Abstract
In spite of advances in immunology on mycobacterial infection, there are few studies on the role of anti-microbial peptides in tuberculosis. The cathelin-related anti-microbial peptide (CRAMP) is the only cathelicidin isolated from mice. In this work we investigated the cellular sources and the production kinetics of this molecule during experimental tuberculosis, using two well-characterized models of latent or chronic infection and progressive disease. The lung of non-infected control mice expressed CRAMP at very low levels. In both models of experimental tuberculosis the main cells immunolabelled for CRAMP were bronchial epithelial cells, macrophages and pneumocytes types II and I. After intratracheal infection with a high bacilli dose (H37Rv strain) in Balb/c mice to produce progressive disease, a high CRAMP gene expression was induced showing three peaks: very early after 1 day of infection, at day 21 when the peak of protective immunity in this model is raised, and at day 28 when the progressive phase starts and the immunoelectronmicroscopy study showed intense immunolabelling in the cell wall and cytoplasm of intracellular bacilli, as well as in cytoplasmic vacuoles. Interestingly, at day 60 post-infection, when advanced progressive disease is well established, characterized by high bacillary loads and extensive tissue damage, CRAMP gene expression decreased but strong CRAMP immunostaining was detected in vacuolated macrophages filled with bacilli. Thus, cathelecidin is highly produced during experimental pulmonary tuberculosis from diverse cellular sources and could have significant participation in its pathogenesis.
Keywords: cathelicidin, Mycobacterium tuberculosis, tuberculosis
Introduction
Tuberculosis (TB) still has a direct detrimental impact on public health worldwide. Indeed, approximately one-third of the world's population is latently infected with Mycobacterium tuberculosis, and there are at present 8 million new cases of active disease per year [1]. During primary tuberculosis infection, bacilli growth is usually well controlled by the immune system; however, it is common that not all bacteria are eliminated. Some bacilli remain in the tissues in a non-replicating or slowly replicating dormant state for the rest of the individual's life [2]. It is not known why some people develop progressive tuberculosis whereas many others do not; perhaps one or several molecules of the innate or acquired immune system are not efficient or are not expressed in sufficient amounts.
Although many advances have been made in the study of the immunological responses during M. tuberculosis infection, scarce information is available about the role of anti-microbial peptides. Some reports have shown the importance of anti-microbial peptides such as defensins during M. tuberculosis infection, mainly in alveolar epithelial cells where these peptides can contribute to the bacilli growth control, particularly during early infection [3], and may contribute to maintain latent infection [4,5]. Another anti-microbial peptide involved in the immunopathogenesis of tuberculosis is LL-37, the only member of the cathelicidin family identified in humans. Besides its direct anti-microbial function, cathelicidin is a multi-functional immunoregulatory factor [6]. Cathelin-related anti-microbial peptide (CRAMP) is the only cathelicidin isolated from mice [7]. According to its gene and peptide structure, cellular processing, anti-microbial spectrum and tissue expression, CRAMP is the LL-37 mouse orthologue [8]. Indeed, this similarity has contributed to the understanding of cathelicidin's role in several human diseases [9]. Recently, several authors reported the importance of cathelicidin in TB control either in the presence or absence of vitamin D [10,11]. We have reported previously that ex vivo alveolar macrophages infection with M. tuberculosis leads to LL-37 production which, associated with surface and cytoplasm of M. tuberculosis, leads to mycobacterial lysis, suggesting that cathelicidin from alveolar macrophages could be an important contributor in the innate immune response during early infection in humans [12]. There are few reports about the role of cathelicidin during primary TB infection and the cell types that participate in its production. The aim of the present study was to investigate the kinetics of the gene expression and protein production of cathelicidin during tuberculosis infection using murine models of latent infection and progressive disease.
Materials and methods
Experimental model of latent TB infection in mice
All animal work was performed in conformity with the Institutional Ethics Committee for Experimentation in Animals. Female B6D2F1 (C57BL/6 × DBA/2J) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). The animals were acclimatized for at least 2 weeks before use. The virulent M. tuberculosis strain H37Rv was cultured in 7H9 broth medium (Difco, Detroit, MI, USA). After 1 month of culture, mycobacteria were harvested, adjusted to 4 × 103 live bacilli in 100 µl phosphate-buffered saline (PBS), aliquoted and maintained at –70°C until use. Before infection, micobacteria were recounted and their viability checked [13].
To induce latent pulmonary tuberculosis infection, 8-week-old mice were anaesthetized intraperitoneally with Pentothal (56 mg/kg). The trachea was exposed via a small mid-line incision and 4 × 103 colony-forming units (CFU) suspended in 100 µl PBS was injected. The incision was then sutured with sterile silk and mice were maintained in groups of five in cages fitted with microisolators connected to negative pressure. Animals were killed at 1, 3, 5 and 7 months post-infection by exsanguination. Three lungs per time-point were prepared for histopathological studies, after having been perfused intratracheally with absolute ethanol and embedded in paraffin. Three more lungs were frozen for determination of cathelicidin expression through real-time PCR.
Experimental model of progressive pulmonary TB
The experimental model of progressive pulmonary tuberculosis has been described in detail previously [14]. Pathogen-free male BALB/c mice were used at 6–8 weeks of age. Each animal was anaesthetized, the trachea was exposed via a small midline incision and 2·5 × 105 viable cells suspended in 100 µl of PBS were injected. After suturing the incision, the mouse was maintained in a vertical position until the effect of anaesthesia had passed. Groups of six animals were killed at 1, 3, 7, 14, 21, 28 and 60 days post-infection by exsanguination. Three lungs per time-point were prepared for histopathological studies. After eliminating hilar lymph nodes and thymic tissues, three more lungs were frozen and kept at –70°C for gene expression studies in two separate experiments.
Kinetics of cathelicidin gene expression determined by real-time PCR in lung homogenates
In two different experiments three lungs, each from different mice, at each time-point during progressive disease or latent infection were processed to isolate RNA with Trizol (Gibco BRL, Gaithersburg, MD, USA), as described previously [5]. Reverse mRNA transcription was performed using 5 µg RNA, 2 µM oligodeoxythymidylic acid [oligo(dT)] 15 primer (Promega, Ontario, Canada), 10 units ribonuclease inhibitor (10 units/µl) (Invitrogen, Carslbad, CA, USA), 1 × reverse transcription (RT) buffer, 0·5 mM of each 2′-deoxynucleosides 5′-triphosphate (dNTP), and 4 units Omniscript Reverse Transcriptase (Qiagen, Mexico City, Mexico). Real-time PCR was performed using the Light Cycler 2·0 (Roche, Mannheim, Germany), Light Cycler TaqMan mastermix, and the specific probe for each gene (Roche). All primers were designed with Universal Probe Library software from Roche for the following targets: CRAMP forward (5′-GCCGCTGATTCTTTTGACAT-3′) and CRAMP reverse (5′-ATTCTTCTCCCCACCTTTGC-3′) and hypoxanthine–guanine phosphoribosyltransferase (HPRT) forward (5′-TCCTCCTCAGACCGCTTTT-3′) and HPRT reverse (5′-CCTGGTTCATCATCGCTAATC-3′). The relative expression of each sample was calculated using mouse mRNA HPRT levels as a reference in all experiments and the ΔΔCT method, as described previously [15].
Preparation of lung tissue for immunohistochemistry and morphometry
Lungs from infected mice were perfused with 100% ethanol via the trachea, fixed for 24 h and embedded in paraffin. Sections 5 µm thick were mounted on silane-coated slides, deparaffinized and the endogenous peroxidase quenched with 0·03% H2O2 in absolute methanol. Then, the sections were washed and blocked with PBS supplemented with 2% human pool serum. Lung sections were incubated for 18 h with goat anti-mouse CRAMP (Santa Cruz Biotechnology, Santa Cruz, CA, USA), then washed and incubated for 2 h with a donkey anti-goat immunoglobulin G (IgG) biotin-labelled antibody. Bound antibodies were detected with avidin–biotin peroxidase (Biocare, Medical, Concord, CA, USA) and counterstained with haematoxylin. For quantification, at least three different mice lungs per time-point in two different experiments were evaluated. Ten random microscopy fields were selected. At ×100 magnification, at least 1200 negative or positive cells from the conducting airway epithelial cells and alveolar epithelium (flat cells corresponded to type I pneumocytes, while cuboidal cells are type II pneumocytes), as well as macrophages located free in the alveolar spaces (alveolar macrophages) and embedded in the alveolar–capillary interstitium (interstitial macrophages), were counted using an image analyser (Axiovision version 4·5·1; Carl Zeiss, Jena, Germany).
For double staining to detect bacilli and CRAMP production, lung sections from infected mice were stained with the conventional Ziehl Neelsen (ZN) method. After ZN staining, slides were subjected to the immunohistochemistry process as described above. To count infected cells, 10 random microscopy fields were selected at ×1000 magnification; at least 300 negative or positive cells per field were counted.
Subcellular localization of CRAMP and lipoarabinomannan (LAM) by immunoelectronmicroscopy in lung sections
To determine the subcellular localization of cathelicidin and LAM, we performed immunoelectronmicroscopy assays using antibodies labelled with different sizes of colloidal gold particles. Lungs from three mice with progressive infection after 28 days of intratracheal inoculation were fixed by perfusion with 4% (v/v) paraformaldehyde dissolved in 0·2 M Sörensen buffer (1 vol NaH2PO4*H2O, 2 vol NaHPO4*7H2O), at pH 7·3, for 4 h at 4°C. Then, small tissue fragments were immersed in 0·5 M ammonium chloride dissolved in PBS for 1 h to block free aldehyde groups. Lung tissue fragments were then dehydrated in graded ethyl alcohol solutions and embedded in LR-white hydrosoluble resin (London Resin Company, London, UK). Thin sections from 70 to 90 nm were placed on nickel grids. The grids were incubated overnight at 4°C with specific polyclonal biotinylated goat anti-CRAMP antibody diluted 1/200 in PBS with 1% bovine serum albumin and 0·5% Tween-20. After rinsing with PBS, the grids were incubated for 1 h at room temperature with streptavidin conjugated with 20 nm gold particles (Sigma Co., St Louis, MO, USA) diluted 1/20 in PBS. After repeated rinsing with PBS, a second overnight incubation was carried out with a polyclonal rabbit anti-LAM antibody diluted 1/30 in the same buffer. After PBS washing, grids were incubated with goat anti-rabbit IgG coupled to 5 nm gold particles at room temperature. The grids were contrasted with uranium salts (Electron Microscopy Sciences, Fort Washington, PA, USA) and examined with an M-10 Zeiss electron microscope (Carl Zeiss). As negative controls, the primary antibody was substituted by normal rabbit serum.
Anti-microbial assay for cathelicidin and binding to M. tuberculosis in culture
CRAMP and LL-37 (kindly donated by Dr Robert Hancock, University of British Columbia, Vancouver, Canada) were tested for anti-microbial activity against M. tuberculosis strain H37Rv by incubating various concentrations of each peptide with 1 × 103M. tuberculosis at 37°C in 100 µl 7H9 broth, viable bacteria were assessed by culturing serial dilutions onto 7H10 agar plates supplemented with oleic acid albumin dextrose complex (OADC) (Beckton Dickinson, Franklin Lakes, NJ, USA) and colonies were counted after 21 days of incubation at 37°C. Anti-microbial assay for LL-37 was compared to the anti-microbial activity against M. tuberculosis with CRAMP.
To determine whether CRAMP was associated with M. tuberculosis cell wall or cytoplasm, 1 × 108 mycobacteria were plated on 200 µl 7H9 broth with or without CRAMP (60 µg) in 24-well culture plates. After 24 h, the cell suspensions were collected and centrifuged at 10 000 g for 15 min, washed in PBS and resuspended in 4% paraformadehyde in 0·1 mM cacodylate buffer overnight at 4°C. After rinsing, cells were embedded in LR-white resin and subjected to immunolectronmicroscopy as described above.
Statistics
Data normality was verified through the Kolgomorov–Smirnov test. Normal distribution data were analysed with two-tailed analysis of variance (anova), using Tukey's test as post-test.
Results
Kinetics of pulmonary CRAMP gene expression during progressive disease and latent TB infection
The quantitative analysis of CRAMP gene expression determined by real-time PCR revealed very low expression in the lungs of non-infected mice. The infected lungs showed higher expression during the whole course of progressive disease, exhibiting three peaks at days 1, 21 and 28 post-infection, the latter being the highest, which showed a 1200-fold higher expression when compared with the control non-infected mice. During late disease, at day 60 post-infection, a significant decrease was seen in expression (Fig. 1a). In contrast, a constant mild expression of CRAMP was seen during experimental latent infection, which was considerably lower than in the lungs from mice with progressive disease (Fig. 1b).
Fig. 1.
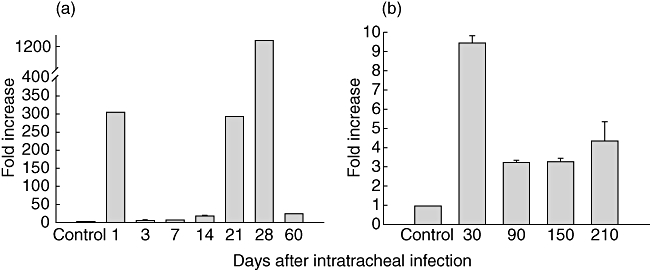
Kinetics of cathelin-related anti-microbial peptide (CRAMP) gene expression determined by quantitative real-time polymerase chain reaction (PCR) in the lung of BALB/c mice during progressive tuberculosis (a) and latent infection in B6D2F1 mice (b). The CRAMP expression during experimental infection is represented as fold increase related to the expression in control non-infected mice. Lungs from three different animals at each time-point were used in two separate experiments. Bars represent mean values ± standard deviation.
Cellular sources and kinetics of CRAMP production during experimental progressive pulmonary TB and latent infection determined by immunohistochemistry
In both experimental models, the cells that showed positive CRAMP immunostaining were bronchial epithelial cells, macrophages and types I and II pneumocytes (Fig. 2). In control non-infected mice, occasional cells of these types showed faint immunostaining (2–6%) in both mice strains (Fig. 2a and b). After 1 day of infection with the high bacilli dose to induce progressive disease, the bronchial epithelium showed the highest percentage of immunostained cells (58–60%), followed by macrophages (45% ± 10), type II pneumocytes (38% ± 13) and type I pneumocytes (3–4%), which showed a low and stable percentage of immunostained cells during the whole infection (Figs 2 and 3). In comparison with day 1 of infection, at days 3, 7 and 14 of infection all these cell types showed a decrease of 30–40% of stained cells, followed by another increase of the percentage of CRAMP immunostained cells at days 21 and 28 that were similar to that observed at day 1 after infection (Fig. 3). At 2 months post-infection, when the progressive phase of the disease was well established, there was a 20–30% decrease of immunostained cells. Vacuolated macrophages located in pneumonic areas showed strong immunostaining that was clearly related to the amount of intracellular bacilli; those cells with numerous bacilli in the cytoplasm showed the strongest immunostaining (Fig. 2g and h).
Fig. 2.
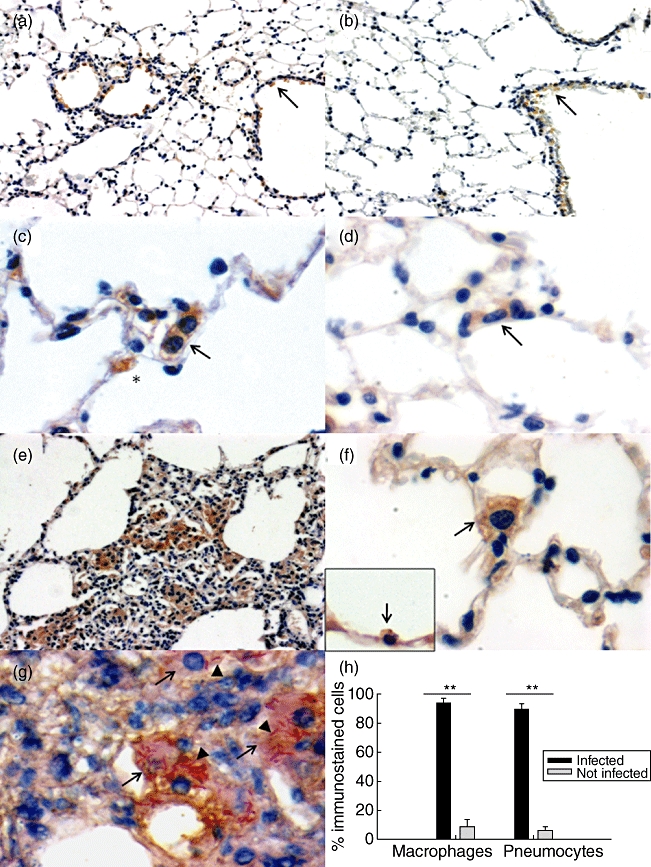
Representative micrographs of cathelin-related anti-microbial peptide (CRAMP) detection by immunohistochemistry during experimental progressive tuberculosis and latent infection. Lungs from BALB/c (a) and B6D2F1 (b) non-infected mice show slight bronchial epithelium CRAMP immunostaining (arrows). In contrast, after day 21 of intratracheal infection with a high bacilli dose to induce progressive disease, there is strong CRAMP immunostaining in interstitial macrophages (arrow) and in cuboidal cells on the alveolar wall surface that correspond to pneumocytes type II (asterisk) (c), or in interstitial macrophages (arrow) after 30 days in animals infected with the low bacilli dose to induce latent infection (d). Pneumonic patches in the lungs of animals after 28 days of high-dose infection show numerous macrophages with strong CRAMP immunostaining (e), whereas after 2 months of infection with the low bacilli dose animals show immunostaining in interstitial macrophages (arrow) and cuboidal cells on the alveolar wall which are pneumocytes type II (inset, arrow) (f). During late progressive disease, day 60 after infection, vacuolated macrophages (arrows) with numerous acid-fast To determine the subcellular localization of cathelicidin and LAM, we performed immunoelectronmicroscopy assays using antibodies labelled with different sizes of colloidal gold particles. Lungs from three mice with progressive infection after 28 days of intratracheal inoculation were fixed by perfusion with 4% (v/v) paraformaldehyde dissolved Ziehl Neelsen (ZN) staining bacilli (arrowheads) show strong CRAMP immunostaining (g). The morphometric study at day 60 post-infection in the pneumonic areas of the progressive disease model showed a significantly higher percentage of immunostaining in infected cells than in non-infected cells (h). Bars represent mean values ± standard deviation. Magnification ×200 (a, b), ×400 (e), ×1000 (c, d, f, g).
Fig. 3.
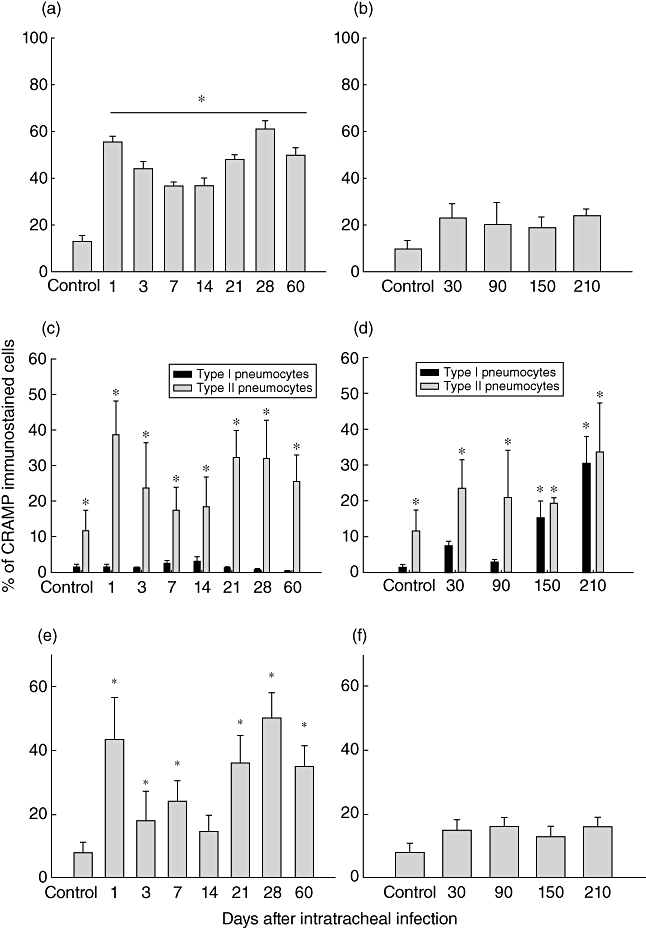
Percentage of cathelin-related anti-microbial peptide (CRAMP) immunostained cells determined by immunohistochemistry and automated morphometry in the lungs of mice during progressive pulmonary tuberculosis (left row) and latent infection (right row). In different cell types such lung epithelial cells (a, b), pneumocytes (c, d) and macrophages (e, f). Ten random microscopy fields were selected at ×1000 magnification, at least 300 negative or positive cells per field were counted and the percentage of the indicated cells was determined. Bars represent mean values ± standard deviation. Asterisks indicate statistical significance when compared with the lungs of control non-infected animals (P < 0·05).
The lungs from mice with latent infection showed a mild and stable percentage of immunostained cells during the whole time–course (7 months) (Fig. 3), the bronchial and alveolar epithelial cells being the most commonly immunostained cells (20–30%), followed by alveolar and interstitial macrophages (8–10%) (Fig. 3e and f).
Subcellular detection of CRAMP by immunoelectronmicroscopy in infected lungs
Complete Freund's adjuvant is used in the production of the CRAMP antibody and its purification is through affinity chromatography (SantaCruz Biotechnologies, personal communication). To check any cross-reaction with M. tuberculosis, we verified whether CRAMP antibody reacted with mycobacterial components through immunoelectronmicroscopy using a secondary anti-goat antibody conjugated with 20-nm gold particles. We did not find cross-reactivity against M. tuberculosis (data not shown).
The immunoelectronmicroscopy study in infected lung sections showed specific CRAMP labelling on the surface and cytoplasm of phagocytized mycobacteria (Fig. 4a). Double staining showed the co-localization of CRAMP and LAM inside cytoplasmic vesicles (Fig. 4a and b). All the studied mycobacteria in the cytoplasm of macrophages showed CRAMP immunolabelling with diverse intensity levels. Scarce immunolabelling was observed in non-infected cells, either macrophages or type II pneumocytes. Uninfected lung tissues or M. tuberculosis-infected lung tissues that were incubated without the primary anti-CRAMP antibody did not show any immunolabelling.
Fig. 4.
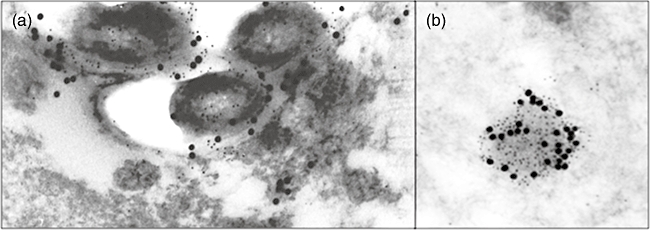
Representative immunoelectronmicroscopy micrographs showing the subcellular localization of cathelin-related anti-microbial peptide (CRAMP) and lipoarabinomannan (LAM), in BALB/c mouse lung after 28 days of intratracheal infection with a high dose of Mycobacterium tuberculosis H37Rv to induce progressive disease. (a) Intracellular bacilli in alveolar macrophage showing CRAMP immunolabelling (small dots) and LAM (large dots) (×40 000). (b) Cytoplasmic subapical vacuoles in alveolar macrophage showing positive immunolabeling to CRAMP (small dots) and LAM (large dots) (×70 000).
In-vitro determination of CRAMP anti-mycobacterial activity
Because LL-37, the human orthologue of CRAMP, has an anti-microbial effect against M. tuberculosis[10,11,16], we determined whether CRAMP also has anti-bacterial activity against M. tuberculosis in vitro. CRAMP was incubated with M. tuberculosis H37Rv strain and after 48 h the effect on M. tuberculosis viability was determined by CFU quantification. This assay showed that the minimum inhibitory concentration for CRAMP is 57 µg and 50 µg for LL-37, so both peptides have similar anti-mycobacterial activity in-vitro (Fig. 5b). Figure 5a shows specific CRAMP labelling on the cell wall and peripheral cytoplasm of mycobacteria, demonstrated by immunoelectronmicroscopy; in areas where the CRAMP labelling was more intense the bacterial cell wall showed dissolution with interruption of the cell membrane bilayer structure.
Fig. 5.
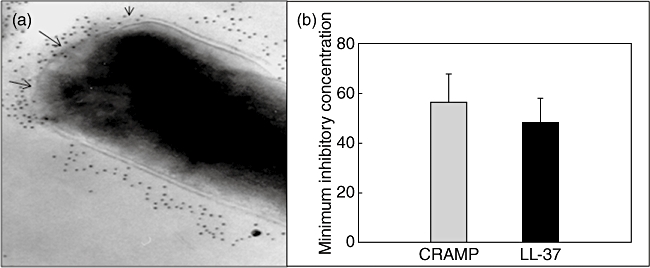
Cathelicidin anti-mycobacterial activity of cathelin-related anti-microbial peptide (CRAMP) and LL-37. (a) The immunolectronmicroscopy study shows strong CRAMP immunolabelling and dissolution of the cell wall (arrows) of mycobacteria incubated in vitro with this anti-microbial peptide (magnification ×75 000). (b) Various concentrations of each peptide were incubated with Mycobacterium tuberculosis strain H37Rv (1 × 103M. tuberculosis at 37°C in 100 µl 7H9 broth), colonies were counted after 21 days of incubation at 37°C to determine minimum inhibitory concentrations. Bars represent mean values ± standard deviation.
Discussion
Several ex vivo studies have been conducted to determine the importance of cathelicidin during M. tuberculosis infection; however, there is no information about in vivo studies. Thus, we sought to determine the cellular sources and kinetic expression of CRAMP during the course of experimental tuberculosis. Our results showed that the lungs of mice that are not infected produced low amounts of CRAMP, confirming that cathelicidin is expressed constitutively [7], and after infection with M. tuberculosis CRAMP gene and protein expression was augmented considerably.
In our experimental model of progressive disease, there is an initial phase of partial resistance dominated by T helper type 1 (Th1) cytokines plus tumour necrosis factor (TNF)-α, which control the infection temporally, followed by a late phase of progressive disease that started after 1 month of infection and is characterized by a drop in the number of cells expressing interferon (IFN)-γ and TNF-α, progressive pneumonia, extensive interstitial fibrosis, high bacillary counts and high levels of immunosuppressive cytokines such as transforming growth factor (TGF)-β, interleukin (IL)-4, IL-10 and IL-13 [7]. High production of CRAMP was seen during early infection, particularly at day 1, when high gene expression and numerous bronchial and bronchiolar epithelial cells, as well as macrophages, showed cathelicidin immunostaining. Although neutrophils produce cathelicidin, and these types of cell have an influx in lung during early stages of mycobacterial infection [17], no significant immunostaining was found in these cells. Other significant bacterial peptides, β-defensin (mBD)-3 and -4, were studied previously in the same animal model; they were also highly expressed during the early phase of the progressive disease from days 1 to 28, the bronchial epithelium being the most important source [5]. Respiratory epithelium was also an important CRAMP source, indicating that not only macrophages but also epithelial cells are important producers of cathelicidin during M. tuberculosis infection [10]. This contrasts with β-defensin expression in the same model, in which only occasional macrophages produced this type of anti-microbial peptide [5].
We do not know the significance of this high CRAMP production at the beginning of the mycobacterial infection, but considering that the usual infecting dose in humans is very low, and the observation that bacteria can be endocytosed by epithelial cells [3,4] inducing anti-microbial peptides, including cathelicidin that lyse bacteria, it is reasonable to suggest that this high anti-microbial peptide production could participate in the early bacilli elimination. This rapid and high production of cathelicidin and other anti-microbial peptides could be related to the well-known observation that M. tuberculosis primary infection leads only rarely to disease. In this regard, it is important to consider that the timing of cathelicidin exposure influences the course of the immune response. Pretreatment of monocytes in vitro with this peptide enhances monocyte-derived dendritic cell functions, such as IL-12 secretion and Th-1 polarized activity [3,4]. However, other studies demonstrated that the simultaneous treatment of immature dendritic cells with cathelicidin and Toll-like receptor (TLR) ligands inhibits dendritic cell maturation [18], and delay of dendritic cell activation is a significant characteristic of this murine model of pulmonary tuberculosis [19]. Thus, overexpression of cathelicidin during early experimental tuberculosis infection could have opposite activity.
The second peak of high cathelicidin production during progressive disease was at day 21 post-infection, when in this murine model the maximal activity of protective adaptive immunity mediated by Th-1 cells occurs [12]. Cathelicidin augments responses to proinflammatory cytokines such as IL-1, boosting signalling pathway activation and chemokine production synergistically [20]. Thus, the high production of cathelicidin at this time-point could contribute to maintain bacilli growth under control. Well-formed granulomas are characteristic of this time-point and showed occasional CRAMP immunostained macrophages, which correlates with our previous findings in human TB granulomas that did not show LL-37 immunostaining [12].
The highest peak of CRAMP expression was detected after 28 days of infection, when the progressive phase of the disease started, announced by the formation of pneumonic patches, and coincided with strong CRAMP immunostaining of epithelial cells and alveolar macrophages. Perhaps is at this time-point when CRAMP exerts its maximal anti-microbial activity, as suggested by the electronmicroscopy study that revealed strong CRAMP immunolabelling in the cell wall and cytoplasm of phagocytosed mycobacteria. Our in vitro study showed that CRAMP has modest anti-mycobacterial activity with similar minimum inhibitory concentration to that of LL-37, which is not better than that reported elsewhere for other anti-microbial peptides, such as human neutrophil peptides (HNPs) [21–24] and β-defensins [23,25]. The ultrastructural study showed cell membrane dissolution in the areas where CRAMP labelling was strong, indicating structural damage.
At day 60 post-infection there are high bacillary loads and extensive pneumonia, indicating a well-established active progressive disease [12]. At this time-point, CRAMP gene expression decreased markedly, but strong CRAMP immunostaining was still observed specifically in highly infected vacuolated macrophages. We have shown previously that these macrophages predominate during late disease, they are heavily infected and constitute a significant source of anti-inflammatory/immunosuppressive molecules, such as TGF-β, prostaglandin E and IL-10 [26,27]. Cathelicidin is the anti-microbial peptide with more immunomodulatory reported functions [6]. It has been demonstrated recently in vitro that LL-37 inhibits cellular responses to IFN-γ, the key cytokine of Th1-polarized immunity and anti-mycobacterial activity [28]. Its strong expression in vacuolated macrophages suggests that CRAMP might have more immunosuppressive effects than anti-microbial activity during advanced disease. Thus, CRAMP may have a split function; during early infection it could be an important factor expressed by lung epithelial cells and alveolar macrophages that contribute to control mycobacteria growth, and during advanced progressive disease CRAMP could be a significant immunomodulatory factor.
Our latent infection model is characterized by low and stable bacillary counts, with few granulomas and small patches of alveolitis without mortality. Indeed, these mice continued to gain weight and appeared healthy for more than 2 years, which resembles the latent infection in humans [29]. In comparison with control non-infected mice, the pulmonary CRAMP production in this model increased, but remained low in comparison with progressive disease. The importance of anti-microbial peptides in TB latent infection has been scarcely studied. We have shown in the same model that mBD-3 is highly expressed [5] and analysis of its subcellular distribution showed high amounts of this defensin, covering intracellular mycobacteria, probably impeding its replication [4]. Perhaps CRAMP and β-defensins contribute to maintaining latent infection, but further studies need to be conducted.
Acknowledgments
This work was supported by the Mexican Institute of Social Security # FIS/IMSS/PROT/C2007/081 and CONACyT Ciencia Basica 82424.
Disclosure
There are no conflicts of interest to declare.
References
- 1.Korenromp EL, Bierrenbach AL, Williams BG, Dye C. The measurement and estimation of tuberculosis mortality. Int J Tuberc Lung Dis. 2009;13:283–303. [PubMed] [Google Scholar]
- 2.Tufariello JM, Chan J, Flynn JL. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect Dis. 2003;3:578–90. doi: 10.1016/s1473-3099(03)00741-2. [DOI] [PubMed] [Google Scholar]
- 3.Rivas-Santiago B, Schwander SK, Sarabia C, et al. Human {beta}-defensin 2 is expressed and associated with Mycobacterium tuberculosis during infection of human alveolar epithelial cells. Infect Immun. 2005;73:4505–11. doi: 10.1128/IAI.73.8.4505-4511.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivas-Santiago B, Contreras JC, Sada E, Hernandez-Pando R. The potential role of lung epithelial cells and beta-defensins in experimental latent tuberculosis. Scand J Immunol. 2008;67:448–52. doi: 10.1111/j.1365-3083.2008.02088.x. [DOI] [PubMed] [Google Scholar]
- 5.Rivas-Santiago B, Sada E, Tsutsumi V, Aguilar-Leon D, Contreras JL, Hernandez-Pando R. beta-Defensin gene expression during the course of experimental tuberculosis infection. J Infect Dis. 2006;194:697–701. doi: 10.1086/506454. [DOI] [PubMed] [Google Scholar]
- 6.Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006:1408–25. doi: 10.1016/j.bbamem.2006.03.030. 1758. [DOI] [PubMed] [Google Scholar]
- 7.Gallo RL, Kim KJ, Bernfield M, et al. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem. 1997;272:13088–93. doi: 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- 8.Pestonjamasp VK, Huttner KH, Gallo RL. Processing site and gene structure for the murine antimicrobial peptide CRAMP. Peptides. 2001;22:1643–50. doi: 10.1016/s0196-9781(01)00499-5. [DOI] [PubMed] [Google Scholar]
- 9.Nijnik A, Hancock RE. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr Opin Hematol. 2009;16:41–7. doi: 10.1097/moh.0b013e32831ac517. [DOI] [PubMed] [Google Scholar]
- 10.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin d-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–3. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 11.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 12.Rivas-Santiago B, Hernandez-Pando R, Carranza C, et al. Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect Immun. 2008;76:935–41. doi: 10.1128/IAI.01218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarnagin JL, Luchsinger DW. The use of fluorescein diacetate and ethidium bromide as a stain for evaluating viability of mycobacteria. Stain Technol. 1980;55:253–8. doi: 10.3109/10520298009067249. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Pando R, Orozcoe H, Sampieri A, et al. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89:26–33. [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol. 2007;103:793–8. doi: 10.1016/j.jsbmb.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 17.Barrios-Payan J, Aguilar-Leon D, Lascurain-Ledezma R, Hernandez-Pando R. [Neutrophil participation in early control and immune activation during experimental pulmonary tuberculosis] Gac Med Mex. 2006;142:273–81. [PubMed] [Google Scholar]
- 18.Kandler K, Shaykhiev R, Kleemann P, et al. The anti-microbial peptide LL-37 inhibits the activation of dendritic cells by TLR ligands. Int Immunol. 2006;18:1729–36. doi: 10.1093/intimm/dxl107. [DOI] [PubMed] [Google Scholar]
- 19.Pedroza-Gonzalez A, Garcia-Romo GS, Aguilar-Leon D, et al. In situ analysis of lung antigen-presenting cells during murine pulmonary infection with virulent Mycobacterium tuberculosis. Int J Exp Pathol. 2004;85:135–45. doi: 10.1111/j.0959-9673.2004.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J, Mookherjee N, Wee K, et al. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1beta, augments immune responses by multiple pathways. J Immunol. 2007;179:7684–91. doi: 10.4049/jimmunol.179.11.7684. [DOI] [PubMed] [Google Scholar]
- 21.Fu LM. The potential of human neutrophil peptides in tuberculosis therapy. Int J Tuberc Lung Dis. 2003;7:1027–32. [PubMed] [Google Scholar]
- 22.Kalita A, Verma I, Khuller GK. Role of human neutrophil peptide-1 as a possible adjunct to antituberculosis chemotherapy. J Infect Dis. 2004;190:1476–80. doi: 10.1086/424463. [DOI] [PubMed] [Google Scholar]
- 23.Miyakawa Y, Ratnakar P, Rao AG, et al. In vitro activity of the antimicrobial peptides human and rabbit defensins and porcine leukocyte protegrin against Mycobacterium tuberculosis. Infect Immun. 1996;64:926–32. doi: 10.1128/iai.64.3.926-932.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma S, Verma I, Khuller GK. Antibacterial activity of human neutrophil peptide-1 against Mycobacterium tuberculosis H37Rv: in vitro and ex vivo study. Eur Respir J. 2000;16:112–17. doi: 10.1034/j.1399-3003.2000.16a20.x. [DOI] [PubMed] [Google Scholar]
- 25.Fattorini L, Gennaro R, Zanetti M, et al. In vitro activity of protegrin-1 and beta-defensin-1, alone and in combination with isoniazid, against Mycobacterium tuberculosis. Peptides. 2004;25:1075–7. doi: 10.1016/j.peptides.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez-Pando R, Orozco H, Arriaga K, Sampieri A, Larriva-Sahd J, Madrid-Marina V. Analysis of the local kinetics and localization of interleukin-1 alpha, tumour necrosis factor-alpha and transforming growth factor-beta, during the course of experimental pulmonary tuberculosis. Immunology. 1997;90:607–17. doi: 10.1046/j.1365-2567.1997.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rangel Moreno J, Estrada Garcia I, De La Luz Garcia Hernandez M, Aguilar Leon D, Marquez R, Hernandez Pando R. The role of prostaglandin E2 in the immunopathogenesis of experimental pulmonary tuberculosis. Immunology. 2002;106:257–66. doi: 10.1046/j.1365-2567.2002.01403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nijnik A, Pistolic J, Wyatt A, Tam S, Hancock RE. Human cathelicidin peptide LL-37 modulates the effects of IFN-gamma on APCs. J Immunol. 2009;183:5788–98. doi: 10.4049/jimmunol.0901491. [DOI] [PubMed] [Google Scholar]
- 29.Arriaga AK, Orozco EH, Aguilar LD, Rook GA, Hernandez Pando R. Immunological and pathological comparative analysis between experimental latent tuberculous infection and progressive pulmonary tuberculosis. Clin Exp Immunol. 2002;128:229–37. doi: 10.1046/j.1365-2249.2002.01832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


