Abstract
Manganese superoxide dismutase (MnSOD) from different species differs in its efficiency in removing high concentrations of superoxide (O2−), due to different levels of product inhibition. Human MnSOD exhibits a substantially higher level of product inhibition than the MnSODs from bacteria. In order to investigate the mechanism of product inhibition and whether it is a feature common to eukaryotic MnSODs, we purified MnSOD from Saccharomyces cerevisiae (ScMnSOD). It was a tetramer with 0.6 equivalents of Mn per monomer. The catalytic activity of ScMnSOD was investigated by pulse radiolysis and compared with human and two bacterial (Escherichia coli and Deinococcus radiodurans) MnSODs. To our surprise, ScMnSOD most efficiently facilitates removal of high concentrations of O2− among these MnSODs. The gating value k2/k3 that characterizes the level of product inhibition scales as ScMnSOD > D. radiodurans MnSOD > E. coli MnSOD > human MnSOD. While most MnSODs rest as the oxidized form, ScMnSOD was isolated in the Mn2+ oxidation state as revealed by its optical and electron paramagnetic resonance spectra. This finding poses the possibility of elucidating the origin of product inhibition by comparing human MnSOD with ScMnSOD
Manganese superoxide dismutase (MnSOD) enzymes catalyze superoxide (O2−) disproportionation by a mechanism that is more complex than those of the other SODs. In particular the reduction of superoxide can proceed via one of two pathways. One pathway dominates when the O2− concentration is low relative to the enzyme concentration (reaction 2) and the other pathway dominates when the ratio [O2−]:[MnSOD] is high (reactions 3 and 4). The paradoxical finding is that MnSOD is a less effective SOD catalyst when O2− levels are elevated.1
| (1) |
| (2) |
| (3) |
| (4) |
The depressed catalytic activity at high O2− concentrations is known to be due to the formation of a product-inhibited Mn3+-peroxo adduct resulting from the inner sphere oxidation of Mn2+SOD by O2− (reaction 3). This pathway is considerably slower than the outer sphere oxidation and protonation pathway described in reaction 2. The relative levels of product inhibition are described kinetically by the value of k2/k3 for different MnSODs (Table I). The contribution from this slower pathway is particularly pronounced in human MnSOD.1 Thus the O2− removal and H2O2 production rates are dependent on the relative levels of MnSOD and O2−, and the degree of product inhibition of the specific MnSOD present.
Table I.
Rate constants for the different MnSODs
Superoxide concentrations are known to be variable in cells; for example, it has recently been shown that transient O2− bursts, termed “superoxide flashes”, are formed in human mitochondria,2 creating the possibility of even greater variability in H2O2 formation rates. However, slower product-inhibited pathway in human MnSOD would allow for more constant H2O2 formation even when O2− concentrations vary.
Low levels of H2O2 play an important role in signaling in mammalian cells, regulating numerous processes including rates of cell growth and division.3 It has been proposed that the slower pathway for human MnSOD appeared in response to an evolutionary pressure to control more tightly intracellular H2O2 levels,1 to reduce H2O2 mediated oxidative damage and to optimize its signaling function.4 The k2/k3 values determined for human and bacterial MnSODs (Table I) are consistent with this hypothesis. However, structural studies of different MnSODs, both wild type and mutant, have yet to reveal why k2/k3 differs so dramatically for this enzyme.1
The budding yeast Saccharomyces cerevisiae is widely used as a single-cell model for higher eukaryotic organisms because it is remarkably similar to mammalian cells. S. cerevisiae also appears to be less sensitive to H2O2 than human cells, and the only currently known H2O2 sensing proteins in S. cerevisiae (YAP1p and Skn7p) are involved in regulating oxidative stress protection; a more general signaling role has yet to be found.3,5 Both human MnSOD and S. cerevisiae MnSOD (ScMnSOD) are tetramers6 and localized to the mitochondrial matrix,7 while most bacterial MnSODs are dimers. Moreover, human MnSOD shares greater sequence similarity with ScMnSOD than with Escherichia coli or Deinococcus radiodurans MnSODs (62.2%, 52.9%, and 54.5% respectively).8 Published reports of the activity of ScMnSOD, do not include a determination of the degree of product inhibition.6,9 We therefore turned our attention to characterizing the catalytic mechanism of ScMnSOD with the expectation that the high contribution from the product-inhibited pathway would prove to be a property common to eukaryotic MnSODs. Surprisingly, we found instead that ScMnSOD is even less product-inhibited than the bacterial MnSODs characterized to date, surpassing even the high activity of MnSOD from the radiation resistant bacterium D. radiodurans.8a
The gene of ScMnSOD,9 which includes the mitochondrial targeting sequence, was inserted into the plasmid YEp352. The enzyme was overexpressed in S. cerevisiae and purified using a protocol modified from that of Fridovich et al. (Supporting Information).6 The protein was isolated as a tetramer with the leader sequence removed and contained 0.6 equivalents of Mn per monomer, as per size exclusion chromatography, mass spectrometry, and ICP-MS, respectively.
Although the fitting of the kinetic data reported here matches the experimental decay of pulsed O2−, out of the four rate constants, only k2 has been measured directly by pulse radiolysis. k2 was measured by oxidizing the resting enzyme with substoichiometric amounts of O2− and following the appearance of Mn3+SOD, which has a characteristic absorption band near 480 nm (Supporting Information). Unlike other MnSODs, ScMnSOD was isolated in the reduced state, precluding direct measurement of k1. k1, k3 and k4 were determined by fitting the observed rate of O2− (ε260=2000 M−1cm−1) loss at multiple enzyme concentrations (1–10 µM) and initial O2− concentrations (2–48 µM) using PRWIN, conditions that, while not physiological, are necessary to study kinetics by pulse radiolysis.1,8 The rate constants are compared to those known for other MnSODs in Table I.
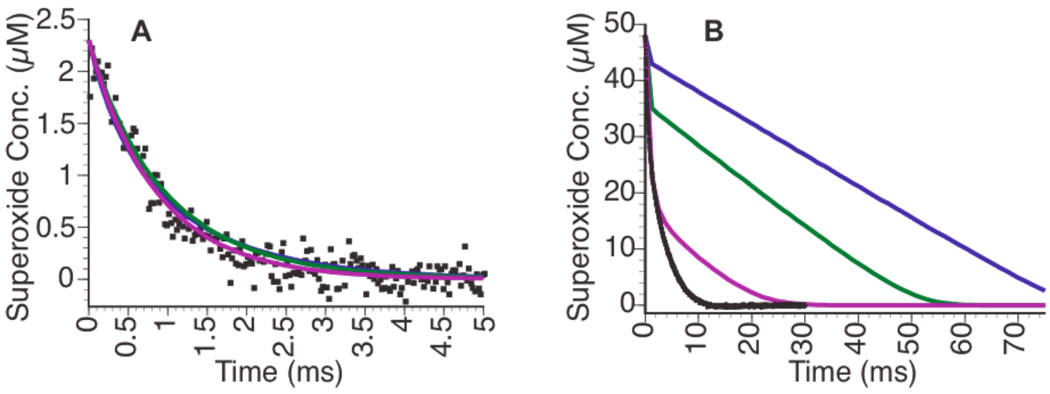
The ability of ScMnSOD10 to catalyze the dismutation of O2− was found to exceed that of the other characterized MnSODs. At low concentrations of O2− ([O2−] ≈ [MnSOD]), the observed rates of disappearance are similar for all the four enzymes (Fig. 1A). However at high concentrations ([O2−] >> [MnSOD]), the differences in activities are pronounced (Fig. 1B). At the highest concentrations of O2− employed in pulse radiolysis, the order of the activities of the enzymes is S. cerevisiae > D. radiodurans > E. coli > human, which is identical to the ordering of k2/k3, indicating that the deciding factor is the tendency to form the product-inhibited state.
Figure 1.
The decay of 2.3-µM (A) and 48-µM (B) O2− concentrations with different types of MnSOD. ScMnSOD pulse radiolysis data are shown in black, while the lines are from Kintecus10 computer modeling using rate constants in Table I: MnSOD from D. radiodurans (purple); E. coli (green); and human (blue); all at 1 µM Mn.
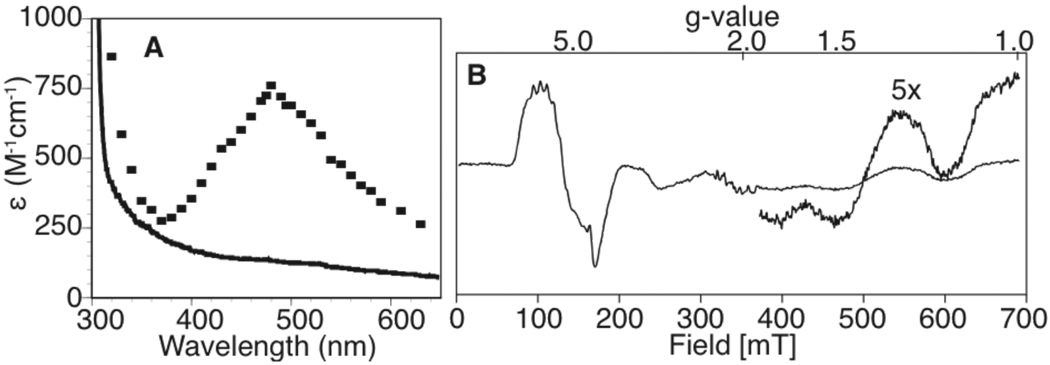
Most known MnSODs rest in the Mn3+ oxidation state,11 but our ScMnSOD was consistently isolated as predominantly reduced Mn2+SOD; the electronic absorption spectrum of as-isolated ScMnSOD lacked the visible absorption band with a maximum near 480 nm. It was reported previously that the optical absorption spectrum of ScMn3+SOD was unusual relative to those of other MnSODs,9 but the spectrum of ScMn3+SOD that we obtained by reaction of as-isolated ScMnSOD with O2− using pulse radiolysis (Fig. 2A, dots) was very similar to those of other Mn3+SODs, with an extinction coefficient of ~800 M−1cm−1. The small measured absorbance at 480 nm (Fig. 2A, line) corresponds to greater than 90% of the as-isolated enzyme being in the Mn2+ state.
Figure 2.
ScMnSOD was isolated in the Mn2+ oxidation state. A) Electronic absorption spectra of as-isolated ScMnSOD (line) and Mn3+SOD obtained after reaction with O2− in pulse radiolysis (dots). B) EPR spectrum of as-isolated ScMnSOD. Instrumental parameters: temperature, 4.7 K; microwave frequency, 9.685 GHz; microwave power, 0.2 mW; modulation amplitude, 0.8 mT.
The EPR spectrum of our as-isolated ScMnSOD also indicates that the enzyme is reduced, since the perpendicular-mode EPR spectrum is similar to those of other Mn2+SODs,12 with the usual six-line hyperfine splitting from the 55Mn nucleus (I = 5/2) seen at geff = 6.0 (Fig 2B). We looked for evidence of the integer spin Mn3+ (S = 2) by parallel-mode EPR, but our spectrum lacked the sextet hyperfine pattern typically displayed by Mn3+SOD (Supporting Information).13
The only other MnSOD enzymes that have been isolated in the Mn2+ oxidation state are mutant MnSODs, most notably the Gln143 mutants of the human enzyme.14 However, the factors that determine the resting oxidation state are unknown.
Increased levels of MnSOD activity have been shown to slow down tumor growth in cultured human cells and in animal studies15, and it has been proposed that this effect is related to cellular H2O2 levels. For that reason and to improve our understanding of the basis of the observed product-inhibition, human MnSOD has been repeatedly mutated in attempts to make its activity resemble that of the bacterial proteins, but with limited success.1,8c As described above, human MnSOD already shares greater sequence similarity with ScMnSOD than with the bacterial enzymes, and the two eukaryotic proteins are tetramers while the bacterial ones are dimers. Also, ScMnSOD resembles the bacterial ones in that k3 is small, but is similar to the human enzyme in that k4 is large. Thus, to improve our understanding of what causes the unusual kinetic properties of human MnSOD, it may be more productive to compare/contrast it with ScMnSOD than with the bacterial MnSODs. Investigation of the slight structural differences between the enzymes may provide a key to understanding the chemical mechanism of product inhibition. We will also continue to study the evolutionary significance of product inhibition by studying MnSOD from other organisms.
Supplementary Material
Acknowledgement
This work was supported by grant DK46828, KOSEF/MEST through WCU project (R31-2008-000-10010-0) to J.S.V and GM48242 to R.D.B. Radiolysis studies were carried out at the Center for Radiation Chemistry Research at BNL, which is funded under contract DE-AC02-98CH10886 with the U.S. Department of Energy and supported by its Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences.
Footnotes
Supporting Information Available: ScMnSOD isolation details, pulse radiolysis, complete ref 2, and parallel mode EPR. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Abreu IA, Cabelli DE. Biochim. Biophys. Acta. 2010;1804:263. doi: 10.1016/j.bbapap.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Wang W, et al. Cell. 2008;134:279. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veal EA, Day AM, Morgan BA. Mol. Cell. 2007;26:1. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Rhee SG. Science. 2006;312:1882. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 5.Stone JR, Yang SP. Antioxid. Redox Signaling. 2006;8:243. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 6.Ravindranath SD, Fridovich I. J. Biol. Chem. 1975;250:6107. [PubMed] [Google Scholar]
- 7.Luk E, Yang M, Jensen LT, Bourbonnais Y, Culotta VC. J. Biol. Chem. 2005;280:22715. doi: 10.1074/jbc.M504257200. [DOI] [PubMed] [Google Scholar]
- 8.a) Abreu IA, Hearn A, An H, Nick HS, Silverman DN, Cabelli DE. Biochemistry. 2008;47:2350. doi: 10.1021/bi7016206. [DOI] [PubMed] [Google Scholar]; b) Hearn AS, Stroupe ME, Cabelli DE, Lepock JR, Tainer JA, Nick HS, Silverman DN. Biochemistry. 2001;40:12051. doi: 10.1021/bi011047f. [DOI] [PubMed] [Google Scholar]; c) Zheng J, Domsic JF, Cabelli D, McKenna R, Silverman DN. Biochemistry. 2007;46:14830. doi: 10.1021/bi7014103. [DOI] [PubMed] [Google Scholar]
- 9.Schrank IS, Sims PFG, Oliver SG. Gene. 1988;73:121. doi: 10.1016/0378-1119(88)90318-6. [DOI] [PubMed] [Google Scholar]
- 10.Ianni JC, Kintecus Windows ver. 3.95. 2008 http://www.kintecus.com/
- 11.Stroupe ME, DiDonato M, Tainer JA. In: Handbook of Metalloproteins. Messerschmidt A, editor. Vol. 2. Chichester, UK: John Wiley & Sons Ltd.; 2001. p. 941. [Google Scholar]
- 12.a) Whittaker JW, Whittaker MM. J. Am. Chem. Soc. 1991;113:5528. [Google Scholar]; b) Whittaker MM, Whittaker JW. J. Biol. Chem. 1999;274:34751. doi: 10.1074/jbc.274.49.34751. [DOI] [PubMed] [Google Scholar]
- 13.Campbell KA, Yikilmaz E, Grant CV, Gregor W, Miller AF, Britt RD. J. Am. Chem. Soc. 1999;121:4714. [Google Scholar]
- 14.Leveque VJP, Stroupe ME, Lepock JR, Cabelli DE, Tainer JA, Nick HS, Silverman DN. Biochemistry. 2000;39:7131. doi: 10.1021/bi9929958. [DOI] [PubMed] [Google Scholar]
- 15.a) Church SL, Grant JW, Ridnour LA, Oberley LW, Swanson PE, Meltzer PS, Trent JM. Proc Natl Acad Sci U S A. 1993;90:3113. doi: 10.1073/pnas.90.7.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Davis CA, Hearn AS, Fletcher B, Bickford J, Garcia JE, Leveque V, Melendez JA, Silverman DN, Zucali J, Agarwal A, Nick HS. J. Biol. Chem. 2004;279:12769. doi: 10.1074/jbc.M310623200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.