Abstract
Alpha-synuclein is the major protein in Lewy bodies, the hallmark pathological finding in Parkinson's disease (PD) and dementia with Lewy bodies (DLB). Although normally intracellular, it also can be secreted, so extracellular alpha-synuclein may contribute to neuronal injury. Serum antibodies to alpha-synuclein could exert protective effects by increasing alpha-synuclein's movement out of the brain and, if they cross the blood–brain barrier, by inhibiting its neurotoxic effects. The objective of this study was to measure antibody concentrations to alpha-synuclein monomer and soluble oligomers in three intravenous immunoglobulin (IVIG) preparations, Gamunex (Talecris Biotherapeutics), Gammagard (Baxter Healthcare) and Flebogamma (Grifols Biologicals). Antibodies were measured in native IVIG preparations and after antibody–antigen complex dissociation. IVIG's non-specific binding was subtracted from its total binding to alpha-synuclein to calculate specific anti-alpha-synuclein antibody concentrations. Specific antibodies to alpha-synuclein monomer and/or soluble oligomers were detected in all IVIG products. In native IVIG preparations, the highest anti-monomer concentrations were in Gammagard and the highest anti-oligomer concentrations were in Gamunex; the extent to which lot-to-lot variation may have contributed to these differences was not determined. Antibody–antigen complex dissociation had variable effects on these antibody levels. The IVIG preparations did not inhibit alpha-synuclein oligomer formation, although they changed the distribution and intensity of some oligomer bands on Western blots. The presence of antibodies to soluble alpha-synuclein conformations in IVIG preparations suggests that their effects should be studied in animal models of synucleinopathies, as a first step to determine their feasibility as a possible treatment for PD and other synucleinopathies.
Keywords: ELISA, intravenous immunoglobulin therapy (IVIG), neuroimmunology, Parkinson's disease, therapy/immunotherapy
Introduction
Alpha-synuclein (α-synuclein) is a 140 amino acid, 17 kDa molecular weight, heat-stable protein located primarily within presynaptic terminals of central nervous system (CNS) neurones [1]. Its native form is unfolded, with little ordered secondary structure [2]. It contains seven tandem repeat sequences in its amino-terminal half, followed by a hydrophobic central region termed the ‘non-β-amyloid component’ (NAC) and an acidic carboxyl terminus [3]. The NAC was reported to be present in amyloid-beta (Aβ)-containing plaques in Alzheimer's disease (AD) [4,5], although some later studies disputed this [6,7]. The role of α-synuclein is unclear; it may be involved in axonal transport [8] and/or dopamine homeostasis [9]. Insoluble α-synuclein fibrils are the main constituents of Lewy bodies, the hallmark pathological finding in individuals with Parkinson's disease (PD) and dementia with Lewy bodies (DLB) [10], and α-synuclein is also present in glial and neuronal inclusions in multiple system atrophy (MSA) [11]. However, soluble α-synuclein oligomers, rather than α-synuclein fibrils, may be the most neurotoxic α-synuclein conformation [12,13]. Increased expression of α-synuclein (gene duplication and triplication) and missense mutations which increase its aggregation have been associated with early-onset, autosomal dominant parkinsonism [14–17]. Although α-synuclein is primarily intracellular, it can also be secreted [18], so its extracellular aggregates may contribute to neuronal injury. Drugs which inhibit α-synuclein's aggregation are therefore being explored as possible therapeutic agents [19,20]. Another potential treatment for PD and other synucleinopathies is intravenous immunoglobulin (IVIG), which consists of purified immunoglobulins from thousands of clinically normal donors. IVIG has been used to treat autoimmune disorders and immunodeficiencies for several decades [21]. Two clinical trials in which IVIG preparations were administered to patients with AD produced encouraging results [22,23], but these drugs have not been evaluated in treatment of patients with other chronic neurodegenerative disorders, with the exception of small numbers of DLB and MSA patients [24,25]. Because serum antibodies to α-synuclein have been reported in some normal subjects as well as PD patients [26], we hypothesized that these antibodies might be present in IVIG. The objective of this study was to measure the concentrations of antibodies to soluble α-synuclein conformations in IVIG preparations.
Materials and methods
α-Synuclein disaggregation
Human recombinant α-synuclein (0·5 mg; rPeptide, Bogart, GA, USA) was disaggregated by suspending in 0·25 ml trifluoroacetic acid (hereafter, TFA; reagent grade TFA; Sigma-Aldrich, Inc., St Louis, MO, USA) followed by an equal volume of hexafluoro-2-propanol (Sigma-Aldrich). After waterbath sonication for 1 h, it was aliquoted into 0·6 ml eppitubes (20 µl/tube), air-dried overnight in a fume hood, and stored at −20°C.
Production of α-synuclein monomer
One eppitube of disaggregated α-synuclein was resuspended in 0·6 ml high performance liquid chromatography (HPLC)-grade water whose pH had been adjusted to 3·0 with TFA (hereafter, ‘TFA water’). This was repeated twice more, yielding 1·8 ml of resuspended α-synuclein; 21·8 mg of Tris base (Trizma base; Sigma-Aldrich) was then added to bring the Tris concentration to 100 mM. The pH of this solution was adjusted to 8·8 by adding 12·1 N HCl. This preparation, whose concentration was measured with the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA) to be 1 µg/ml, was centrifuged (11 752 g for 5 min), passed through a 0·2 µm filter (GHP Acrodisc 13 mm Syringe Filter with 0·2 µm GHP Membrane; Pall Life Sciences, East Hills, NY, USA), and used immediately.
Production of α-synuclein oligomers
Two eppitubes of previously disaggregated α-synuclein were resuspended in a total of 5 µl of phosphate-buffered saline (PBS, 0·01 M, pH 7·2); 50·3 µl of PBS was then added and the α-synuclein preparation was divided equally between two tubes. The protein concentration of this preparation was measured to be 43 µg/ml. The tubes were incubated in a shaking waterbath at 37°C for 4 days before use.
Western blot evaluation of α-synuclein conformations
α-Synuclein preparations were electrophoresed under reducing and denaturing conditions through 4–20% Tris-HCl Ready Gels (Bio-Rad). Twenty µl of the 1 µg/ml monomer preparation or 24 µl of the 43 µg/ml oligomer preparation was mixed with an equal volume of Laemmli Sample Buffer (Bio-Rad), boiled briefly, and loaded onto the gel. After electrophoresis, the proteins were transferred to Westran S polyvinylidene fluoride (PVDF) membranes (Whatman International Ltd, Maidstone, UK). Membranes were then blocked with blocking buffer for near infra-red fluorescent Western blotting (Rockland Immunocytochemicals, Gilbertsville, PA, USA) for 1 h at room temperature and incubated (overnight, 4°C, with agitation) in mouse monoclonal anti-α-synuclein antibody clone syn 211 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; 1:200 dilution), followed by IRDye 800 conjugated affinity purified rabbit anti-mouse IgG (LI-COR Biosciences, Lincoln, NE; 1:15 000 dilution) for 1 h at room temperature. Bands were visualized with LI-COR's Odyssey Infrared Imaging System, and densitometric scanning was subsequently performed using LI-COR's Odyssey Infrared Imaging System.
IVIG preparations
Three IVIG preparations were evaluated: Gamunex immune globulin intravenous (human), 10% (Talecris Biotherapeutics, Inc., Research Triangle Park, NC, USA), Gammagard liquid [immune globulin intravenous (human)] 10% (Baxter Healthcare Corp., Westlake Village, CA, USA) and immune globulin intravenous (human) Flebogamma 5% DIF 2·5 g (Grifols Biologicals Inc., Los Angeles, CA, USA).
Dissociation of antibody–antigen complexes in IVIG preparations
The procedure described by Li et al. [27] for antibody–antigen dissociation (hereafter, ‘dissociation’) was followed with slight modifications, as reported in our previous study [28]. Anti-α-synuclein antibodies were measured in dissociated, as well as native, IVIG preparations because an earlier study [27] found that dissociation of anti-Aβ antibodies from Aβ by brief incubation of serum under acidic conditions (pH 3·5) led to an increase in detectable anti-Aβ antibody levels, apparently by unmasking antibody previously bound to antigen. This procedure should be applicable for other antibodies as well as those to Aβ.
Measurement of IVIG antibodies to α-synuclein monomer
Antibody (IgG) concentrations to α-synuclein monomer were measured by indirect enzyme-linked immunosorbent assay (ELISA) in four experiments for each IVIG preparation. IVIG preparations were randomized as to the order in which these experiments were performed. α-Synuclein monomer was resuspended to 1 µg/ml in Tris buffer (0·1 M, pH 8·8), passed through a 0·2 µm filter, and incubated on a 96-well Nunc Maxisorp plate (Nalge Nunc International, Rochester, NY, USA). As a ‘specificity control’, the same concentration of bovine serum albumin (BSA, 98%; Sigma-Aldrich) in Tris buffer was filtered and placed in adjacent wells. After incubation overnight at 4°C, wells were washed three times with PBS with 0·1% Tween-20 (Sigma-Aldrich) (hereafter, PBS-T; this wash step was repeated after all subsequent incubations), then treated with SuperBlock (SuperBlock Blocking Buffer in PBS; Thermo Scientific, Rockford, IL, USA), as per the manufacturer's instructions, followed by native or dissociated IVIG preparations, diluted in PBS-T with 1% BSA (hereafter, PBS-T-BSA). Two dilutions of each IVIG preparation were evaluated in each experiment, as follows: Gamunex, 1:100 and 1:200; Gammagard, 1:100 and 1:200; and Flebogamma, 1:50 and 1:100. Three wells were incubated for each condition. Fourfold dilutions of rabbit anti-α-synuclein antibody (Millipore, Billerica, MA, USA), from 1:1000 (2000 ng/ml) to 1:256 000 (7·8 ng/ml) were included for the standard curve on each plate; blank wells received PBS-T-BSA only. Secondary anti-sera were biotinylated goat anti-human IgG (Jackson Immunoresearch Laboratories, West Grove, PA, USA; 1:1000 dilution) for wells with IVIG preparations and biotinylated goat anti-rabbit IgG (Vector Laboratories, Inc., Burlingame, CA, USa; 1:1000 dilution) for wells with the standard curve. The remaining steps in the ELISA were as described in our previous study [28]. To calculate specific anti-α-synuclein monomer antibody concentrations, the mean antibody concentration measured when each IVIG preparation was incubated on BSA-coated wells was subtracted from antibody concentrations measured on wells coated with α-synuclein monomer.
Measurement of IVIG antibodies antibodies to α-synuclein soluble oligomers
In separate experiments (four for each IVIG preparation) in which specific antibodies were measured to α-synuclein oligomers, the procedure was as described above for anti-monomer antibodies except that ELISA plates were coated initially with both the α-synuclein monomer and the oligomer preparations at 1 µg/ml. Because densitometric analysis of Western blots indicated that approximately 50% of the total band intensity in the oligomer preparation was due to α-synuclein monomer (see below), 50% of the mean anti-monomer antibody concentration was subtracted from the specific antibody concentrations to the oligomer preparation.
Evaluation of binding curves of α-synuclein monomer to IVIG and BSA: specific versus non-specific binding
A sandwich ELISA was performed to determine if binding curve characteristics could be used to distinguish between α-synuclein's specific binding to wells coated with monoclonal anti-mouse α-synuclein, its non-specific binding to wells coated with BSA and its binding to wells coated with one of the IVIG products, Gammagard. Gammagard was chosen because our studies indicated that it contained the highest specific antibody levels to α-synuclein monomer among the IVIG products studied (see Results, Fig. 3). Specific, receptor-mediated binding of antigen by antibody should be saturable; when antigen is in excess then this binding should plateau, and with increasing antigen concentrations the binding should decrease because of the prozone effect [29]. The ELISA plate was coated overnight with mouse monoclonal clone 211 anti-α-synuclein [diluted to 1:160 (1·25 µg/ml), because a preliminary experiment showed this dilution to be in excess when the α-synuclein concentration was 1 µg/ml (data not shown)], Gammagard (1:100 dilution; = 1 mg/ml, or BSA (1 mg/ml). After overnight incubation, the plate was treated with Superblock, then incubated overnight with α-synuclein monomer serially diluted from 10 µg/ml to 78 ng/ml in Tris buffer. All wells then received rabbit anti-α-synuclein (1:500; 1 h, 37°C), followed by biotinylated goat anti-rabbit IgG (Vector), avidin-conjugated alkaline phosphatase and PNPP as described above.
Fig. 3.

Specific anti-α-synuclein monomer concentrations (means ± standard error of the mean), n = 6 wells/condition; µg of specific antibody/g total protein) in native and antibody–antigen complex-dissociated intravenous immunoglobulin (IVIG) preparations. aP < 0·01 versus dissociated Gamunex; bP < 0·01 versus dissociated Gammagard; cP < 0·01 versus dissociated Flebogamma; dP < 0·01 versus native Gamunex; eP < 0·01 versus native Gammagard; fP < 0·01 versus native Flebogamma (no specific antibodies to α-synuclein monomer were detected in native Gamunex and dissociated Flebogamma).
Influence of IVIG preparations on α-synuclein oligomer formation
Gammagard, Gamunex and Flebogamma were diluted 1:50, 1:50 and 1:25, respectively, in PBS (0·01 M, pH 7·4, with 0·02% sodium azide) to obtain total protein concentrations of 2 mg/ml. Rabbit anti-α-synuclein was diluted 1:250 (= 500 ng/ml) in PBS/azide, because preliminary experiments found this concentration to be in excess when incubated with 1 µg/ml of α-synuclein monomer. Mouse monoclonal anti-α-synuclein 5C2 (Santa Cruz Biotechnology) was diluted 1:80 (= 1·25 µg/ml) and normal mouse immunoglobulin (clone MOPC-21, IgG1 kappa from murine myeloma; Sigma-Aldrich) was diluted 1:720 (1·25 µg/ml) in PBS/azide. Of these antibodies, 27·7 µl (and, as a negative control, PBS alone) were placed in tubes containing 1·8 µg of disaggregated α-synuclein, which was then vortexed to resuspend it. These preparations were placed in a shaking waterbath (37°C) for 4–5 days, then evaluated by Western blot as described above.
Statistics
Data from typical experiments were analysed. Between-group significance was evaluated for antibody concentrations (µg of specific antibody/g total protein) to monomeric and oligomeric α-synuclein between IVIG products. Two dilutions for each IVIG were used in each experiment (1:100 and 1:200 for both Gamunex and Gammagard, and 1:50 and 1:100 for Flebogamma). Because the calculated antibody concentrations were similar for the two dilutions, the data from both dilutions were pooled, resulting in six values per condition in each experiment. To compare results between experiments, data from each experiment were adjusted for interassay variation by multiplying them by a normalization factor. This factor was derived by first determining the observed (calculated) concentration in each experiment of ‘positive control’ wells which had received a 1:16 000 dilution (125 ng/ml) of rabbit anti-α-synuclein antibody. To calculate the normalization factor for each experiment, the mean calculated concentration for this antibody for all experiments was divided by its observed concentration in each experiment. Data for anti-α-synuclein monomer antibodies were normalized separately from those for anti-oligomer antibodies. The distribution of each data set was first analysed to determine if it met the assumptions of the statistical tests proposed to analyse it. Either standard one-way analysis of variance (anova) or the Welch test was used to assess differences among the three IVIG preparations. Post-hoc pairwise comparisons of anti-monomer antibodies between dissociated IVIG preparations used Tukey's multiple comparison procedures, and other post-hoc comparisons used the T3′ procedure for pairwise multiple comparisons for normal data with unequal variances [30,31].
To determine statistical significance between mean optical density (OD) values for binding of IVIG products to wells coated with α-synuclein preparations versus wells coated with buffer alone, P-values were obtained by applying the Holm procedure (stepdown Bonferroni) to the P-values obtained from two-sample t-tests with the Satterthwaite approximation, to adjust for multiple comparisons. Statistical analyses involving OD values were performed on the more concentrated dilution for each IVIG preparation (1:100 for Gamunex and Gammagard, and 1:50 for Flebogamma).
Statistical analyses were performed using the sas system for Windows version 9·2 and Minitab Release 14. P-values ≤ 0·05 were considered statistically significant; all P-values were two-tailed.
Results
Production of α-synuclein monomer and soluble oligomers
The α-synuclein monomer preparation, generated as described above, produced one band on Western blot with a molecular weight of approximately 16–17 kDa. The α-synuclein oligomer preparation contained monomer, dimer and trimer bands, as well as faint higher molecular weight bands (Fig. 1).
Fig. 1.
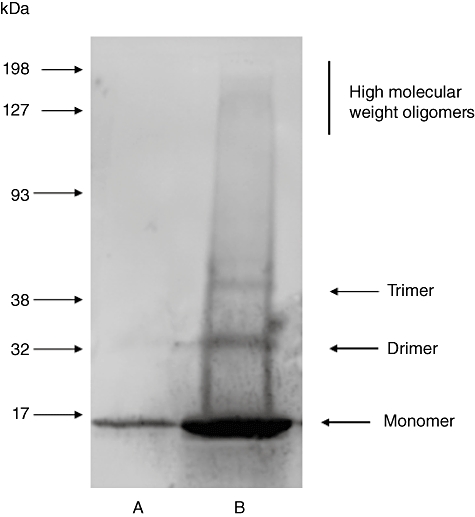
Western blot: α-synuclein monomer (lane A; 0·02 µg) and oligomer (lane B; 1·03 µg) preparations. The oligomer preparation was not pure; densitometric analysis indicated that approximately 50% of the total band density in this preparation was due to α-synuclein monomer.
IVIG antibodies to α-synuclein monomer
Specific antibody concentrations in IVIG preparations to α-synuclein monomer were calculated by subtracting their binding to BSA-coated wells from binding to wells coated with the same concentration of α-synuclein monomer. Although serum antibodies to BSA have been reported in some normal subjects [32,33], we found no difference between the binding of IVIG preparations to wells coated with BSA versus wells coated with buffer in more than 20 ELISAs which we performed in our previous study [28]. Thus, if these IVIG preparations contain anti-BSA antibodies, then the levels are so low as to be undetectable in our ELISA. IVIG binding to BSA-coated wells in the present study was therefore considered be non-specific. This accounted for 98% of native Gamunex's binding, 44% of native Gammagard's binding and 67% of native Flebogamma's binding to α-synuclein monomer (mean OD values for binding of native IVIG preparations to α-synuclein monomer and BSA are shown in Fig. 2). The IVIG preparations differed in their mean concentrations of specific anti-monomer antibodies (P < 0·01 for all pairwise comparisons); the highest levels were in Gammagard and the lowest were in Gamunex (Fig. 3). Because only one IVIG dilution could be used for the statistical analysis of OD data in Fig. 2, this resulted in a discrepancy with the data for specific anti-monomer antibody concentrations shown in Fig. 3, in which data from both dilutions were used. In Fig. 2, binding of dissociated Flebogamma to monomeric α-synuclein achieved marginal statistical significance compared with its binding to BSA (P = 0·042) but, as shown in Fig. 3, when all six OD values were converted to specific antibody concentrations, no specific binding for dissociated Flebogamma to monomeric α-synuclein was found. Measurements of specific anti-α-synuclein monomer antibodies in IVIG after dissociation of antibody–antigen complexes also required subtraction of non-specific binding, as indicated by the OD values produced when the dissociated IVIG products were incubated on BSA-coated wells (Fig. 2). Non-specific binding accounted for 26% of dissociated Gamunex's binding, 46% of dissociated Gammagard's binding and 89% of dissociated Flebogamma's binding to α-synuclein monomer. Dissociation increased the detectable anti-monomer antibody concentrations in Gamunex, while decreasing them in the other IVIG products (all P < 0·01; Fig. 3). The antibody levels to α-synuclein monomer differed between the three dissociated IVIG products (P = 0·0011 for Gamunex versus Gammagard; P < 0·0001 for comparisons involving Flebogamma), with the highest levels in Gamunex and the lowest levels in Flebogamma.
Fig. 2.

Assessment of specific versus non-specific immunoreactivity of intravenous immunoglobulin (IVIG) preparations in enzyme-linked immunosorbent assay (ELISA) measuring antibodies to α-synuclein monomer. Data shown are optical density values (means ± standard error of the mean) for binding of native and antibody-antigen-dissociated IVIG preparations to wells coated with α-synuclein monomer or bovine serum albumin (BSA). aP < 0·001 versus BSA; bP < 0·01 versus BSA; cP < 0·05 versus BSA (GX = Gamunex, 1:100 dilution; GG = Gammagard, 1:100 dilution; FG = Flebogamma, 1:50 dilution; n = 2 for binding of dissociated Gammagard to BSA, n = 3 for other conditions).
IVIG antibodies to α-synuclein soluble oligomers
Extensive non-specific binding of the IVIG products (e.g. to BSA-coated as well as oligomer-coated wells) was present, similar to the ELISAs for antibodies to α-synuclein monomer (data not shown). Specific anti-α-synuclein oligomer concentrations in native IVIG products were significantly different from each other, with the highest levels in Gamunex and the lowest ones in Flebogamma (Fig. 4, P < 0·01 for all comparisons). Antibody–antigen complex dissociation significantly altered the anti-oligomer concentrations in all IVIG preparations, increasing them only in Flebogamma (P < 0·01 for Gamunex and Gammagard; P < 0·05 for Flebogamma). For dissociated IVIG products, the highest anti-α-synuclein oligomer concentrations were in Flebogamma and Gammagard (differences between these two did not achieve significance); statistical significance was present only between Flebogamma and Gamunex (Flebogamma > Gamunex, P < 0·05).
Fig. 4.

Specific anti-α-synuclein oligomer concentrations (means ± standard error of the mean), n = 6 wells/condition; µg of specific antibody/g total protein) in native and antibody–antigen complex-dissociated intravenous immunoglobulin (IVIG) preparations. aP < 0·01 versus dissociated Gamunex; bP < 0·01 versus dissociated Gammagard; cP < 0·05 versus dissociated Flebogamma; dP < 0·01 versus native Gamunex; eP < 0·01 versus native Gammagard; fP < 0·01 versus native Flebogamma; gP < 0·05 versus dissociated Gamunex; hP < 0·05 versus native Flebogamma.
IVIG's binding curve to α-synuclein monomer
Using a sandwich ELISA, α-synuclein's binding to wells coated with the IVIG product Gammagard was compared to its binding to wells coated with mouse monoclonal anti-α-synuclein clone syn 211 or with BSA, to determine if Gammagard's binding to α-synuclein could be characterized as specific or non-specific on the basis of its binding curve. However, all three curves followed a similar pattern, levelling-off with increased α-synuclein concentrations (Fig. 5). [A similar curve was seen when wells were coated with buffer alone, although the plateau occurred at lower concentrations of α-synuclein (data not shown).]
Fig. 5.

Binding curves for wells coated with intravenous immunoglobulin (IVIG) (Gammagard, 1 mg/ml), mouse monoclonal anti-α-synuclein clone syn 211 (1·25 µg/ml), or bovine serum albumin (BSA) (1 mg/ml), followed by increasing concentrations of α-synuclein monomer. Levelling-off of the curve with increasing α-synuclein concentrations was observed with all three conditions.
Influence of IVIG preparations on α-synuclein oligomer formation
Disaggregated α-synuclein was incubated for up to 4 days at 37°C with IVIG preparations (Gammagard 1:50, Gamunex 1:50, Flebogamma 1:25), mouse monoclonal anti-α-synuclein (clone 5C2), rabbit anti-α-synuclein, normal mouse IgG (MOPC-21 mouse myeloma) or PBS, then analysed with Western blot (Fig. 6). Oligomer formation was detected in all conditions, although incubation of α-synuclein with the IVIG preparations changed the distribution of the oligomer bands. Similar results were obtained with all three IVIG preparations. The band migrating between the 28 kDa and 39 kDa standards (probable dimer) was unchanged after incubation with Gammagard or Gamunex (lanes D and E, respectively), but appeared darker after incubation with Flebogamma (lane F). The band which ran slightly below the 60 kDa standard (probable trimer) was detected when α-synuclein was incubated in the presence of commercial anti-α-synuclein antibodies, but was not seen when it was incubated with the IVIG preparations. In contrast, the band which migrated between the 60 kDa and 84 kDa standards (probable tetramer) was more prominent when α-synuclein was incubated with IVIG products than with the commercial anti-α-synuclein antibodies or PBS.
Fig. 6.

Effect of incubation intravenous immunoglobulin (IVIG) preparations on α-synuclein oligomer formation. Western blot shows products resulting from incubation of disaggregated α-synuclein with IVIG products, commercial anti-α-synuclein antibodies, normal mouse IgG or buffer. Lane A: α-synuclein monomer (0·02 µg), prepared as described in text; lanes B–I, α-synuclein oligomer preparations (total α-synuclein loaded in each lane: 1·6 µg) after incubation with the following (duration of incubation: lane B, 1 day; other lanes, 4 days): lanes B and C, phosphate-buffered saline (PBS); lane D, Gammagard (1:50 dilution); lane E, Gamunex (1:50); lane F, Flebogamma (1:25); lane G, rabbit-anti-α-synuclein (1:250); lane H, mouse monoclonal anti-α-synuclein 5C2 (1:50), lane I, MOPC-21 [normal mouse IgG; 1:720 (1·25 µg/ml)].
Discussion
The major finding in this study is that IVIG products contain specific antibodies to α-synuclein monomer and soluble oligomers. There were marked differences between the native IVIG products with respect to these antibody levels, and their relative order differed between anti-monomer and anti-oligomer levels. The issue of variability in specific antibody concentrations between IVIG preparations has been raised by previous investigators [34–36], and may be due to differences in preparation methods and/or donor populations. Because lot-to-lot variability for specific antibody levels in IVIG preparations has also been reported [37,38], the possibility cannot be ruled out that the differences we detected in anti-α-synuclein antibody levels between IVIG preparations could be due, at least in part, to lot-to-lot variation. Examination of the lot-to-lot variability of the three IVIG preparations for antibodies to α-synuclein monomer and soluble oligomers was beyond the scope of this study.
None of the IVIG preparations prevented formation of α-synuclein soluble oligomers, and neither did commercial anti-α-synuclein sera. This contrasts with our earlier finding that Gamunex (1:100 dilution) prevented formation of Aβ soluble oligomers [39] (the effects of the other IVIG products on Aβ oligomer formation were not evaluated in that study), as well as a previous report that some antibodies are able to prevent α-synuclein aggregation [40]. Interference with the C-terminal region of α-synuclein or its hydrophobic core region can influence α-synuclein folding and aggregation [41], so perhaps these regions of α-synuclein were not bound by IVIG; or, if they were bound, then the affinity of this binding was not sufficient to prevent oligomer formation. This does not explain why the rabbit and mouse anti-α-synuclein antibodies in our study, which were generated against amino acid residues 111–131 and 61–95, respectively, did not inhibit oligomer formation; perhaps higher antibody concentrations were required. Determination of the significance of the changes that were seen in α-synuclein oligomer bands when oligomer development was allowed to proceed in the presence of IVIG (in particular, the increased intensity of the tetramer band) requires further study.
Because α-synuclein is produced primarily by neurones [42], its presence in peripheral blood [43] suggests that it may move across the blood–brain barrier in a bidirectional manner. Anti-α-synuclein antibodies in peripheral blood, whether naturally occurring or due to IVIG treatment, could reduce CNS α-synuclein levels by increasing its efflux from the brain, similar to the ‘peripheral sink’ hypothesis proposed for Aβ[44], and/or by reducing the entrance of circulating α-synuclein into the brain through the formation of antibody–antigen complexes. If antibodies to α-synuclein are able to enter the brain in synucleinopathies, then they could block α-synuclein's activation of glial cells [45,46] or reduce the neurotoxicity of extracellular α-synuclein. The possible benefits of anti-α-synuclein antibodies for treatment of synucleinopathies were suggested by a study by Masliah et al. [47], in which vaccination with human α-synuclein in transgenic mice expressing the gene for this protein prevented synaptic loss; this protective effect was associated with production of high-affinity anti-α-synuclein antibodies.
Much of the binding of IVIG products to α-synuclein-coated wells was non-specific. We reported similar findings in our measurements of anti-Aβ antibodies in these same IVIG preparations [39]. This non-specific binding must be subtracted in order to measure accurately IVIG's specific antibodies to α-synuclein. We performed a sandwich ELISA to determine if the curve for the IVIG product Gammagard's binding to α-synuclein would be indicative of specific or non-specific binding. Levelling-off of the binding curve occurred with either Gammagard or mouse anti-α-synuclein as the capture antibody, but it was also present when α-synuclein was incubated on wells coated initially with BSA; the plateau in this case was due probably to steric hindrance. This indicates that binding curve characteristics are not of value for determining the specificity of Gammagard's binding to α-synuclein.
We conclude that specific antibodies to soluble conformations of α-synuclein are present in, and may differ between, IVIG preparations. These results suggest that an investigation of IVIG's therapeutic effects in animal models of synucleinopathies, and perhaps eventually in patients with these disorders, is warranted.
Acknowledgments
This study was supported by a generous donation from Mr Norman Merollis.
Disclosure
The authors have no conflicts of interest or financial interest in the products used in this work.
References
- 1.Giasson BI, Murray IV, Trojanowski JQ, Lee VM. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–6. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 2.Uversky VN, Lee HJ, Li J, Fink AL, Lee SJ. Stabilization of partially folded conformation during alpha-synuclein oligomerization in both purified and cytosolic preparations. J Biol Chem. 2001;276:43495–8. doi: 10.1074/jbc.C100551200. [DOI] [PubMed] [Google Scholar]
- 3.Miake H, Mizusawa H, Iwatsubo T, Hasegawa M. Biochemical characterization of the core structure of alpha-synuclein filaments. J Biol Chem. 2002;277:19213–19. doi: 10.1074/jbc.M110551200. [DOI] [PubMed] [Google Scholar]
- 4.Uéda K, Fukushima H, Masliah E, et al. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:11282–6. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masliah E, Iwai A, Mallory M, Uéda K, Saitoh T. Altered presynaptic protein NACP is associated with plaque formation and neurodegeneration in Alzheimer's disease. Am J Pathol. 1996;148:201–10. [PMC free article] [PubMed] [Google Scholar]
- 6.Culvenor JG, McLean CA, Cutt S, et al. Non-Abeta component of Alzheimer's disease amyloid (NAC) revisited. NAC and alpha-synuclein are not associated with Abeta amyloid. Am J Pathol. 1999;155:1173–81. doi: 10.1016/s0002-9440(10)65220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayer TA, Jäkälä P, Hartmann T, et al. Alpha-synuclein accumulates in Lewy bodies in Parkinson's disease and dementia with Lewy bodies but not in Alzheimer's disease beta-amyloid plaque cores. Neurosci Lett. 1999;266:213–16. doi: 10.1016/s0304-3940(99)00311-0. [DOI] [PubMed] [Google Scholar]
- 8.Clayton DF, George JM. Synucleins in synaptic plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58:120–9. [PubMed] [Google Scholar]
- 9.Sidhu A, Wersinger C, Vernier P. Alpha-synuclein regulation of the dopaminergic transporter: a possible role in the pathogenesis of Parkinson's disease. FEBS Lett. 2004;565:1–5. doi: 10.1016/j.febslet.2004.03.063. [DOI] [PubMed] [Google Scholar]
- 10.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–73. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H. Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett. 1998;249:180–2. doi: 10.1016/s0304-3940(98)00407-8. [DOI] [PubMed] [Google Scholar]
- 12.Trojanowski JQ, Lee VM. ‘Fatal attractions’ of proteins. A comprehensive hypothetical mechanism underlying Alzheimer's disease and other neurodegenerative disorders. Ann NY Acad Sci. 2000;924:62–7. doi: 10.1111/j.1749-6632.2000.tb05561.x. [DOI] [PubMed] [Google Scholar]
- 13.Periquet M, Fulga T, Myllykangas L, Schlossmacher MG, Feany MB. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J Neurosci. 2007;27:3338–46. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasser T. Genetics of Parkinson's disease. Curr Opin Neurol. 2005;18:363–9. doi: 10.1097/01.wco.0000170951.08924.3d. [DOI] [PubMed] [Google Scholar]
- 15.Chartier-Harlin MC, Kachergus J, Roumier C, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–9. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 16.Singleton AB, Farrer M, Johnson J, et al. Alpha-synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 17.Krüger R, Kuhn W, Müller T, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–8. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ. Origins and effects of extracellular alpha-synuclein: implications in Parkinson's disease. J Mol Neurosci. 2008;34:17–22. doi: 10.1007/s12031-007-0012-9. [DOI] [PubMed] [Google Scholar]
- 19.El-Agnaf OM, Paleologou KE, Greer B, et al. A strategy for designing inhibitors of alpha-synuclein aggregation and toxicity as a novel treatment for Parkinson's disease and related disorders. FASEB J. 2004;18:1315–17. doi: 10.1096/fj.03-1346fje. [DOI] [PubMed] [Google Scholar]
- 20.Amer DA, Irvine GB, El-Agnaf OM. Inhibitors of alpha-synuclein oligomerization and toxicity: a future therapeutic strategy for Parkinson's disease and related disorders. Exp Brain Res. 2006;173:223–33. doi: 10.1007/s00221-006-0539-y. [DOI] [PubMed] [Google Scholar]
- 21.Scheinfeld NS, Godwin JE. Intravenous immunoglobulin. Emedicine. 2008 Last updated: 22 June 2010. Available at: http://emedicine.medscape.com/article/210367-overview (accessed 18 May 2010. [Google Scholar]
- 22.Dodel RC, Du Y, Depboylu C, et al. Intravenous immunoglobulins containing antibodies against beta-amyloid for the treatment of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2004;75:1472–4. doi: 10.1136/jnnp.2003.033399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Relkin NR, Szabo P, Adamiak B, et al. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging. 2009;30:1728–36. doi: 10.1016/j.neurobiolaging.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Shankle WR, Hara J. Longitudinal measure of IVIG treatment effect in patients with Alzheimer's and Lewy body disease. Alzheimers Dement. 2009;5:P430. [Google Scholar]
- 25.Nanri K, Okita M, Takeguchi M, et al. Intravenous immunoglobulin therapy for autoantibody-positive cerebellar ataxia. Intern Med. 2009;48:783–90. doi: 10.2169/internalmedicine.48.1802. [DOI] [PubMed] [Google Scholar]
- 26.Papachroni KK, Ninkina N, Papapanagiotou A, et al. Autoantibodies to alpha-synuclein in inherited Parkinson's disease. J Neurochem. 2007;101:749–56. doi: 10.1111/j.1471-4159.2006.04365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Gordon M, Cao C, Ugen KE, Morgan D. Improvement of a low pH antigen–antibody dissociation procedure for ELISA measurement of circulating anti-Abeta antibodies. BMC Neurosci. 2007;8:22. doi: 10.1186/1471-2202-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klaver AC, Finke JM, Digambaranath J, Balasubramaniam M, Loeffler DA. Antibody concentrations to Aβ1-42 monomer and soluble oligomers in untreated and antibody–antigen-dissociated in intravenous immunoglobulin preparations. Int Immunopharmacol. 2010;10:115–19. doi: 10.1016/j.intimp.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Heidelberger M, Kendall FE. A quantitative theory of the precipitin reaction. III. The reaction between crystalline egg albumin and its homologous antibody. J Exp Med. 1935;62:697–720. doi: 10.1084/jem.62.5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunnett CW. Pairwise multiple comparisons in the unequal variance case. J Am Stat Assoc. 1980;75:796–800. [Google Scholar]
- 31.Korhonen MP. On the performance of some multiple comparison procedures with unequal variances. Scand J Statist. 1982;9:241–7. [Google Scholar]
- 32.Beck DA, Rossen RD, Cangir A, DuBois DB. Correlation of immune complexes in disseminated neuroblastoma with serum antibody to bovine serum albumin. Cancer Res. 1983;43:879–85. [PubMed] [Google Scholar]
- 33.Karjalainen J, Saukkonen T, Savilahti E, Dosch HM. Disease-associated anti-bovine serum albumin antibodies in type 1 (insulin-dependent) diabetes mellitus are detected by particle concentration fluoroimmunoassay, and not by enzyme linked immunoassay. Diabetologia. 1992;35:985–90. doi: 10.1007/BF00401430. [DOI] [PubMed] [Google Scholar]
- 34.Lamari F, Anastassiou ED, Tsegenidis T, Dimitracopoulos G, Karamanos NK. An enzyme immunoassay to determine the levels of specific antibodies toward bacterial surface antigens in human immunoglobulin preparations and blood serum. J Pharm Biomed Anal. 1999;20:913–20. doi: 10.1016/s0731-7085(99)00087-4. [DOI] [PubMed] [Google Scholar]
- 35.Krause I, Wu R, Sherer Y, Patanik M, Peter JB, Shoenfeld Y. In vitro antiviral and antibacterial activity of commercial intravenous immunoglobulin preparations – a potential role for adjuvant intravenous immunoglobulin therapy in infectious diseases. Transfus Med. 2002;12:133–9. doi: 10.1046/j.1365-3148.2002.00360.x. [DOI] [PubMed] [Google Scholar]
- 36.Khan S, Sims S, Raine D, Sewell WA. Both autoantibodies and pathogen-specific antibodies are present in immunoglobulin preparations and reflect characteristics of the donor population. J Am Acad Dermatol. 2008;59:1089–90. doi: 10.1016/j.jaad.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 37.Lejtenyi D, Mazer B. Consistency of protective antibody levels across lots of intravenous immunoglobulin preparations. J Allergy Clin Immunol. 2008;121:254–5. doi: 10.1016/j.jaci.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Reipert BM, Stellamor MT, Poell M, et al. Variation of anti-Fas antibodies in different lots of intravenous immunoglobulin. Vox Sang. 2008;94:334–41. doi: 10.1111/j.1423-0410.2008.001036.x. [DOI] [PubMed] [Google Scholar]
- 39.Klaver AC, Patrias LM, Coffey MP, Finke JM, Loeffler DA. Measurement of anti-Aβ1-42 antibodies in intravenous immunoglobulin with indirect ELISA: the problem of nonspecific binding. J Neurosci Methods. 2010;187:263–9. doi: 10.1016/j.jneumeth.2010.01.018. Epub 25 January 2010. [DOI] [PubMed] [Google Scholar]
- 40.Beyer K, Ariza A. The therapeutical potential of alpha-synuclein antiaggregatory agents for dementia with Lewy bodies. Curr Med Chem. 2008;15:2748–59. doi: 10.2174/092986708786242868. [DOI] [PubMed] [Google Scholar]
- 41.Emadi S, Liu R, Yuan B, et al. Inhibiting aggregation of alpha-synuclein with human single chain antibody fragments. Biochemistry. 2004;43:2871–8. doi: 10.1021/bi036281f. [DOI] [PubMed] [Google Scholar]
- 42.Iwai A, Masliah E, Yoshimoto M, et al. The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–75. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 43.El-Agnaf OM, Salem SA, Paleologou KE, et al. Alpha-synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003;17:1945–7. doi: 10.1096/fj.03-0098fje. [DOI] [PubMed] [Google Scholar]
- 44.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2001;98:8850–5. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W, Wang T, Pei Z, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J. 2005;19:533–42. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 46.Klegeris A, Giasson BI, Zhang H, Maguire J, Pelech S, McGeer PL. Alpha-synuclein and its disease-causing mutants induce ICAM-1 and IL-6 in human astrocytes and astrocytoma cells. FASEB J. 2006;20:2000–8. doi: 10.1096/fj.06-6183com. [DOI] [PubMed] [Google Scholar]
- 47.Masliah E, Rockenstein E, Adame A, et al. Effects of alpha-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005;46:857–68. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]


