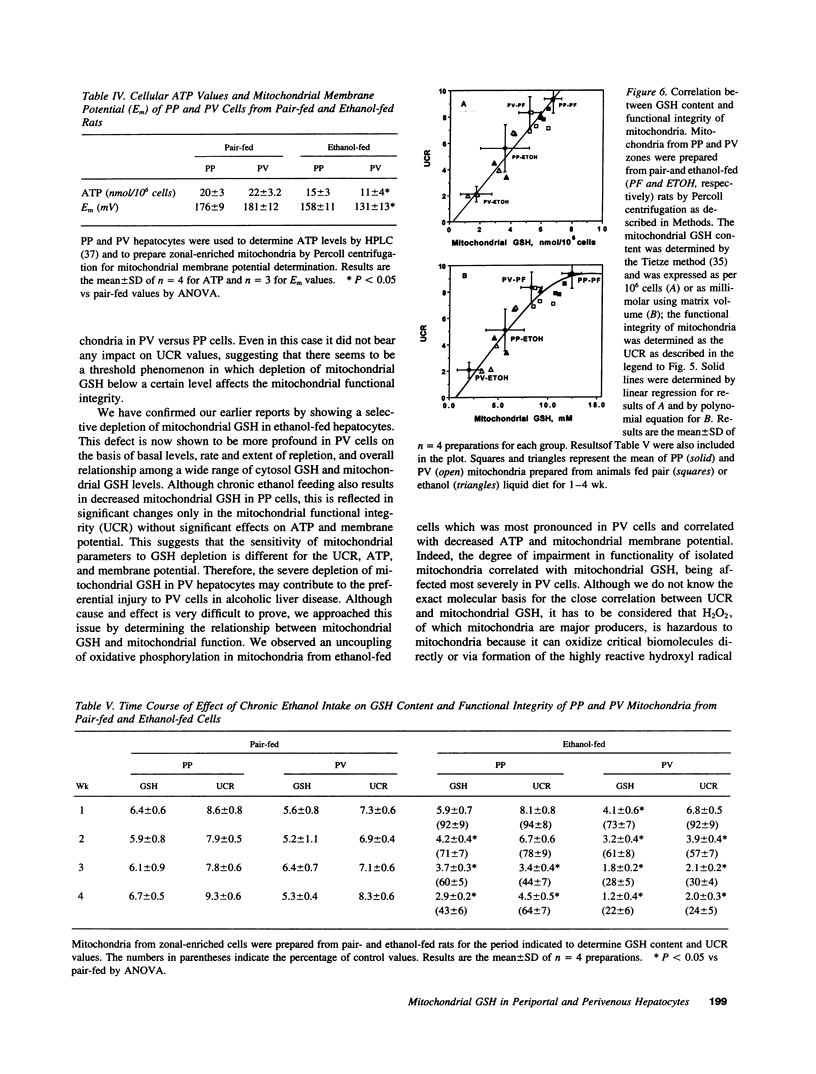
Chronic ethanol feeding selectively impairs the translocation of cytosol GSH into the mitochondrial matrix. Since ethanol-induced liver cell injury is preferentially localized in the centrilobular area, we examined the hepatic acinar distribution of mitochondrial GSH transport in ethanol-fed rats. Enriched periportal (PP) and perivenous (PV) hepatocytes from pair- and ethanol-fed rats were prepared as well as mitochondria from these cells. The mitochondrial pool size of GSH was decreased in both PP and PV cells from ethanol-fed rats either as expressed per 10(6) cells or per microliter of mitochondrial matrix volume. The rate of reaccumulation of mitochondrial GSH and the linear relationship of mitochondrial to cytosol GSH from ethanol-fed mitochondria were lower for both PP and PV cells, effects observed more prominently in the PV cells. Mitochondrial functional integrity was lower in both PP and PV ethanol-fed rats, which was associated with decreased cellular ATP levels and mitochondrial membrane potential, effects which were greater in the PV cells. Mitochondrial GSH depletion by ethanol feeding preceded the onset of functional changes in mitochondria, suggesting that mitochondrial GSH is critical in maintaining a functionally competent organelle and that the greater depletion of mitochondrial GSH by ethanol feeding in PV cells could contribute to the pathogenesis of alcoholic liver disease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braakman I., Keij J., Hardonk M. J., Meijer D. K., Groothuis G. M. Separation of periportal and perivenous rat hepatocytes by fluorescence-activated cell sorting: confirmation with colloidal gold as an exogenous marker. Hepatology. 1991 Jan;13(1):73–82. [PubMed] [Google Scholar]
- Cederbaum A. I., Rubin E. Molecular injury to mitochondria produced by ethanol and acetaldehyde. Fed Proc. 1975 Oct;34(11):2045–2051. [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Chen L., Sidner R. A., Lumeng L. Distribution of alcohol dehydrogenase and the low Km form of aldehyde dehydrogenase in isolated perivenous and periportal hepatocytes in rats. Alcohol Clin Exp Res. 1992 Feb;16(1):23–29. doi: 10.1111/j.1530-0277.1992.tb00630.x. [DOI] [PubMed] [Google Scholar]
- Cunningham C. C., Coleman W. B., Spach P. I. The effects of chronic ethanol consumption on hepatic mitochondrial energy metabolism. Alcohol Alcohol. 1990;25(2-3):127–136. doi: 10.1093/oxfordjournals.alcalc.a044987. [DOI] [PubMed] [Google Scholar]
- Fariss M. W., Reed D. J. High-performance liquid chromatography of thiols and disulfides: dinitrophenol derivatives. Methods Enzymol. 1987;143:101–109. doi: 10.1016/0076-6879(87)43018-8. [DOI] [PubMed] [Google Scholar]
- Fernandez-Checa J. C., Ookhtens M., Kaplowitz N. Effect of chronic ethanol feeding on rat hepatocytic glutathione. Compartmentation, efflux, and response to incubation with ethanol. J Clin Invest. 1987 Jul;80(1):57–62. doi: 10.1172/JCI113063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Checa J. C., Ookhtens M., Kaplowitz N. Effects of chronic ethanol feeding on rat hepatocytic glutathione. Relationship of cytosolic glutathione to efflux and mitochondrial sequestration. J Clin Invest. 1989 Apr;83(4):1247–1252. doi: 10.1172/JCI114008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Checa J. C., Ren C., Aw T. Y., Ookhtens M., Kaplowitz N. Effect of membrane potential and cellular ATP on glutathione efflux from isolated rat hepatocytes. Am J Physiol. 1988 Oct;255(4 Pt 1):G403–G408. doi: 10.1152/ajpgi.1988.255.4.G403. [DOI] [PubMed] [Google Scholar]
- Fernández-Checa J. C., García-Ruiz C., Ookhtens M., Kaplowitz N. Impaired uptake of glutathione by hepatic mitochondria from chronic ethanol-fed rats. Tracer kinetic studies in vitro and in vivo and susceptibility to oxidant stress. J Clin Invest. 1991 Feb;87(2):397–405. doi: 10.1172/JCI115010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Checa J. C., Kaplowitz N. The use of monochlorobimane to determine hepatic GSH levels and synthesis. Anal Biochem. 1990 Nov 1;190(2):212–219. doi: 10.1016/0003-2697(90)90183-a. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y., Stiggall D. L. Preparation and properties of succinate: ubiquinone oxidoreductase (complex II). Methods Enzymol. 1978;53:21–27. doi: 10.1016/s0076-6879(78)53008-5. [DOI] [PubMed] [Google Scholar]
- Hirano T., Kaplowitz N., Tsukamoto H., Kamimura S., Fernandez-Checa J. C. Hepatic mitochondrial glutathione depletion and progression of experimental alcoholic liver disease in rats. Hepatology. 1992 Dec;16(6):1423–1427. doi: 10.1002/hep.1840160619. [DOI] [PubMed] [Google Scholar]
- Jones D. P. Determination of pyridine dinucleotides in cell extracts by high-performance liquid chromatography. J Chromatogr. 1981 Oct 9;225(2):446–449. doi: 10.1016/s0378-4347(00)80293-5. [DOI] [PubMed] [Google Scholar]
- KIESSLING K. H., TOBE U. DEGENERATION OF LIVER MITOCHONDRIA IN RATS AFTER PROLONGED ALCOHOL CONSUMPTION. Exp Cell Res. 1964 Jan;33:350–354. doi: 10.1016/s0014-4827(64)81040-5. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N., Aw T. Y., Ookhtens M. The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol. 1985;25:715–744. doi: 10.1146/annurev.pa.25.040185.003435. [DOI] [PubMed] [Google Scholar]
- Kurosawa K., Hayashi N., Sato N., Kamada T., Tagawa K. Transport of glutathione across the mitochondrial membranes. Biochem Biophys Res Commun. 1990 Feb 28;167(1):367–372. doi: 10.1016/0006-291x(90)91774-m. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L., Vercesi A., Bababunmi E. A. Regulation of Ca2+ release from mitochondria by the oxidation-reduction state of pyridine nucleotides. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1690–1694. doi: 10.1073/pnas.75.4.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindros K. O., Penttilä K. E. Digitonin-collagenase perfusion for efficient separation of periportal or perivenous hepatocytes. Biochem J. 1985 Jun 15;228(3):757–760. doi: 10.1042/bj2280757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötscher H. R., Winterhalter K. H., Carafoli E., Richter C. Hydroperoxide-induced loss of pyridine nucleotides and release of calcium from rat liver mitochondria. J Biol Chem. 1980 Oct 10;255(19):9325–9330. [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meredith M. J., Reed D. J. Depletion in vitro of mitochondrial glutathione in rat hepatocytes and enhancement of lipid peroxidation by adriamycin and 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU). Biochem Pharmacol. 1983 Apr 15;32(8):1383–1388. doi: 10.1016/0006-2952(83)90451-3. [DOI] [PubMed] [Google Scholar]
- Moldéus P., Högberg J., Orrenius S. Isolation and use of liver cells. Methods Enzymol. 1978;52:60–71. doi: 10.1016/s0076-6879(78)52006-5. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Frayer W., Meister A. Glutathione metabolism in the lung: inhibition of its synthesis leads to lamellar body and mitochondrial defects. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5296–5300. doi: 10.1073/pnas.86.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Lai J. C., Meister A. High-affinity transport of glutathione is part of a multicomponent system essential for mitochondrial function. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7185–7189. doi: 10.1073/pnas.87.18.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Lai J. C., Meister A. High-affinity transport of glutathione is part of a multicomponent system essential for mitochondrial function. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7185–7189. doi: 10.1073/pnas.87.18.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Meister A. Mitochondrial damage in muscle occurs after marked depletion of glutathione and is prevented by giving glutathione monoester. Proc Natl Acad Sci U S A. 1989 Jan;86(2):471–475. doi: 10.1073/pnas.86.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUBERT D., WOJTCZAK A. B., LEHNINGER A. L. Purification and enzymatic identity of mitochondrial contraction-factors I and II. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1651–1658. doi: 10.1073/pnas.48.9.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper H., Lieber C. S. Histogenesis of alcoholic fibrosis and cirrhosis in the baboon. Am J Pathol. 1980 Mar;98(3):695–716. [PMC free article] [PubMed] [Google Scholar]
- Porta E. A., Hartroft W. S., De la Iglesia F. A. Hepatic changes associated with chronic alcoholism in rats. Lab Invest. 1965 Aug;14(8):1437–1455. [PubMed] [Google Scholar]
- Porter R. K., Scott J. M., Brand M. D. Choline transport into rat liver mitochondria. Characterization and kinetics of a specific transporter. J Biol Chem. 1992 Jul 25;267(21):14637–14646. [PubMed] [Google Scholar]
- Radi R., Bush K. M., Freeman B. A. The role of cytochrome c and mitochondrial catalase in hydroperoxide-induced heart mitochondrial lipid peroxidation. Arch Biochem Biophys. 1993 Jan;300(1):409–415. doi: 10.1006/abbi.1993.1055. [DOI] [PubMed] [Google Scholar]
- Radi R., Turrens J. F., Chang L. Y., Bush K. M., Crapo J. D., Freeman B. A. Detection of catalase in rat heart mitochondria. J Biol Chem. 1991 Nov 15;266(32):22028–22034. [PubMed] [Google Scholar]
- Reed D. J. Regulation of reductive processes by glutathione. Biochem Pharmacol. 1986 Jan 1;35(1):7–13. doi: 10.1016/0006-2952(86)90545-9. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. A procedure for the rapid preparation of mitochondria from rat liver. Biochem J. 1982 Jun 15;204(3):731–735. doi: 10.1042/bj2040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X., Aw T. Y., Shapira R., Jones D. P. Oxygen dependence of glutathione synthesis in hepatocytes. Toxicol Appl Pharmacol. 1989 Nov;101(2):261–270. doi: 10.1016/0041-008x(89)90275-5. [DOI] [PubMed] [Google Scholar]
- Sies H., Moss K. M. A role of mitochondrial glutathione peroxidase in modulating mitochondrial oxidations in liver. Eur J Biochem. 1978 Mar 15;84(2):377–383. doi: 10.1111/j.1432-1033.1978.tb12178.x. [DOI] [PubMed] [Google Scholar]
- Thayer W. S., Rubin E. Effects of chronic ethanol intoxication on oxidative phosphorylation in rat liver submitochondrial particles. J Biol Chem. 1979 Aug 25;254(16):7717–7723. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Wang T. T., Farrés J., Weiner H. Liver mitochondrial aldehyde dehydrogenase: in vitro expression, in vitro import, and effect of alcohols on import. Arch Biochem Biophys. 1989 Aug 1;272(2):440–449. doi: 10.1016/0003-9861(89)90238-5. [DOI] [PubMed] [Google Scholar]
- Wellner V. P., Meister A. Binding of adenosine triphosphate and adenosine diphosphate by glutamine synthetase. Biochemistry. 1966 Mar;5(3):872–879. doi: 10.1021/bi00867a010. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Marcillat O., Giulivi C., Ernster L., Davies K. J. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J Biol Chem. 1990 Sep 25;265(27):16330–16336. [PubMed] [Google Scholar]



