Abstract
A pyrrolyl-based triazolophane, incorporating CH and NH donor groups, acts as a receptor for the pyrophosphate anion in chloroform solution. It shows selectivity for this trianion, followed by HSO4- > H2PO4- > Cl- > Br- (all as the corresponding tetrabutylammonium salts), with NH-anion interactions being more important than CH-anion interactions. In the solid state, the receptor binds the pyrophosphate anion in a clip-like slot via NH and CH hydrogen bonds.
Pyrophosphate detection has aroused interest in the scientific community not only because pyrophosphate is the product of ATP hydrolysis under cellular conditions,1 but also because it could afford a means of effecting real-time DNA sequencing.2 Pyrophosphate monitoring may also have a role to play in cancer research since this anion is involved in DNA replication catalyzed by DNA polymerase.3 Pyrophosphate is also of interest as a larger, more highly charged analogue of inorganic phosphate (H2PO4-/HPO42-), which has physiological relevance in energy storage and signal transduction, in addition to being a structural component in teeth and bones.4 Not surprisingly, therefore, considerable effort has been devoted recently to the development of synthetic receptors that allow for the recognition, detection, or extraction of pyrophosphate and related species, such as inorganic phosphate.5 Systems incorporating neutral or cationic NH hydrogen bond donor groups (e.g., pyrrole, indole, ammonium, and guanidinium) or cationic CH hydrogen bond donor motifs (e.g. imidazolium and triazolium) have been particularly effective in this regard. However, to the best of our knowledge, receptors with neutral CH H-bond donors have yet to be exploited for the purpose of pyrophosphate (or phosphate) anion recognition.
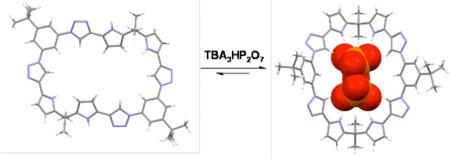
CH bonds are present in the overwhelming majority (97%) of chemical compounds.6 Nevertheless, it is only recently that the importance of CH H-bond in biological and artificial anion recognition has come to be appreciated.7 In recent pioneering work, Flood and co-workers reported the synthesis and anion binding properties of [34]triazolophanes. These new macrocycles have a diameter of about 3.8 Å, and display a high affinity for the chloride ion.7(c) On the basis of this and previous theoretical and experimental studies,8 it was suggested that the strength of neutral, triazole-derived C-H···X- (X- = halide) bonds can approach those of more traditional NH donors, such as pyrrole. We thus considered it of interest to combine both these recognition motifs within the same macrocyclic framework. Here, we report the first such system, namely the calix[2]1,3-bis(pyrro-2-yl)(1,4)-1,2,3-triazolo-phane (1), and show that it acts as a highly effective receptor for the pyrophosphate anion, both in the solid state and in organic media.9 Our findings, supported by theory, provide support for the conclusion that NH-anion bonding interactions are more important than CH-anion interactions.
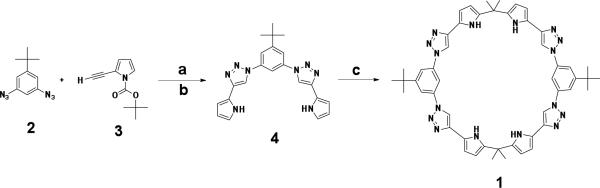
The synthesis of 1 is shown in Scheme 1. Using “click” chemistry conditions, a copper-catalyzed Huisgen 1,3-dipolar cycloaddition10 was used to couple 2 with 3. This was followed by t-BOC deprotection11 to give 1,3-bis(pyrro-2-yl)-(1,4)-1,2,3-triazolo-benzene (4) in 24% combined yield for two steps. Condensation of 4 with acetone12 produced 1 in 10% yield.
Scheme 1.
Synthesis of macrocycle 1. a) CuSO4, sodium ascorbate, EtOH / H2O / toluene (7:3:1); b) NaOC(CH3)3, anhydrous THF; c) acetone, TFA, Ar, R.T.
NOESY spectroscopic studies provided support for the notion that 1 has a flexible conformation at ambient temperature. This was further verified by variable temperature 1H NMR spectroscopic studies; specifically, signals that became broader with decreasing temperature were observed (cf. ESI).
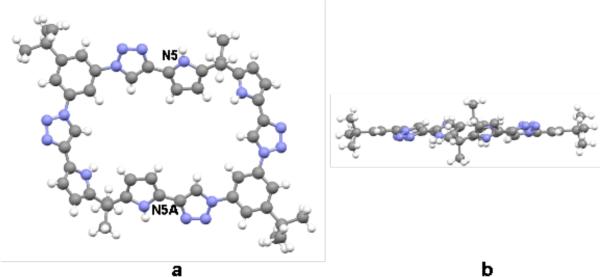
A single crystal X-ray diffraction analysis of 1 revealed a nearly flat structure, wherein the pyrrole NH protons on N5 and N5A point out from the center of the core. The distance between the exocyclic C-H or N-H and the nitrogen atoms on the neighboring triazole ring generally proved to be less than 3 Å. This leads us to suggest that intramolecular H-bonding interactions on the exterior of the ring,7(r) help stabilize the observed planar conformation in the solid state (cf. Figure 1).
Figure 1.
a) Top and b) side views of the single crystal X-ray structure of 1·4CH3OH·H2O. All solvent molecules have been omitted for clarity.
The anion properties of 1 in solution were first analyzed using UV-Vis spectroscopy. Standard titrations, associated curve fittings,13 and Job plots provided support for pyrophosphate (as the tetrabutylammonium (TBA) salt) being bound to 1 strongly in chloroform at 300 K (Ka = (2.30 ± 0.40) × 106 M-1) and with a 1:1 binding stoichiometry. Mass spectrometric analyses also provide support for the formation of stable complexes (cf. ESI).
The association constants (Ka) between receptor 1 and various other test anions were also determined in chloroform via UV-Vis absorption titrations. Among the test anions considered, receptor 1 displays selectivity for the trianionic pyrophosphate anion, followed by HSO4- ((4.98±0.40)×105) > H2PO4- ((2.38±0.25)×105) > Cl- ((1.18±0.07)×104) > Br- ((2.64±0.09)×103) (all as TBA salts; cf. ESI). Macrocycle 1 thus displays a high pyrophosphate/dihydrogenphosphate selectivity (Ka(HP2O73-)/Ka(H2PO4-) = 10).
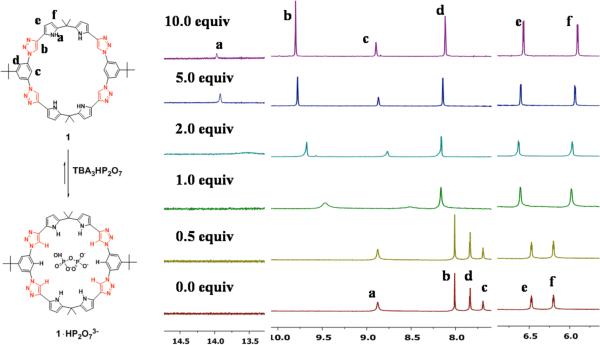
The host-guest interactions between macrocycle 1 and the pyrophosphate anion were further analyzed by 1H NMR spectroscopy. Specifically, a solution of 1 (5 mM, CDCl3) was titrated with up to 10 equivalents of TBA3HP2O7 (Figure 2). The hydrogen signals for the pyrrole NH, the triazole CH, and the endocyclic hydrogen atom of the N1-linked phenyl unit were seen to shift downfield by 5.09 ppm, 1.96 ppm, and 1.22 ppm, respectively (Figure 2). These changes followed the sequence of expected H acidity, namely pyrrole NH > triazole CH > benzene CH.14 Thus the trends observed in the NMR spectral studies are consistent with the intrinsic strength of the various H-bond donor groups as inferred from electronic structure calculations carried out with chloride anion. Binding energies (MP2/aug-cc-pVDZ) computed for complexes of chloride with pyrrole N-H, triazole C-H, and benzene C-H donors are -22.50, -18.94, and -8.42 kcal mol–1, respectively (cf. ESI).15 They are also consistent with the hydrogen bond distances observed in the solid state (see below). Nevertheless, it is important to appreciate that all three hydrogen bond donor motifs play a role in the observed anion recognition. In contrast, only a small downfield shift was seen for the signals ascribed to the exocyclic hydrogen atoms on the phenyl and pyrrole rings (Δδ = 0.09 ppm and 0.10 ppm, respectively); apparently these C-H bonds play little role in anion recognition.
Figure 2.
1H NMR spectra (aromatic region) of 1 recorded upon titration with (TBA)3HP2O7; CDCl3, 300K.
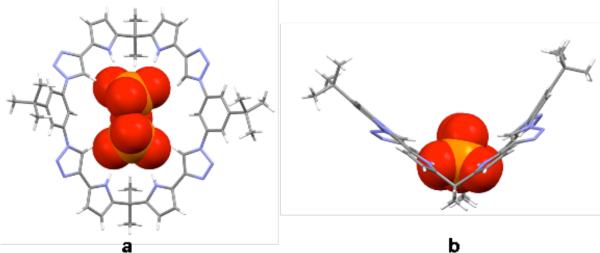
Additional support for the suggestion that both NH and neutral CH interactions combine to effect pyrophosphate anion recognition in the case of receptor 1 came from a single crystal X-ray diffraction analysis of the complex formed between pyrophosphate and 1 (1·TBA3HP2O7·3H2O) (Figure 3). In this complex, the macrocycle displays a folded conformation; this provides a clip-like slot into which the pyrophosphate anion inserts. All the pyrrole NH, triazole CH, and endocyclic benzene CH protons point into the center of the ring and are involved in hydrogen bond interactions with the bound pyrophosphate guest, as inferred from bond distances (pyrrole NH···O ca. 1.9 Ǻ, triazole CH···O ca. 2.3 Ǻ, benzene CH···O ca. 3.7 Ǻ ). The resulting conformation thus stands in contrast to what is seen in the case of the free host 1.
Figure 3.
a) Top and b) side views of a single-crystal X-ray diffraction structure of 1·TBA3HP2O7·3H2O in which the pyrophosphate anion is in a space filling representation. Solvent molecules and TBA cations have been omitted for clarity.
In summary, the pyrrolyl-based receptor 1 has been prepared in a moderate yield. It binds the pyrophosphate anion in the solid state and displays a high selectivity for this trianion relative to various test monoanions in chloroform solution. The present results thus serve to underscore the utility of new recognition motifs in the design of anion binding agents, particularly those that combine both CH- and NH-anion interactions within a single framework. The present work also helps calibrate directly the relative importance of these interactions. It thus provides a foundation for future receptor design.
Supplementary Material
Acknowledgement
This work was supported by the National Institutes of Health (Grant No. GM58907 to J.L.S.) and the National Science Foundation (Grants No. 0749571 to J.L.S. and 0741973 for the X-ray diffractometer). B.P.H. acknowledges support from the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences, U.S. DOE at Oak Ridge National Laboratory. Support under the WCU (World Class University) program (R32-2008-000-10217-0) is also acknowledged.
Footnotes
Supporting Information Available: Details describing the synthesis and characterization of compounds 4 and 1, details of fitting binding curves, details of electronic structure calculations and crystallographic data (CIF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Mathews CP, van Hold KE. Biochemistry. The Benjamin/Cummings Publishing Company, Inc.; Redwood City, CA: 1990. [Google Scholar]
- 2.Ronaghi M, Karamohamed S, Pettersson B, Uhlén M, Nyrén P. Anal. Biochem. 1996;242:84–89. doi: 10.1006/abio.1996.0432. [DOI] [PubMed] [Google Scholar]
- 3.Xu S, He M, Yu H, Cai X, Tan X, Lu B, Shu B. Anal. Biochem. 2001;299:188–193. doi: 10.1006/abio.2001.5418. [DOI] [PubMed] [Google Scholar]
- 4.Saenger W. Principles of Nucleic Acid Structure. Springer-Verlag; New York: 1988. [Google Scholar]
- 5.For review, see Sessler JL, Gale PA, Cho W-S. Anion Receptor Chemistry. RSC Publishing; Cambridge, U.K.: 2006. Kim S-K, Lee D-H, Hong J-I, Yoon J-Y. Acc. Chem. Res. 2009;42:23–31. doi: 10.1021/ar800003f.; For recent reports, see Kumar A, Pandey PS. Org. Lett. 2008;10:165–168. doi: 10.1021/ol702457w. Chen K-H, Liao J-H, Chan H-Y, Fang J-M. J. Org. Chem. 2009;74:895–898. doi: 10.1021/jo802173b.; Xu Z, Singh NJ, Lim J, Pan J, Kim H-N, Park SS, Kim KS, Yoon J-Y. J. Am. Chem. Soc. 2009;131:15528–15533. doi: 10.1021/ja906855a.
- 6.Of the 18,426,559 molecules in PubChem (March 2008), 17,947,719 (97%) contained one or more CH units. Li Y, Flood AH. J. Am. Chem. Soc. 2008;130:12111–12122. doi: 10.1021/ja803341y.
- 7.a Bryantsev VS, Hay BP. Org. Lett. 2005;7:5031–5034. doi: 10.1021/ol0520119. [DOI] [PubMed] [Google Scholar]; b Bryantsev VS, Hay BP. J. Am. Chem. Soc. 2005;127:8282–8283. doi: 10.1021/ja0518272. [DOI] [PubMed] [Google Scholar]; c Li Y, Flood AH. Angew. Chem., Int. Ed. 2008;47:2649–2652. doi: 10.1002/anie.200704717. [DOI] [PubMed] [Google Scholar]; d Yoon DW, Gross DE, Lynch VM, Sessler JL, Hay BP, Lee C-H. Angew. Chem., Int. Ed. 2008;47:5038–5042. doi: 10.1002/anie.200801426. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Li Y, Pink M, Karty JA, Flood AH. J. Am. Chem. Soc. 2008;130:17293–17295. doi: 10.1021/ja8077329. [DOI] [PubMed] [Google Scholar]; f Zhu SS, Staats H, Brandhorst K, Grunenberg J, Gruppi F, Dalcanale E, Lützen A, Rissanen K, Schalley CA. Angew. Chem., Int. Ed. 2008;47:788–792. doi: 10.1002/anie.200703451. [DOI] [PubMed] [Google Scholar]; g Juwarker H, Lenhardt JM, Pham DM, Craig SL. Angew. Chem., Int. Ed. 2008;47:3740–3743. doi: 10.1002/anie.200800548. [DOI] [PubMed] [Google Scholar]; h Meudtner RM, Hecht S. Angew. Chem., Int. Ed. 2008;47:4926–4930. doi: 10.1002/anie.200800796. [DOI] [PubMed] [Google Scholar]; i Berryman OB, Sather AC, Hay BP, Meisner JS, Johnson DW. J. Am. Chem. Soc. 2008;130:10895–10897. doi: 10.1021/ja8035652. [DOI] [PubMed] [Google Scholar]; j Maeda H, Mihashi Y, Haketa Y. Org. Lett. 2008;10:3179–3182. doi: 10.1021/ol801014z. [DOI] [PubMed] [Google Scholar]; k Hay BP, Bryantsev VS. Chem. Commun. 2008:2417–2428. doi: 10.1039/b800055g. [DOI] [PubMed] [Google Scholar]; l Romero T, Caballero A, Tárraga A, Molina P. Org. Lett. 2009;11:3466–3469. doi: 10.1021/ol901308z. [DOI] [PubMed] [Google Scholar]; m Mullen KM, Mercurio J, Serpell CJ, Beer PD. Angew. Chem., Int. Ed. 2009;48:4781–4784. doi: 10.1002/anie.200901313. [DOI] [PubMed] [Google Scholar]; n Juwarker H, Lenhardt JM, Castillo JC, Craig SL. J. Org. Chem. 2009;74:8924–8934. doi: 10.1021/jo901966f. [DOI] [PubMed] [Google Scholar]; o Fisher MG, Gale PA, Hiscock JR, Hursthouse MB, Light ME, Schmidtchen FP, Tong CC. Chem. Commun. 2009:3017–3019. doi: 10.1039/b904089g. [DOI] [PubMed] [Google Scholar]; p Pedzisa L, Hay BP. J. Org. Chem. 2009;74:2554–2560. doi: 10.1021/jo900018u. [DOI] [PubMed] [Google Scholar]; q Schulze B, Friebe C, Hager MD, Günther W, Köhn U, Jahn BO, Görls H, Schubert US. Org. Lett. 2010;12:2710–2713. doi: 10.1021/ol100776x. [DOI] [PubMed] [Google Scholar]; r Lee S, Hua Y, Park H, Flood AH. Org. Lett. 2010;12:2100–2102. doi: 10.1021/ol1005856. [DOI] [PubMed] [Google Scholar]; s Yano M, Tong CC, Light ME, Schmidtchen FP, Gale PA. Org. Biomol. Chem. 2010 doi: 10.1039/c0ob00128g. in press (DOI: 10.1039/C0OB00128G) [DOI] [PubMed] [Google Scholar]
- 8.Hua Y, Flood AH. Chem. Soc. Rev. 2010;39:1262–1271. doi: 10.1039/b818033b. [DOI] [PubMed] [Google Scholar]
- 9.Although it does not contain triazole and pyrrole in the same macrocyclic ring, it is to be noted that Gale recently reported a triazole strapped calixpyrrole and showed it to be effective for chloride anion recognition. See: ref. 7(o) and 7(s);
- 10.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem,. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Tom NJ, Simon WM, Frost HN, Ewing M. Tetrahedron Lett. 2004;45:905–906. [Google Scholar]
- 12.Kim SK, Sessler JL, Gross DE, Lee C-H, Kim JS, Lynch VM, Delmau LH, Hay BP. J. Am. Chem. Soc. 2010;132:5827–5836. doi: 10.1021/ja100715e. [DOI] [PubMed] [Google Scholar]
- 13.Bourson J, Pouget J, Valeur B. J. Phys. Chem. 1993;97:4552–4557. [Google Scholar]
- 14.Katritzky AR. Handbook of Heterocyclic Chemistry. Pergamon Press; p. 2000. [Google Scholar]
- 15.The CH binding energies are consistent with a prior description of individual hydrogen-bond contributions within a triazolophane*Cl- complex, which implicated energies of -10 kcal/mol per triazole CH and -3 to -4 kcal/mol per aryl CH: Bandyopadhyay I, Raghavachari K, Flood AH. Chem. Phys. Chem. 2009;10:2535–2540. doi: 10.1002/cphc.200900476.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.