Abstract
Immunosuppression therapy following lung transplant fails to prevent chronic rejection/bronchiolitis obliterans syndrome, which we have shown is associated with lack of suppression of peripheral blood T cell granzyme B, interferon (IFN)-γ and tumour necrosis factor (TNF)-α. We hypothesized that these proinflammatory mediators may increase with time post-transplant in otherwise stable patients before clinical signs of declining lung function, and patients experiencing declining lung function would show a further increase in these mediators. Intracellular cytokine profiles and granzyme B were investigated in T cells in whole blood and airways from lung transplant patients using flow cytometry. There was a significant negative correlation between forced expiratory volume in 1 s (FEV1), drug dose and time post-transplant. A significant correlation between increased granzyme B, IFN-γ, interleukin (IL)-2 and TNF-α and time post-transplant was noted in peripheral blood T cells but not lung T cells from stable patients. Patients with similar drug dose but experiencing declining FEV1 showed a further increase in peripheral blood T cell IFN-γ, IL-2 and TNF-α. Time post-lung transplant correlates with increasing peripheral blood T cell granzyme B and proinflammatory cytokines. Declining FEV1 is associated with a further increase in these proinflammatory mediators. Drugs that reduce these inflammatory mediators effectively may reduce the incidence of chronic graft rejection.
Keywords: IFN-γ, lung transplant, T cell granzyme B, time post-transplant, TNF-α
Introduction
Immunosuppression therapy following lung transplant is inadequate at preventing chronic graft failure in many patients with 5-year survival rates less than 60% [1] and incidence of bronchiolitis obliterans syndrome 50% at 5 years [2]. T cell T helper type 1 (Th1) proinflammatory cytokines are involved in transplant rejection and are a target of current immunosuppression strategies [3]. Our previous studies of stable lung transplant patients at early post-transplant showed that, while T cell proinflammatory cytokines were reduced significantly in peripheral blood consistent with the effect of immunosuppressive agents [4], there was inadequate immunosuppression in the airways and allograft [5,6].
Importantly, we also showed that inhibition of proinflammatory cytokines by CD8+ T cells in stable lung transplant recipients was less effective [4,5]. Our studies of lung transplant patients undergoing acute rejection episodes showed adequate immunosuppression of T cell proinflammatory cytokines in the blood, but not in the airways [6]. Furthermore, patients with culture-positive bronchoalveolar lavage (BAL) also showed adequate immunosuppression of T cell proinflammatory cytokines in the blood, but decreased proinflammatory response in the airways [7]. In all these studies, systemic drug levels were within ‘therapeutic range’, suggesting that intracellular T cell cytokine levels may provide a more physiological indication of immunosuppression than drug levels. We have shown recently that bronchiolitis obliterans syndrome is associated with lack of suppression of peripheral blood T cell granzyme B, interferon (IFN)-γ and tumour necrosis factor (TNF)-α[8,9]. Furthermore, we have shown recently that lymphocytic bronchiolitis, an important risk factor for the development of obliterative bronchiolitis [10], was also associated with increased granzyme B, IFN-γ and TNF-α[11]. Importantly, several patients who were followed longitudinally showed increased levels of T cell granzyme B, IFN-γ and TNF-α preceding the clinical and/or histological diagnosis of lymphocytic bronchiolitis and bronchiolitis obliterans [8,9,11]. These findings suggest that patients who show lack of immunosuppression of these proinflammatory mediators, either through reduced therapy due to their toxic side effects, altered pharmacokinetics or pharmacodynamics, are at risk of progressing to graft rejection. We hypothesized these proinflammatory mediators may increase with time post-transplant in otherwise stable patients before clinical signs of declining lung function. We hypothesized further that patients showing declining lung function would show a further increase in these proinflammatory mediators.
To investigate this hypothesis, T cell granzyme B and intracellular cytokine production by CD8+ and CD8– (CD4+) T cell subsets were determined from whole blood, BAL and bronchial brushings from stable lung transplant patients and a group of patients experiencing declining lung function using multi-parameter flow cytometry.
Materials and methods
Patient and control groups
Lung transplant patients undergoing surveillance or diagnostic post-transplant bronchoscopy and transbronchial biopsy were invited to participate in the study. Fully informed consent was obtained following institutional ethical approval. Seventy-seven lung transplant recipients with no histopathological and/or clinical evidence of current acute or chronic rejection were enrolled. Thirty-three of these patients had stable forced expiratory volume in 1 s (FEV1) and 44 patients experienced a fall in FEV1 of >10%. Fourteen stable patients and 31 patients experiencing a fall in FEV1 had clinically significant culture-positive BAL. All patients were submitted to the same protocol and analysis performed retrospectively. All patients were tested for cytomegalovirus (CMV) [histopathologically, rapid viral culture and CMV polymerase chain reaction (PCR) of BAL] and bacterial and fungal infection (BAL culture). Stable patients were 22 ± 25 months [mean ± standard deviation (s.d.)] post-transplant. Patients experiencing a fall in FEV1 were 27 ± 35 months (mean ± s.d.) post-transplant. Some of the data from 10 patients with early transplant times have been used in a different context in previous publications [4–7]. Immunosuppression therapy comprised combinations of either cyclosporin A (CsA) or tacrolimus (Tac) with prednisolone, and azathioprine or mycophenolate mofetil. Trough plasma drug levels of either CsA or Tac were within or above recommended therapeutic ranges [range for CsA (80–250 µg/l) or CsA C2 levels (600–1000 µg/l) and Tac (5–15 µg/l)]. Venous blood was collected into 10 U/ml preservative free sodium heparin (DBL, Sydney, Australia) and blood, BAL and bronchial brushing samples were maintained at 4°C until processing.
BAL
Aspirated BAL samples (3 × 50 ml aliquots) were collected as described previously [5] and transferred to 50-ml polypropylene tubes. For each collection from an individual, the first aliquot was processed for microbiological and viral testing. BAL specimens 2 and 3 were pooled and cell counts determined as described above and cells were then pelleted by centrifugation at 500 g for 5 min. Supernatant was discarded and cells resuspended at 4 × 105 cells/ml in RPMI-1640 media supplemented with 125 U/ml penicillin and 125 U/ml streptomycin (Gibco, New York, NY, USA).
Bronchial brushings
Flexible bronchoscopy was performed as described previously [6,7]. Cells were obtained from the third- or fourth-order bronchi with several passages of the brush into each airway in order to avoid bleeding. Cells were deposited by washing the brush in 5 ml RPMI in 10 ml conical polypropylene tubes (Johns Professional Products, Sydney, Australia) and kept on ice until processed.
CD4+ and CD8+ T cell counts
One hundred microlitres of peripheral blood, BAL and bronchial brushings were stained with appropriately diluted fluorescently conjugated monoclonal antibodies to CD8 fluorescein isothiocyanate (FITC) (BD Biosciences, Sydney, Australia) (BD), CD4 phycoerythrin (PE) (BD) and CD3 phycoerythrincyanin 5 (PC5) (Beckman Coulter, Sydney, Australia), as described previously [4–6]. Briefly, samples were analysed by gating using forward scatter (FSC) versus side scatter (SSC) to exclude platelets and debris. Gated cells were analysed with CD45/CD14 (BD) to ascertain that cells were of lymphoid origin, as reported previously [4]. Control staining of leucocytes with anti-mouse immunoglobulin G1 (IgG1)–FITC (BD)/IgG1a–PE (BD)/IgG1–PC5 (Beckman Coulter) was performed on each sample and background readings of <2% were obtained. A minimum of 10 000 (from blood cultures) and 3000 CD3-positive, low SSC events from BAL and bronchial brushing samples were acquired in list-mode format for analysis.
Peripheral blood T cell granzyme B
Granzyme B was enumerated in peripheral blood T cells, as described previously [13].
T cell cytokine production and granzyme B following stimulation
T cell cytokine production was assessed as described previously [4,9,10]. The same technique was used to stimulate production of granzyme B in peripheral blood but not in BAL or in bronchial brushings due to limited sample volume. Two-ml aliquots of prepared BAL, bronchial brushing or 1-ml aliquots of blood (diluted 1:2 with RPMI-1640 medium) were placed in 10-ml sterile conical PVC tubes (Johns Professional Products). Phorbol myristate (25 ng/ml) (Sigma, Sydney, Australia) and ionomycin (1 µg/ml) (Sigma) were added to stimulate T cell cytokine and granzyme B production. Brefeldin A (10 µg/ml) was added as a ‘Golgi block’ (Sigma) and the tubes reincubated in a humidified 5% CO2/95% air atmosphere at 37°C. At 16 h 100 µl 20 mM ethylenediamine tetraacetic acid/phosphate-buffered saline (EDTA/PBS) was added to the culture tubes, which were vortexed vigorously for 20 s to remove adherent cells. Three hundred microlitre aliquots of cells were treated with 500 µl FACSPerm for 10 min. Two ml 0·5% bovine serum albumin (BSA) (Sigma) in IsoFlow (Beckman Coulter) was then added and the tubes centrifuged at 300 g for 5 min. After decanting supernatant, Fc receptors were blocked with 10 µl human immunoglobulin (Intragam, CSL, Parville, NSW, Australia) for 10 min at room temperature. Five µl of appropriately diluted anti-CD8 (BD), anti-CD3 PC5 (Beckman Coulter) and PE-conjugated anti-cytokine monoclonal antibodies to interleukin (IL)-2, IL4, IFN-γ, TNF-α (BD), granzyme B (Serotec, Sydney, Australia) or isotype control monoclonal antibody was added for 15 min in the dark at room temperature. Events were acquired and analysed as described above.
Statistical analysis
Statistical analysis was performed using the non-parametric Mann–Whitney U-test and Spearman's rho correlation tests using spss software and differences between groups of P < 0·05 considered significant.
Results
Correlation between FEV1, FEV1/forced vital capacity (FVC) and time post-transplant
There was a significant negative correlation between FEV1 and time post-transplant (in months) for stable patients (R = −0·342, P = 0·036) (Fig. 1). There was a significant negative correlation between FEV1/FVC and time post-transplant for stable patients (R = −0·390, P = 0·002).
Fig. 1.
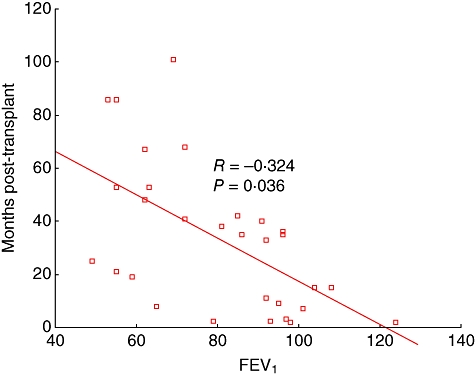
Graph showing a significant negative correlation between forced expiratory volume in 1 s (FEV1) and time post-transplant for stable patients.
There was a significant negative correlation between FEV1 and time post-transplant for patients with declining FEV1 (R = −0·780, P = 0·032). There was a significant correlation between FEV1/FVC and time post-transplant for patients with declining FEV1 (R = −0·790, P = 0·000).
CD4+ and CD8+ T cell percentages and time post-transplant
There was no significant correlation between the percentages of CD4 or CD8+ T cells in peripheral blood, BAL or bronchial brushings and time post-transplant for stable patients (R = −0·106, P = 0·918 and R = 0·106, P = 0·918 for CD4 or CD8+ T cells in peripheral blood, respectively) (R = −0·216, P = 0·188 and R = 0·204, P = 0·357 for CD4 or CD8+ T cells in BAL) and (R = −0·041, P = 0·931 and R = 0·332, P = 0·291 for CD4 or CD8+ T cells in bronchial brushings). There was no significant correlation between the percentages of CD4 or CD8+ T cells in peripheral blood, BAL or bronchial brushings and time post-transplant for patients with declining FEV1 (P > 0·05) (data not shown).
Correlation between peripheral blood T cell subset cytokine production and time post-transplant
There was a significant correlation between the percentage of CD4 and CD8+ T cell subsets producing IFN-γ, IL-2 and TNF-α and time post-transplant for stable patients. Data for the percentage of CD3+ T cells producing these cytokines and time post-transplant are shown in Fig. 2. There was no correlation between the percentage of CD4+ and CD8+ T cell subsets producing IL-4 and time post-transplant for stable patients (R = 0·059, P = 0·724 and R = 0·085, P = 0·610 for CD4 or CD8+ T cells in peripheral blood, respectively).
Fig. 2.
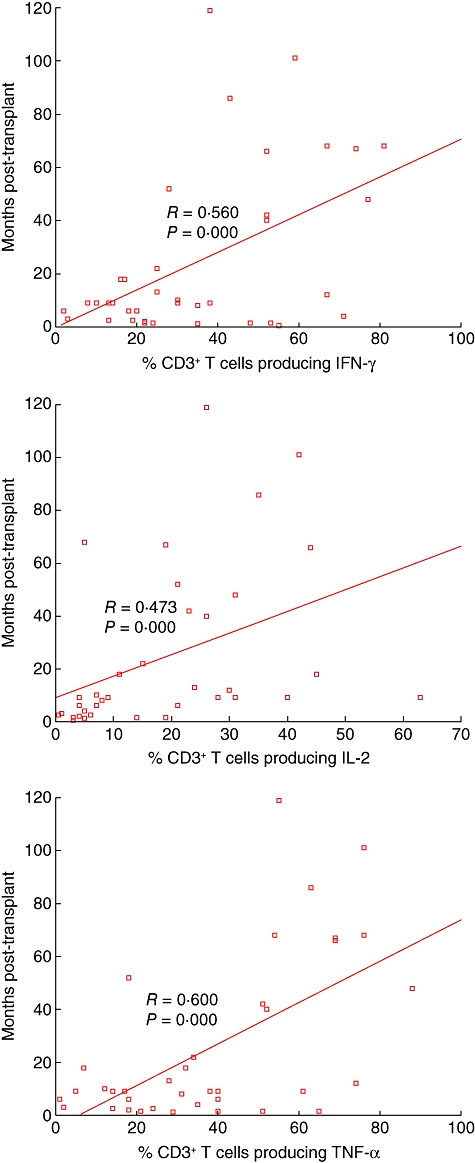
Graphs showing a significant correlation between the percentage of peripheral blood T cells producing interferon (IFN)-γ, interleukin (IL)-2 and tumour necrosis factor (TNF)-α and time post-transplant for stable patients.
There was a significant correlation between the percentage of CD4 and CD8+ T cell subsets producing IFN-γ (R = 0·714, P = 0·000 and R = 0·815, P = 0·001 for CD4 and CD8 T cells, respectively), IL-2 (R = 0·844, P = 0·000 and R = 0·626, P = 0·005 for CD4 and CD8 T cells, respectively) and TNF-α (R = 0·816, P = 0·000 and R = 0·489, P = 0·015 for CD4 and CD8 T cells, respectively) and time post-transplant for patients with declining FEV1. The was no correlation between the percentage of CD4+ and CD8+ T cell subsets producing IL-4 and time post-transplant for patients with declining FEV1 (data not shown).
BAL T cell subset cytokine production and time post-transplant
There were no correlations between the percentage of CD4 or CD8 T cell subsets producing IFN-γ, IL-2, IL-4 or TNF-α and time post-transplant for the stable group or the group with declining FEV1 (P > 0·05). Although patients with declining FEV1 showed an increase Th1 T cell cytokines in BAL, there were no significant differences in the percentage of CD4 or CD8 T cell subsets producing IFN-γ, IL-2, IL-4 or TNF-α between the stable group or in patients with declining FEV1 (Table 1).
Table 1.
The percentage of T cells producing intracellular cytokines (median and range) in bronchoalveolar lavage (BAL) of stable patients (S) and transplant patients with declining forced expiratory volume in 1 s (FEV1) (D). There were no changes in the percentage of CD4+ or CD8+ T cells or the percentage of T cells producing interferon (IFN)-γ, interleukin (IL)-2, IL-4 or tumour necrosis factor (TNF)-α between the groups.
| IFN-γ |
IL-2 |
IL-4 |
TNF-α |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | |
| S | 45 | 54 | 9 | 16 | 5 | 4 | 4 | 7 | 13 | 12 |
| 35–56 | 44–59 | 0–18 | 2–30 | 1–9 | 0–8 | 1–9 | 0–15 | 3–23 | 2–22 | |
| D | 48 | 52 | 14 | 21 | 13 | 7 | 2 | 5 | 20 | 19 |
| 40–56 | 42–61 | 2–26 | 5–37 | 1–29 | 1–17 | 1–4 | 1–9 | 2–42 | 2–35 | |
| P | 0·890 | 0·890 | 0·500 | 0·486 | 0·536 | 0·330 | 0·311 | 0·377 | 0·452 | 0·452 |
Intraepithelial T cell subset cytokine production and time post-transplant
There were no correlations between the percentage of CD4 or CD8 T cell subsets producing IFN-γ, IL-2, IL-4 or TNF-α and time post-transplant for the stable group or the group with declining FEV1 (P > 0·5). There were no differences in the percentage of CD4 or CD8 T cell subsets producing IFN-γ, IL-2, IL-4 or TNF-α between the stable group or the group with declining FEV1 (Table 2).
Table 2.
The percentage of intraepithelial T cells producing intracellular cytokines (median and range) in bronchial brushings of stable patients (S) and transplant patients with a declining forced expiratory volume in 1 s (FEV1) (D). There were no changes in the percentage of CD4+ or CD8+ T cells or the percentage of T cells producing interferon (IFN)-γ, interleukin (IL)-2, IL-4 or tumour necrosis factor (TNF)-α between the groups.
| IFN-γ |
IL-2 |
IL-4 |
TNF-α |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | |
| S | 28 | 73 | 8 | 33 | 4 | 2 | 1 | 3 | 9 | 30 |
| 14–39 | 56–84 | 3–13 | 13–46 | 2–7 | 0–4 | 0–3 | 0–6 | 2–19 | 14–45 | |
| D | 26 | 74 | 7 | 33 | 4 | 2 | 1 | 4 | 8 | 31 |
| 12–41 | 51–82 | 2–15 | 14–51 | 1–9 | 0–5 | 0–4 | 0–5 | 1–21 | 9–47 | |
| P | 0·980 | 0·980 | 0·860 | 0·890 | 0·990 | 0·878 | 0·980 | 0·980 | 0·780 | 0·790 |
Correlation between peripheral blood granzyme B and time post-transplant
There was no correlation between the percentage of CD3+ T cells expressing granzyme B and time post-transplant for stable patients (R = 0·181, P = 0·257). However, the percentage of CD3+ T cells producing granzyme B following stimulation correlated significantly with time post-transplant for stable patients (R = 0·430, P = 0·046).
There was no correlation between the percentage of CD3+ T cells expressing granzyme B and time post-transplant for patients with declining FEV1 (R = 0·167, P = 0·495). However, the percentage of CD3+ T cells producing granzyme B following stimulation correlated significantly with time post-transplant for patients with declining FEV1 (R = 0·480, P = 0·041).
Increased peripheral blood T cell cytokine production in patients with a fall in FEV1
There was a significant increase in the percentage of CD4+ and CD8+ T cells producing IFN-γ, IL-2 and TNF-α in the peripheral blood of patients with declining FEV1 compared with stable patients (Fig. 3). There were no significant differences in time post-transplant, FEV1, FVC, FEV1/FVC or the percentage of T cell subsets producing any other cytokine or granzyme B in blood, BAL or bronchial brushings between stable or patients with declining FEV1 (P > 0·05). Representative plots showing an increased percentage of peripheral blood T cells producing IFN-γ, IL-2 and TNF-α in a patient with declining FEV1 compared with a stable patient is shown in Fig. 4.
Fig. 3.
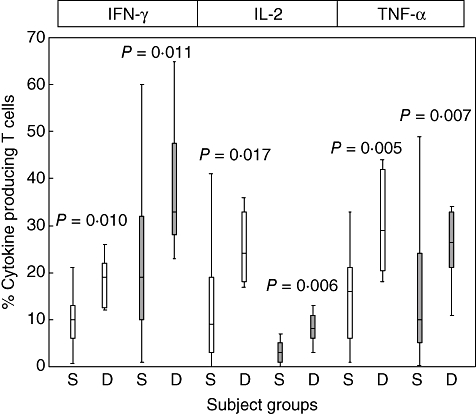
Box plot graphs showing the percentage of blood CD4+ (clear bars) and CD8+ (grey bars) T cells producing intracellular cytokines from stable lung transplant patients (S) and lung transplant patients with declining forced expiratory volume in 1 s (FEV1) (D) following in vitro stimulation (mean ± standard deviation and range). There was a significant increase in the percentage of CD4+ and CD8+ T cells producing IFN-γ, IL-2 and TNF-α in patients with declining FEV1.
Fig. 4.
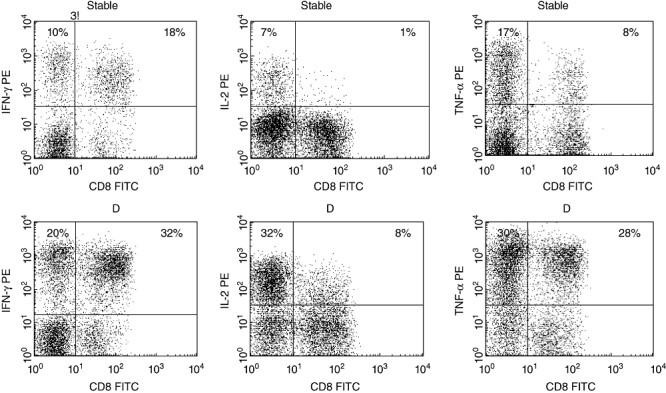
Representative dot plots showing interferon (IFN)-γ, interleukin (IL)-2 and tumour necrosis factor (TNF)-α production by CD8+ and CD8– (CD4+) T cells from blood from a stable patient and a lung transplant patient with declining forced expiratory volume in 1 s (FEV1) (D). T cells were identified by CD3 phycoerythrincyanin 5 (PC5)versus side-scatter characteristics. There was a significant increase in blood CD8+ and CD8– (CD4+) T cell IFN-γ, IL-2 and TNF-α in patients with declining FEV1 compared with the stable patient group.
Correlation between drug levels and time post-transplant
There was a significant negative correlation between time post-transplant for all transplant patients and plasma levels of tacrolimus (R = −0·386, P = 0·007), dose of mycophenolate mofetil (R = −0·448, P = 0·000), dose of prednisolone (R = −0·476, P = 0·000) and a trend for cyclosporin A plasma levels (R = −0·415, P = 0·077). There was no difference in plasma levels or dose of any of the drugs between stable patients and patients with declining FEV1 (P > 0·05 for all drugs, data not shown). There was no correlation between plasma level or dose of any drug and the percentage of T cells expressing any cytokine or granzyme B (data not shown).
Discussion
These novel findings of a correlation between increasing proinflammatory peripheral blood cytokine profiles and granzyme B with time following lung transplant provides important evidence for lack of immunosuppression in these patients.
Immunosuppression therapy following lung transplant is inadequate at preventing chronic rejection in many patients, with resulting 5-year survival rates less than 60% [1]. Th1 proinflammatory cytokines are involved in transplant rejection and are a target of current immunosuppression strategies [3]. Our previous studies in stable lung transplant patients with early post-transplant times showed significantly reduced T cell proinflammatory cytokines in peripheral blood consistent with adequate immunosuppression [4,5], although inhibition of proinflammatory cytokines by CD8+ T cells was less effective [4]. Our present study of a much larger cohort of patients shows that immunosuppression of these proinflammatory mediators is much less effective in the peripheral blood of patients with longer post-transplant times. Our findings of a correlation between decreasing drug dose to reduce potential associated toxicity, and time post-transplant, may help to explain these findings.
However, we also provide novel evidence that the patient group with declining FEV1, receiving similar drug dosage, had significantly increased percentages of peripheral blood T cells producing IFN-γ, IL-2 and TNF-α compared with the stable group. These findings may indicate development of drug resistance in these patients, something we are currently investigating.
Our compartmental approach to cytokine profiling in lung transplant recipients has elucidated several interesting findings. Studies from airway-derived cells showed that CD8 subsets in BAL and intraepithelial T cells obtained by bronchial brushings were not suppressed, suggesting inadequate immunosuppression in the airways and allograft [4–6]. Furthermore, studies of lung transplant patients undergoing acute rejection episodes showed adequate immunosuppression of T cell proinflammatory cytokines in the blood, but not in the airways of these patients [12]. Interestingly, our previous studies in patients with culture-positive BAL showed immunosuppression of Th1 cytokines [7] in the peripheral blood, but cytokine profiles in BAL showed a reduced Th1 profile, consistent with the theory that an adequate Th1 response is necessary to fight these pathogens [14], and that there is a delicate balance between immunosuppression and adequate immune effector function in these patients to reduce both rejection and risk of infection. In our current study there is no evidence for increased proinflammatory T cell cytokines in BAL or in intraepithelial cells obtained from bronchial brushings. This suggests that circulating proinflammatory T cells may be homing to an unsampled region of the allograft, such as the subepithelium or the small airway epithelium. An interesting addition to our study would be investigation of these proinflammatory mediators in T cells from lung biopsies or small airways, and we are currently investigating T cell cytokine profiles in small airway brushings to elucidate this further.
As our studies have shown previously that bronchiolitis obliterans syndrome and lymphocytic bronchiolitis are associated with increased granzyme B, IFN-γ and TNF-α[8,9,11], our common finding in all these studies is the association of increased proinflammatory mediators with patient morbidity and standard indicators of decline. These findings are consistent with those of others that have shown post-transplant inflammation promotes chronic allograft rejection [15]. One could speculate that patients in our current study showing the highest levels of these proinflammatory mediators may be at greater risk of increased morbidity and possibly progression to these diseases, and we are currently following these patients longitudinally to determine this.
Our ability to predict and eliminate graft rejection is limited severely by the insensitivity and inaccuracy of our currently available tools, lung of function testing and transbronchial biopsies [16]. Our previous and current findings argue for the possibility of monitoring immunosuppression levels by measuring granzyme B and cytokine profiles in peripheral blood T cells, a significantly less invasive technique than histological assessment of transbronchial biopsies. The data presented herein suggest that this strategy may identify patients at risk of increased morbidity and possible progression to subsequent graft loss at an early stage before a fall in lung function or histological evidence of chronic rejection.
In conclusion, time post-lung transplant is associated with increasing granzyme B and proinflammatory cytokines in peripheral blood T cells, but not in the airways. This minimally invasive technique allows more frequent profiling of patients and may allow early intervention before clinical or histological evidence of graft deterioration. Drugs that reduce these inflammatory mediators effectively may improve patient morbidity and possibly reduce the incidence of graft rejection. The risk of morbidity due to infection and drug side effects in more immunosuppressed patients would need to be studied closely in this clinical scenario.
The clinical relevance of this work is being pursued further with longitudinal follow-up of this and a much larger patient cohort including T cell profiles in small airway brushings.
Disclosure
The authors have no conflict of interest in relation to the manuscript.
References
- 1.Lama R, Santos F, Alvarez A, et al. Analysis of lung transplant recipients surviving beyond 5 years. Transplant Proc. 2005;37:1523–5. doi: 10.1016/j.transproceed.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Hertz MI, Aurora P, Chistie JD, et al. Scientific Registry of the International Society of Heart and Lung Transplantation: Introduction to the 2009 Annual Reports. J Heart Lung Transplant. 2009;28:989–92. doi: 10.1016/j.healun.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Corris PA, Kirby JA. A role for cytokine measurement in therapeutic monitoring of immunosuppressive drugs following lung transplantation. Clin Exp Immunol. 2005;139:176–8. doi: 10.1111/j.1365-2249.2005.02711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodge G, Hodge S, Reynolds P, Holmes M. Intracellular cytokines in blood T cells in lung transplant patients – a more relevant indicator of immunosuppression than drug levels. Clin Exp Immunol. 2005;139:159–64. doi: 10.1111/j.1365-2249.2005.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodge G, Hodge S, Reynolds P, Holmes M. Increased intracellular pro- and anti-inflammatory cytokines in bronchoalveolar lavage T cells of stable lung transplant patients. Transplantation. 2005;80:1040–5. doi: 10.1097/01.tp.0000173997.92753.25. [DOI] [PubMed] [Google Scholar]
- 6.Hodge G, Hodge S, Reynolds P, Holmes M. Compartmentalisation of intracellular proinflammatory cytokines in bronchial intraepithelial T cells of stable lung transplant patients. Clin Exp Immunol. 2006;145:413–19. doi: 10.1111/j.1365-2249.2006.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodge G, Hodge S, Reynolds P, Holmes M. Airway infection is associated with decreased intracellular Th1 cytokines in bronchoalveolar lavage CD8+ T cells of stable lung transplant patients. Transpl Infect Dis. 2007;10:99–105. doi: 10.1111/j.1399-3062.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- 8.Hodge G, Hodge S, Chambers D, Reynolds PN, Holmes M. Bronchiolitis obliterans syndrome is associated with absence of suppression of peripheral blood Th1 pro-inflammatory cytokines. Transplantation. 2009;88:211–18. doi: 10.1097/TP.0b013e3181ac170f. [DOI] [PubMed] [Google Scholar]
- 9.Hodge S, Hodge G, Ahern J, et al. Increased levels of T-cell granzyme B in BOS are not adequately suppressed by current immunosuppressive regimens. Clin Exp Immunol. 2009;158:230–6. doi: 10.1111/j.1365-2249.2009.04008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glanville AR, Aboyoun CL, Havryk A, Plit M, Rainer S, Malouf MA. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med. 2008;177:1033–40. doi: 10.1164/rccm.200706-951OC. [DOI] [PubMed] [Google Scholar]
- 11.Hodge G, Hodge S, Liew CL, et al. Lymphocytic bronchiolitis is associated with inadequate suppression of blood T-cell granzyme B, IFNγ and TNFα. Transplantation. 2010 doi: 10.1097/TP.0b013e3181d75971. in press. [DOI] [PubMed] [Google Scholar]
- 12.Hodge G, Hodge S, Chambers D, Reynolds P, Holmes M. Acute lung transplant rejection is associated with localised increase in T cell IFNγ and TNFα pro-inflammatory cytokines in the airways. Transplantation. 2007;84:1452–8. doi: 10.1097/01.tp.0000290679.94163.e1. [DOI] [PubMed] [Google Scholar]
- 13.Hodge S, Hodge G, Nairn J, Holmes M, Reynolds P. Increased airway granzyme b and perforin in current and ex-smoking COPD subjects. COPD. 2006;3:179–86. doi: 10.1080/15412550600976868. [DOI] [PubMed] [Google Scholar]
- 14.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–6. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 15.Bharat A, Narayanan K, Street T, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic allograft rejection. Transplantation. 2007;83:150–8. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 16.Chamberlain D, Maurer J, Chaparro C, Idolor L. Evaluation of lung biopsy specimens in the diagnosis of bronchiolitis obliterans after lung transplantation. J Heart Lung Transplant. 1994;13:963–71. [PubMed] [Google Scholar]


