Abstract
There are two major aspects to a spinal cord injury (SCI): an acute, primary mechanical trauma and a progressive phase of secondary tissue damage provoked by inflammation, excitotoxicity, apoptosis and demyelination. MicroRNAs (miRs) are small, ~22 nucleotide, non-protein-coding RNAs that function at the post-transcriptional level to regulate gene expression. They have important roles in homeostatic processes such as cell proliferation and programmed cell death. In the injured rat spinal cord we performed an expression analysis of miRs and their downstream targets involved in apoptotic pathways and used post-injury cycling exercise to test for activity dependent plasticity of miR expression. We show that SCI results in increased expression of miR Let-7a and miR16 while exercise leads to elevated levels of miR21 and decreased levels of miR15b. These changes in miR expression are correlated with changes in expression of their target genes: pro-apoptotic (decreased PTEN, PDCD4 and RAS mRNA) and anti-apoptotic (increased Bcl-2 mRNA) target genes. This is accompanied by a down regulation of mRNA for caspase-7 and caspase-9 and reduced levels of caspase-7 protein. These results indicates possible beneficial effects of exercise through action on multiple miRs and their targets that contribute to the functional regulation of apoptosis after SCI.
Keywords: apoptosis, microRNAs, target gene expression, activity-dependent plasticity, spinal cord injury
Introduction
MicroRNAs (miRs) are a class of small, non-coding RNAs whose mature products are ~22 nucleotides long (Griffiths-Jones, 2004; Griffiths-Jones et al., 2006; Griffiths-Jones et al., 2008). Sequence and function conservation between distantly related organisms suggest that this class of small RNAs is an integral part of essential cellular processes (Pasquinelli et al., 2000). Microarrays performed after contusion spinal cord injury (SCI) identified 60 miRs up- or down-regulated at moderate to high levels compared to uninjured spinal cord tissue (Liu et al., 2009). Cluster analysis indicated that many of these miRs were involved in pathophysiological events secondary to SCI, such as inflammation, oxidation and apoptosis.
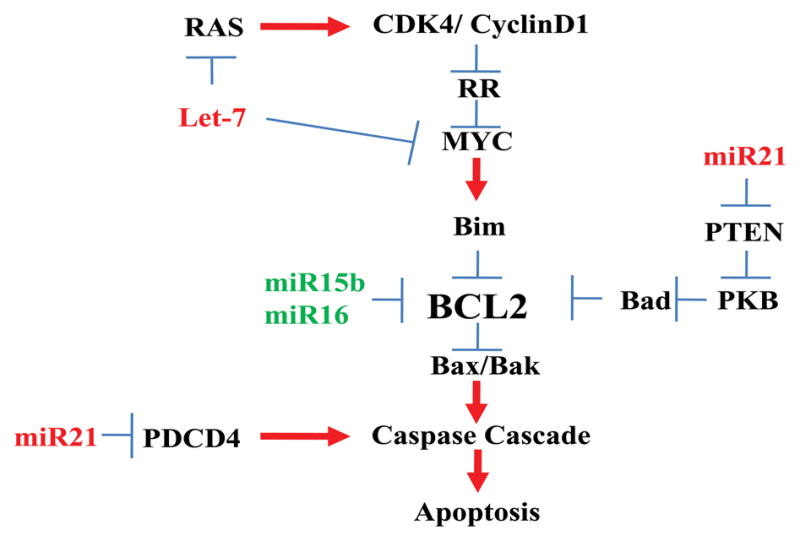
Apoptosis is an active form of cell death known to occur during development and following trauma to the central nervous system. Much of the early data regarding apoptotic death was confined to the study of neurons but it occurs also in oligodendrocytes and microglial cells (Beattie et al., 2000). The loss of oligodendrocytes in white matter tracts continues for many weeks after spinal cord injury (SCI) and may contribute to progressive post-injury demyelination (Crowe et al., 1997; Shuman et al., 1997). As an important contributor to secondary tissue damage after SCI we focused on the expression of miRs known to be associated with apoptotic pathways (Figure 1). MiR21 functions as an anti-apoptotic factor by inhibiting the expression of the pro-apoptotic proteins phosphatase and tensin homolog (PTEN) and programmed cell death 4 (PDCD4) (Chan et al., 2005; Sayed et al., 2010). The miR Let-7a may function as a pro-apoptotic factor by its effects on the anti-apoptotic genes RAS and MYC (Johnson et al., 2005; Sampson et al., 2007). The miRs 15b and 16 appear to function as pro-apoptotic mediators of cell death and their upregulation is correlated with the reduced expression of the anti-apoptotic factor Bcl-2 and increased expression of caspases 3, 8 and 9 (Guo et al., 2009). Bcl-2 and related cytoplasmic proteins are well established key regulators of apoptosis (Adams et al., 1998; Danial, 2007). Caspases are the final effectors in the apoptotic pathway and thus are key mediators of programmed cell death (Eldadah and Fadden, 2000). Caspase-9 is one of several initiator caspases that cleaves inactive pro-forms of effector caspases such as caspase-3 and caspase-7. In turn, active caspase-3, a major mediator of apoptosis following injury, can cleave caspase-9 as well as itself.
Figure 1.
Diagram of miRNA regulation of apoptosis. MicroRNA 21 can affect the caspase cascade by 2 paths, by inhibiting PDCD4 and/or PTEN, leading to reduced apoptosis. The miR15 and miR16 directly influence BCL-2 expression, leading in increased apoptosis. The role of Let-7a in apoptosis is less direct as it influences expression of RAS and MYC which are further upstream of Bcl2.
Here we sought evidence for change in expression of miRs which are involved in fine-tuning of downstream apoptosis-related genes and tested whether exercise would affect changes in miR expression associated with programmed cell death after SCI. As a non-invasive therapy, exercise maintains muscle mass of paralyzed hindlimbs (Houle et al., 1999), stabilizes rhythmic firing patterns of spinal motoneurons (Beaumont et al., 2004), leads to anatomical and biochemical plasticity in the spinal cord (Tillakaratne et al., 2000) and results in increased levels of intraspinal neurotrophic factors in muscle and spinal cord tissue (Gomez-Pinilla et al., 2002; Dupont-Versteegden et al., 2004; Ying et al., 2005). We show that exercise therapy influences multiple miRs with target genes that are key regulators of caspase gene expression, greatly expanding our knowledge of possible mechanisms by which this treatment approach may have neuroprotective and/or neuroregenerative effects after SCI.
Materials and Methods
Animal groups
Adult female Sprague-Dawley rats (225–250g) were divided into 5 groups (n=6 for each group, 30 total): Uninjured control, transected for 10 days (Tx10d), transected for 10d with cycling exercise (Tx+Ex 10d), transected for 31d (Tx 31d), and transected for 31d with cycling exercise (Tx+Ex 31d), (Supplemental Figure 1A). The experimental protocol was approved by Drexel University s Institutional Animal Care and Use Committee (IACUC) and all animal procedures followed National Institute of Health (NIH) guidelines for the care and use of laboratory animals.
Spinal cord transection
Inhalation anesthesia with isoflurane was used for all animals. Depth of anesthesia was confirmed by the absence of corneal reflexes and no response to a paw pinch. Using sterile techniques laminectomy of the ninth thoracic vertebra was performed to expose the dorsal surface of the T10 spinal cord. Meningeal membranes were opened longitudinally and gentle aspiration through a glass micropipette was used to create a 2 mm long complete transection lesion cavity that was confirmed microscopically. The cavity was filled with gel foam to achieve hemostasis, after which gel foam was removed, the dura closed with 10-0 sutures and overlying muscles and fascia closed in layers with 4-0 sutures. Michel wound clips were used to close the skin. After surgery, bladders were manually expressed 2–3 times daily until reflex voiding returned (about 2 weeks post injury). Ampicillin (100 mg/kg, sc) was administered twice daily for 7 days to prevent infection, Buprenorphin (0.05 mg/kg, im) was given 3 days post injury for analgesic effect. Sterile lactated Ringer s solution (5 ml daily, sc) was provided for 7d to reduce the possibility of dehydration. Special absorbent bedding was provided to prevent pressure ulceration and animals were housed 3/cage and provided food and water ad.lib.
Cycling exercise
Details of this passive form of hindlimb exercise have been provided previously (Skinner et al., 1996; Houle et al., 1999). In brief, animals were supported in an adjustable sling with their hind limbs hanging beneath them. Surgical tape was used to secure the hind paw to motor-driven foot pedals. Exercise began 5 days after spinal cord transection and continued on a daily basis, 5 days per week. Full range of motion occurred through each cycle of the pedals, the rate was 45 rpm and there were two 30 minute exercise sessions separated by a 10 minute rest period each day. Animals in the Tx+Ex 10d group were exercised for 5d, while animals in the Tx+Ex 31d group were exercised for a total of 20 days.
Tissue collection
Animals in the exercise groups were sacrificed approximately 1 hour after their final training session, at either 10 or 31 days after injury. Animals were anesthetized with a lethal dose of Euthasol (390 mg/kg, J.A. Webster, Sterling, MA.) and lumbar spinal cord segments L4–L6 were removed and divided at the midline into two equal pieces (Supplementary Figure 1B). These pieces were immediately frozen in liquid nitrogen and stored separately at −80°C for further processing.
Total RNA isolation and quantitative PCR (Q-PCR)
Total RNA from one half of the L4-6 spinal cord was isolated with an RNeasy Mini kit (QIAGEN, Valencia, CA). cDNA synthesis was carried out with 500ng of total RNA using a RT First Strand Kit (SABiosciences, Frederick, MD). Setup for the cDNA PCR reaction was standard for all samples: all primers were ordered from Integrated DNA Technologies Inc. (San Diego, CA) and are listed in Supplemental Table 1. Each 25μL reaction contained 12.5μL of iQ SYBR Green Supermix (Bio-Rad. Hercules, CA), 1.5μL (4μM) each of forward and reverse primers, 1μL of cDNA, and 8.5μL of nuclease free water. The miRs QPCR system from Applied Biosystems (Foster City, CA) was used in a 2-step reaction: reverse transcription (RT) and PCR. The RT reactions used 30ng of total RNA, 3μl 5X miRNA RT looped-primers, 1.5μl 10X RT buffer, 1ul reverse transcriptase, and 0.188μl RNase inhibitor and water to a 15μl final volume, RT reactions were incubated for 30 min at 16°C and at 42°C. The miRs Q-PCR reaction contained the following: 10μl of TaqMan 2X universal PCR master mix, 1μl of 20X TaqMan miRNA primers, 1.33μl RT product and 7.67μl nuclease free water. Q-PCR primers and their sequence are listed in Supplemental Table 2. All samples were run in duplicate and crossing thresholds were averaged for each animal. RealTime PCR was conducted with a MyiQ RealTime Detection System (Bio-Rad). Data was analyzed by software supplied with the MyiQ system which uses a modified 2−ΔΔCT method described by Vandesompele et al. (2002).
All mRNA expression data was normalized to reference RNAs GAPDH and 18s, and all the miRNA expression data was normalized to reference miR U6 (Applied Biosystems). Control group levels were set at a relative expression of 1 and experimental group expression was normalized to this level. All values are expressed as mean±SD.
Western Blot Analysis
Protein was extracted from the other half of the L4-6 spinal cord using ice cold RIPA buffer (50 mM Tris buffer, pH 7.4, 150 mM NaCL, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1mM EDTA, 10 mM PMSF (phenyl-methyl sulphonyl-fluoride) in the presence of protease and phosphatase inhibitors (Roche). Samples were homogenized by sonication and centrifuged at 14,000 rpm at 4°C for 30 min. Supernatants were collected and stored at −80°C. Standard Laemmli buffer was added and samples were boiled for 5 min. Equal amounts of protein were resolved in gradient 10–20% SDS-PAGE gel and transferred to a polyvinylidene difluoride (PVDF) membrane (BioRad, Hercules, CA) overnight. Membranes were blocked with 5% non-fat milk in Tris buffer saline, 0.1% Tween (TTBS) for 1 hour, Membranes were incubated at 4°C overnight with one of the following rabbit primary antibodies: anti-caspase-3 (1:2,000, Imgenex, San Diego, CA), anti-caspase-7 (1:1,000, Cell Signaling, Beverly, MA) and anti-caspase-9 (1:3,000, Imgenex). Goat anti-rabbit HRP-conjugated immunoglobulins (Covance, Berkeley, CA) were used as the secondary antibody (dilution 1:16,000) for 1 hour at room temperature. Membranes were washed and immunopositive bands were visualized using Western Lightning ECL (PerkinElmer, Waltham, Massachusetts) and Blue Basic Autorad film (ISC Bio Express. Kaysville, UT). After stripping, membranes were re-probed with mouse monoclonal antibody to β-actin (dilution 1:15,000, Sigma, St. Louis, MO) as an internal control for loading and transfer of proteins. Optical densities of immunopositive bands were analyzed using GeneSnap and GeneTools (Syngene, Frederick, MD) and normalized to actin levels. All values are expressed as mean±SD.
Statistical Analysis
Statistical analysis was performed with PASW Statistics v.17 (SPSS Inc., Chicago, IL). ANOVA was used to determine whether or not a significant interaction was present. Positive results were followed up with Tukey s post-hoc test with an alpha of less than 0.05 considered significant. In all cases the term Control refers to the non-injured animal group.
Results
MiR21, Let-7a, miR15b and miR16 expression
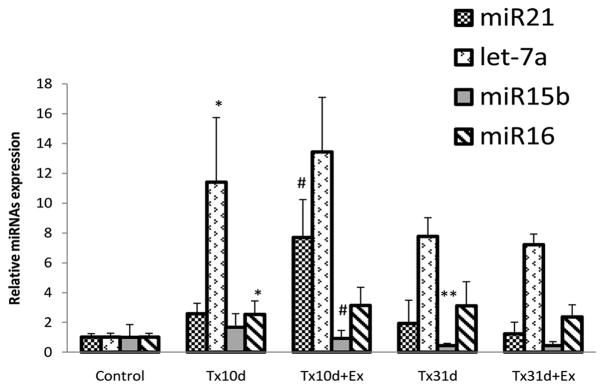
Spinal cord injury at a lower thoracic level resulted in a significant increase in the expression of 2 pro-apoptotic miRs; Let-7a and miR16, compared to uninjured, control values (Fig. 2). The expression of Let-7a was increased over 11 fold compared to the control group (Control: 1.00±0.27, Tx10d: 11.40±4.33. p<0.0001), and the expression of miR16 was increased 2.5 fold compared to the control group (control: 1.00±0.26, Tx10d: 2.54±0.89. p<0.05). There was no significant change in expression of miR21 or miR15b with injury only. Over a longer post-injury period (31d total) there was no change in the expression of miR21, Let-7a or miR16 compared to Tx10d levels, but miR15b levels were significantly decreased.
Figure 2.
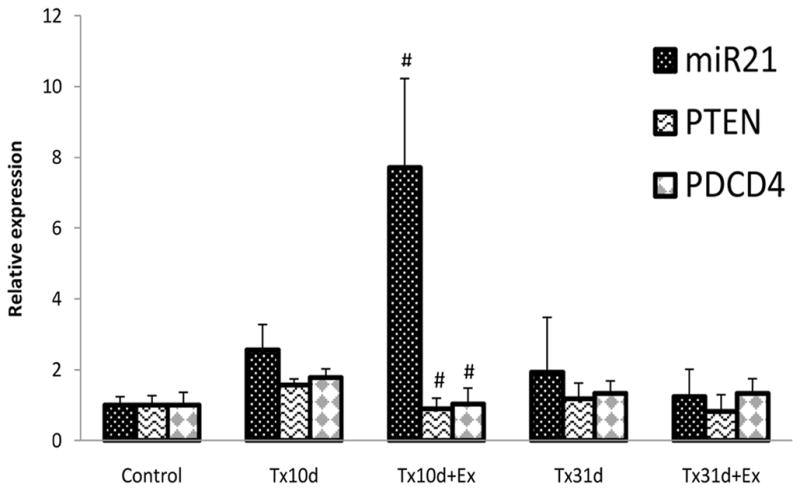
Expression of 4 miRs related to the apoptosis pathway in uninjured, control animals and animals subjected to spinal transection with or without exercise. Animals were sacrificed either 10 or 31 days after transection injury. Short term exercise led to a significant increase of miR21 and decrease of miR15b. Long term exercise had no effect on miR expression. *indicates significant difference between Tx10d and control group; # indicates significant difference betweenTx10d+Ex and Tx10d group.** indicates significant difference between Tx31d and Tx10d group. Values are mean±SD.
Five days of exercise after SCI resulted in a significant increase in the expression of anti-apoptotic miR21 compared to the 10d injury alone group (Tx10d: 2.57±0.71, Tx10d+EX: 7.7±2.53. p<0.0001) and a greater than 50% decrease in the expression of miR15b (Tx10d: 1.64±0.78 and Tx10d+EX: 0.72±0.38. p<0.05) (Fig. 2). Exercise did not change the expression of Let-7a or miR16, as both remained significantly increased over the control group, but unchanged from the Tx10d group. An extended exercise regimen had no effect on the expression of any miRs tested at 31 days after injury when compared to the Tx31d alone group.
Expression of miR21and its target genes PTEN and PDCD4
Inhibition of PTEN and PDCD4, whose down-regulation will release its inhibition on protein kinase B (PKB), results in significantly reduced apoptosis in cancer cells (Griffiths-Jones, 2004). SCI did not change the expression of miR21 or its 2 target genes, PTEN or PDCD4 (Fig. 3) at 10d or 31d. There was a significant increase in miR21 observed after cycling exercise for 5 days after injury which was associated with mRNA expression for PTEN and PDCD4 (PTEN - Tx10d: 1.56±0.18, Tx10d+Ex: 0.90±0.30. p<0.05; PDCD4 – Tx10d: 1.78±0.24, Tx10d+Ex: 1.04±0.44. p<0.05) below that detected for injury only animals. This indicates an activity dependent depression of apoptotic genes with short bouts of exercise, but with a longer exercise regimen there was no effect on the expression of miR21 or mRNA for PTEN or PDCD4.
Figure 3.
Expression of miR21 and its target genes PDCD4 and PTEN. Increased level of miR21 with exercise correlates with reduced expression of PDCD4 and PTEN. # indicates significant difference between Tx10d+EX group and Tx10d group. Values are mean±SD.
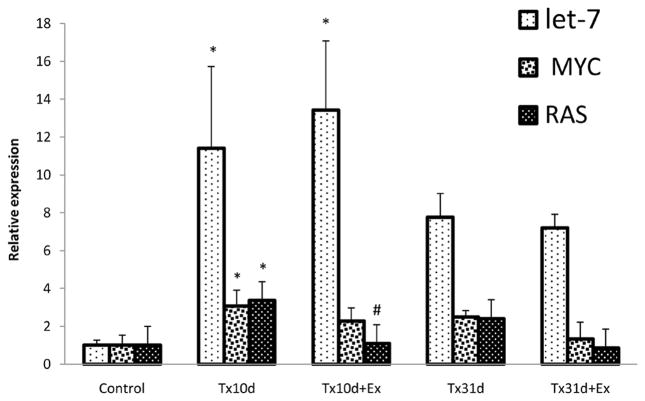
Expression of Let-7a and its target genes RAS and MYC
After 10 days of injury there was a significant increase in expression of Let-7a and two of its target genes compared to the control values (Fig. 4). At 31 days post-injury, expression of Let-7a remained elevated, but expression of MYC and RAS mRNAs were unchanged from uninjured, control levels (Fig. 4).
Figure 4.
Expression of miR Let-7a, and its target genes RAS and MYC. Let-7a and its target genes are increased with spinal cord injury but with exercise there is a decrease in RAS mRNA expression. *indicates significant difference between Tx10d and Tx10d+EX groups and control group; # indicates significant difference between Tx10d+EX group and Tx10d group. Values are mean±SD.
Cycling exercise for 5d did not affect the increase in Let-7a or MYC mRNA, but RAS mRNA was significantly decreased (Tx10d: 3.08±0.83, Tx10d+Ex: 1.08±0.18. p<0.05). Exercise for four weeks following injury did not affect the expression of Let-7a or expression of MYC or RAS mRNAs compared to Tx31d only (Fig. 4).
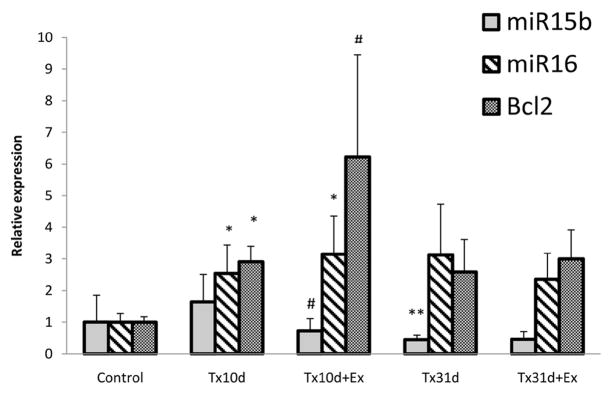
Expression of miR15b and miR16 and their target gene Bcl-2
At 10d after SCI there was an increase in the expression of miR16 but not miR15b. Exercise decreased the post-injury expression of miR15b but had no effect on miR16 expression compared with the injury only group (Fig. 5). Injury alone led to a significant increase in the expression of Bcl-2 mRNA, but levels were even greater after 5 days of exercise (Tx10d: 2.91±0.49, Tx10d+Ex: 6.22±3.24. p<0.05).
Figure 5.
Expression of miR15b and miR16 and their target gene BCL-2. There is no effect of injury on miR15b but miR 16 is significantly increased with subsequent increase in Bcl2 mRNA. Exercise significantly decreases miR15b levels, does not alter miR16 expression and significantly increase Bcl2 mRNA expression. * indicates significant difference between Tx10d and Tx10d+EX groups with control group; # indicates significant difference between Tx10d+EX group and Tx10d group.** indicates significant difference between Tx31d group and to Tx10d group. Values are mean±SD.
At 31 days of injury, expression of miR15b was significantly reduced in the injury only group compared to 10d injury alone and exercise had no further effect on its expression (Fig. 5). The expression of miR16 was unaffected over the longer post injury interval with or without exercise and remained comparable to 10d injury levels. In the 31 day injury only group, mRNA levels of Bcl-2 remained at approximately the same level as the 10 day injury only group and long term exercise had no effect on mRNA expression compared to the Tx10d+Ex group or the Tx31d group.
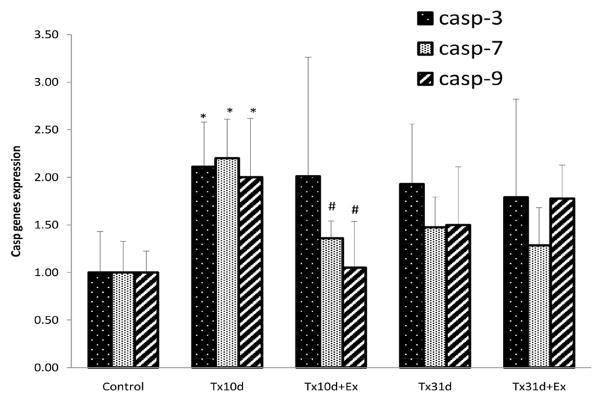
Expression of caspase-3, -7, and -9 mRNA after SCI and Exercise
At 10d of injury there was a significant increase in the expression of mRNA for caspase-3, caspase-7, and caspase-9, when compared with uninjured control (Fig. 6, caspase-3-Control: 1.00±043, Tx10d: 2.11±0.47. p<0.05; caspase-7-Control: 1.00±0.27, Tx10d: 2.2±0.41. p<0.05; caspase-9-Control: 1.00±0.23, Tx10d: 2.00±0.62. p<0.05). There was no significant change in mRNA expression for any of the three caspases at the longer post-injury period (Tx31d). Five days of exercise had no effect on the expression of caspase-3 mRNA, but expression of caspase-7 and caspase-9 mRNA was significantly less than the Tx10d group (caspase-7 Tx10d: 2.2±0.41, Tx10d+EX 1.36±0.18. p<0.05; caspase-9 Tx10d: 2±0.61, Tx10d+EX 1.05±0.48. p<0.05) and not significantly different from uninjured, control values. There was no change in caspase mRNA expression with a longer period of EX (Tx31d+EX).
Figure 6.
Expression of mRNA for caspases -3, -7, and -9. These caspases are involved in cellular apoptosis and are increased in response to spinal cord injury. Exercised animals express significantly less caspase mRNA than non-exercised animals. * indicates significant difference between Tx10d and control group; # indicates significant difference between Tx10d+EX group and Tx10d group. Values are mean±SD.
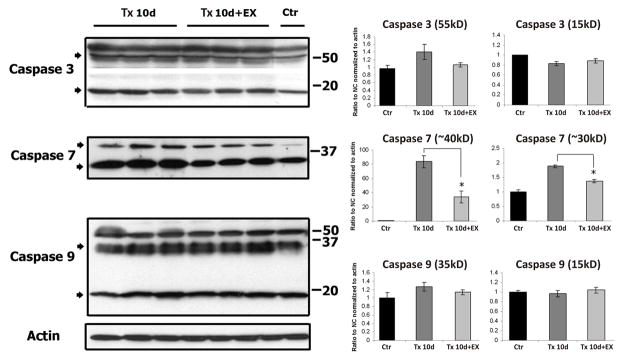
Since caspase activity is regulated at a post-translational level the expression of caspase-3, -7 and -9 protein at 10 days after injury and exercise were assessed by semi-quantitative Western blot analyses (Fig.7). Anti-Caspase-3 antibody recognized several major protein bands with molecular weights ranging from ~55 kDa (pro-caspase) to ~15kDa (cleaved caspase). Ten days after SCI there was no significant change in the expression of pro-caspase-3 or cleaved caspase-3 in the lumbar cord from in the injury only or exercise groups. Anti-caspase-7 antibody detected two prominent immunopositive bands, ~40kDa and ~30kDa (pro-caspase) and 10 days after SCI their expression was elevated 90-fold and ~2-fold, respectively, compared to control levels. The expression of both forms of caspase-7 protein in the Tx10d+Ex group was significantly downregulated compared to the injury only group. The expression of cleaved fragment of caspase-7 (~20kDa) was below the level of detection in all groups for the antibody that was used. Anti-caspase-9 antibody recognized several major protein bands of ~50kDa, ~35 kDa (pro-form) and ~15kDa (cleaved form). No significant differences were detected in the expression of these caspase-9 isoforms between experimental groups.
Figure 7.
Western blot analysis of caspase-3, -7 and -9 protein expression 10 days after the injury with or without exercise. Spinal cord injury alone or with exercise had no affect on levels of either intact caspase 3 (55kDa) or cleaved caspase 3 (15 kDa). The level of caspase-7 proteins was significantly elevated in the injury only group but exercise led to significant decrease in forms of caspase-7. Levels of caspase-9 were not affected by injury or exercise. Values are mean±SD.
We reasoned that the absence of change in expression of caspase mRNAs at the 31d post-injury period would preclude us from examining these samples for changes in levels of caspase proteins.
Discussion
Programmed cell death of neurons and glial cells after SCI results in secondary loss of tissue after the initial insult (Schwab et al., 1996; Hulsebosch et al., 2002). We found significant changes in the expression of miRs and their downstream or target genes associated with pathways leading to cell apoptosis after a complete transection injury. Exercise therapy often is used to provide rhythmic input to the spinal cord and there is evidence of activity dependent “retraining” or plasticity of spinal cord functions after SCI (Edgerton et al., 2008; Ichiyama et al., 2008; Dunlop, 2008; Edgerton and Roy, 2009). When cycling exercise was provided to our SCI animals we found a significant decrease in several miRs in the apoptosis pathway, changes in the expression of target genes of these miRs and a significant increase in the expression of mRNA for the anti-apoptotic marker Bcl-2. The high level of Bcl2 mRNA expression was coincident with reduced expression of caspase-7 and -9 mRNAs and levels of caspase-7 protein were significantly reduced. This supports observations that multiple miRs have vital roles in homeostatic processes for cell proliferation and cell death and suggests that short term exercise may have a neuroprotective role by reducing apoptosis after SCI, possibly leading to reduced structural and/or functional deficit after the initial insult. We appreciate that further studies using pharmacological and/or antisense knockdown are necessary to establish a direct effect of miR inhibition on apoptosis after SCI.
Exercise may affect apoptosis following SCI through expression of miRs
Previous studies of exercise after spinal cord transection found that our cycling regimen affects biochemical and physiological features of the lumbar spinal cord, below the level of the lesion. Activity-dependent plasticity was indicated by the upregulation of mRNA expression of neurotrophic factors (Côté et al., 2009, Abstract, International Symposium on Neural Regeneration) and return of frequency dependent depression of the H reflex (Skinner et al., 1996; Reese et al., 2006). Beneficial effects of exercise on the attenuation of oxidative stress and caspase activity have been shown in different animal models (Lee et al., 2006; Hoffman-Goetz et al., 2007; Gomez-Cabrera et al., 2008) and it is known that inhibition of caspases has neuroprotective effects in rat models of SCI (Citron et al., 2008; Colak et al., 2005; Siniscalco et al., 2008; Knoblach et al., 2002). After a thoracic contusion injury, Liu et al. (2009) found changes in miRs known to be associated with apoptosis, oxidative stress and inflammation. For those miRs with pro-apoptosis gene targets (such as caspase 3 and calpains -1 and -2) there was significant increase at 4h after injury but this did not persist at 1d or 7d. Most of the identified miRs with Bcl-2 as a potential target were not elevated until 7d post injury. After SCI we found similar changes in expression of miRs that regulate the caspase cascade, leading to an increase in expression of caspase -3, -7 and -9 mRNA. Exercise had no affect on the expression of caspase-3 mRNA but did reduce the expression of caspase-7 and -9 mRNA, and reduced caspase-7 protein levels compared to the Tx10d group.
Information about the role of caspases in secondary injury after SCI relates to their expression at the injury site in the first few hours and up to seven days after injury (Emery et al., 1998; Citron et al., 2000; Knobloch et al., 2005). Early components of secondary injury, including glutamate release and generation of free radicals, are implicated in the activation of caspases soon after SCI. At the protein level, while the robust over expression of caspase-7 in the injured cord was significantly reduced after exercise compared to the Tx10d group there was no change in levels of caspase-3 or -9. The absence of caspase-9 protein upregulation after Tx or Tx+Ex could reflect the accelerated rate of selective proteolytic degradation of pro-domain and a cleaved product of caspase-9 (Siegel. 2006; Lavrik et al., 2005;). Data from other models of CNS injury suggest that multiple caspases may be involved in spinal cord trauma (Knoblach et al, 2005) and our findings suggest that caspases 3, 7 and 9 differentially contribute to the outcome of the SCI and exercise. Long term changes in expression of miRs associated with apoptotic pathways were identified but extended exercise treatment had no effect on this expression, unlike previously identified long term changes, such as increase in neurotrophic factors (Ying et al., 2005; Cote et al., 2009), after exercise. A positive effect over at least 1 week of exercise was observed but it is not clear whether this was the limit of an exercise effect or that there was a gradual diminution of the exercise effect over time.
The anti-apoptotic factor miR21 targets PTEN and PDCD4
MiR21 was first implicated as an anti-apoptotic factor by the observation that knockdown of miR21 increased cell death in human and mouse models of glioblastoma (Chan et al., 2005; Si et al., 2005). Impairment of PTEN regulation is thought to play a role in oncogenic transformation (Maehama et al., 2007) and MiR21 directly targets this gene, down-regulating its expression, leading to a reduction of PTEN s inhibition of protein kinase B (PKB) and reduced apoptosis in cancer cells. MiR21 also targets PDCD4, a pro-apoptotic gene that is frequently down-regulated in hepatocellular carcinoma (Zhang et al., 2006). PDCD4 is a tumor suppressor protein that is over expressed during apoptosis (Lankat-Buttgereit et al., 2003) and it s down regulation in a number of human cancers correlates with poor survival prognosis in lung and colorectal cancers ( Chen et al., 2003; Mudduluru et al., 2007).
In the Tx+Ex10d group we found significantly increased expression of miR21and decreased expression of its target genes, PTEN and PDCD4, compared to the Tx10d group, indicating 2 pathways by which an increase in miR21 might affect caspase gene activation.
Interest in the role of PTEN in neuroplasticity has increased greatly with the observation that deletion of PTEN in mice leads to significant upregulation of intrinsic growth control pathways in neurons and extensive regeneration of axons in the optic nerve after injury (Park et al., 2010). PTEN acts as a negative regulator of mTOR (the mammalian target of rapamycin), but when PTEN is deleted there is an accumulation of PIP3 [phosphatidylinositol (3, 4, 5) triphosphate], activation of AKT and increased protein synthesis and process growth. Exercise after SCI thus may stimulate multiple effects through the down regulation of PTEN, including regulation of genes in the apoptotic pathway and influence on axon growth (sprouting or regenerative).
Let-7a targets RAS and MYC and may cooperate with miR-21
The Let-7 family of miRs is a highly conserved group containing 12 closely related members. Let-7a can target both RAS (Asangani et al., 2008; Johnson et al., 2005) and MYC (Sampson et al., 2007) and inhibit their expression under normal physiological conditions. The RAS genes encode a family of membrane-bound 21-kd guanosine triphosphate (GTP)-binding proteins that regulate cell growth, differentiation, and apoptosis by interacting with multiple effectors, including those in the MAPK (mitogen-activated protein kinase), STAT (signal transducer and activator of transcription), and PI3K (phosphoinositide 3-kinase) signaling cascades ( Downward, 1998; Shields et al.,2000; Vojtek et al.,1998). Growth and proliferation potentiated by deregulated MYC oncogene expression is balanced by MYC-induced apoptosis. Blocking of this apoptotic pathway in MYC over-expressing cells leads to cancer progression (Secombe et al., 2004).
In the Tx10d and Tx10d+Ex groups we found enhanced Let-7a expression compared to the control group and that one of its target genes, RAS, decreased expression by 68% with exercise therapy compared to the Tx10d alone group. Let-7a over expression could regulate RAS gene activation resulting in decreased caspase gene expression. MYC decreased expression about 26% compared to the Tx10d group but this was not a significant change. Activity-dependent reduction in RAS mRNA expression and downstream increase in the expression of the anti-apoptotic gene Bcl-2 indicates another pathway by which exercise may lead to reduced apoptosis after SCI.
The pro-apoptotic miRs 15b and 16 target the BCL-2 gene
Several target prediction programs (Cimmino et al., 2005; Krek et al., 2005) identified miR15b and miR16 as putative regulators of Bcl-2 based on being complementary at the 3 - UTR and observing that endogenous levels of miR15b and miR16 correlate inversely with Bcl-2 protein levels. Bcl-2 is an anti-apoptotic factor that is a major target of post-transcriptional repression by miR15b and miR16 and a downstream target of miR21 expression. miR15b expression was significantly decreased after cycling exercise and expression of one of its target genes Bcl-2 was significantly increased compared to the Tx10d group. These data indicate that one week of exercise inhibited miR15b but not miR16 mRNA expression, with a net result of increased expression of Bcl-2 mRNA.
In summary, we have identified changes in the expression of miRs and their downstream targets that are associated with the apoptotic pathway that is activated after spinal cord injury. There are multiple routes by which the cascade of caspase activity may be regulated by miRs and we have shown that SCI modifies the expression of several of these miRs. Of prime interest is the observation that cycling exercise of paralyzed hindlimbs results in changes in miR expression that is associated with the downregulation of caspase mRNA and protein. This study identifies possible regulatory molecules of apoptosis that are upregulated after SCI that may be targeted by the rhythmic input to the spinal cord that is provided by cycling exercise, suggesting a possible neuroprotective effect of this treatment strategy for SCI.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Health Grant NS 055976. We gratefully acknowledge Kassi Miller for postoperative care and bicycle training of animals and Rachel Siegfried for assistance with surgery and tissue preparation. Dr. Lisa Stein (Imgenex) provided important technical information about the detection of caspases by Western blot techniques. Drs. Marion Murray and Veronica Tom provided valuable comments about data presentation and interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JM, Cory S. The Bcl-2 Protein Family: Arbiters of Cell Survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Farooqui AA, Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma. 2000;17(10):915–925. doi: 10.1089/neu.2000.17.915. [DOI] [PubMed] [Google Scholar]
- Beaumont E, Houle JD, Peterson CA, Gardiner PF. Passive exercise and fetal spinal cord transplant both help to restore motoneuronal properties after spinal cord transection. Muscle & Nerve. 2004;29:234–242. doi: 10.1002/mus.10539. [DOI] [PubMed] [Google Scholar]
- Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an anti-apoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- Chen Y, Knösel T, Kristiansen G, Pietas A, Garber ME, Matsuhashi S, Ozaki I, Petersen I. Loss of PDCD4 expression in human lung cancer correlates with tumour progression and prognosis. J Pathol. 2003;200:640–646. doi: 10.1002/path.1378. [DOI] [PubMed] [Google Scholar]
- Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2006;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron BA, Arnold PM, Sebastian C, Qin F, Malladi S, Ameenuddin S, Landis ME, Festoff BW. Rapid upregulation of caspase-3 in rat spinal cord after injury: mRNA, protein, and cellular localization correlate with apoptotic cell death. Exp Neurol. 2000;166:213–226. doi: 10.1006/exnr.2000.7523. [DOI] [PubMed] [Google Scholar]
- Citron BA, Arnold PM, Haynes NG, Ameenuddin S, Farooque M, Santacruz K, Festoff BW. Neuroprotective effects of caspase-3 inhibition on functional recovery and tissue sparing after acute spinal cord injury. Spine. 2008;33:2269–2277. doi: 10.1097/BRS.0b013e3181831f7e. [DOI] [PubMed] [Google Scholar]
- Colak A, Karao lan A, Barut S, Köktürk S, Akyildiz AI, Ta yurekli M. Neuroprotection and functional recovery after application of the caspase 89 inhibitor z-LEDH-fmk in a rat model of traumatic spinal cord injury. J Neurosurg Spine. 2005;2:327–334. doi: 10.3171/spi.2005.2.3.0327. [DOI] [PubMed] [Google Scholar]
- Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nature Medicine. 1997;3:173–176. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- Danial NN. BCL-2 family proteins: critical checkpoints of apoptotic cell death. Clin Cancer Res. 2007;13:7254–7263. doi: 10.1158/1078-0432.CCR-07-1598. [DOI] [PubMed] [Google Scholar]
- Downward J. Signal transduction. New exchange, new target. Nature. 1998;396:474–477. doi: 10.1038/24743. [DOI] [PubMed] [Google Scholar]
- Dunlop SA. Activity-dependent plasticity: implications for recovery after spinal cord injury. Trends Neurosci. 2008;31:410–418. doi: 10.1016/j.tins.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, Houle JD, Dennis RA, Zhang J, Know M, Wagoner G, Peterson CA. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle Nerve. 2004;29:73–81. doi: 10.1002/mus.10511. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Courtine G, Gerasimenko YP, Lavrov I, Ichiyama RM, Fong AJ, Cai LL, Otoshi CK, Tillakaratne NJ, Burdick JW, Roy RR. Training locomotor networks. Brain Res Rev. 2008;57:241–254. doi: 10.1016/j.brainresrev.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Roy RR. Activity-dependent plasticity of spinal locomotion: implications for sensory processing. Exercise Sport Sci Rev. 2009;37:171–178. doi: 10.1097/JES.0b013e3181b7b932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldadah BA, Fadden AI. Caspase pathways, neuronal apoptosis, and CNS injury. J Neurotrauma. 2000;17:811–829. doi: 10.1089/neu.2000.17.811. [DOI] [PubMed] [Google Scholar]
- Emery E, Aldana P, Bunge MB, Puckett W, Srinivasan A, Keane RW, Bethea J, Levi AD. Apoptosis after traumatic human spinal cord injury. J Neurosurg. 1998;89:911–920. doi: 10.3171/jns.1998.89.6.0911. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Domenech E, Viña J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44(2):126–31. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S. The microRNA Registry. Nucleic Acids Res. 2004;32 (Database issue):D109–111. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34 (Database issue):D140–144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36 (Database issue):D154–158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CJ, Pan Q, Li DG, Sun H, Liu BW. miR-15b and miR-16 are implicated in activation of the rat hepatic stellate cell: an essential role for apoptosis. J Hepatol. 2009;50:766–778. doi: 10.1016/j.jhep.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Hoffman-Goetz L, Spagnuolo PA. Freewheel exercise training modifies pro-and anti-apoptotic protein expression in mouse splenic lymphocytes. Int J Sports Med. 2007;28:787–91. doi: 10.1055/s-2007-964857. [DOI] [PubMed] [Google Scholar]
- Houle JD, Morris K, Skinner RD, Garcia-Rill E, Peterson CA. Effects of fetal spinal cord tissue transplants and cycling exercise on the soleus muscle in spinalized rats. Muscle Nerve. 1999;22:846–856. doi: 10.1002/(sici)1097-4598(199907)22:7<846::aid-mus6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE. Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Education. 2002;26:238–255. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- Ichiyama RM, Courtine G, Gerasimenko YP, Yang GJ, van den Brand R, Lavrov IA, Zhong H, Roy RR, Edgerton VR. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci. 2008;28:7370–7375. doi: 10.1523/JNEUROSCI.1881-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the Let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Knoblach SM, Nikolaeva M, Huang X, Fan L, Krajewski S, Reed JC, Faden AI. Multiple caspases are activated after traumatic brain injury: evidence for involvement in functional outcome. J Neurotrauma. 2002;19:1155–1170. doi: 10.1089/08977150260337967. [DOI] [PubMed] [Google Scholar]
- Knoblach SM, Huang X, VanGelderen J, Calva-Cerqueira D, Faden AI. Selective caspase activation may contribute to neurological dysfunction after experimental spinal cord injury. J Neurosci Res. 2005;80:369–380. doi: 10.1002/jnr.20465. [DOI] [PubMed] [Google Scholar]
- Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial micro-RNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- Lankat-Buttgereit B, Göke R. Programmed cell death protein 4 (pdcd4): a novel target for anti-neoplastic therapy? Biol Cell. 2003;95:515–519. doi: 10.1016/j.biolcel.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Lavrik IN, Golks A, Krammer PH. Caspases: pharmacological manipulation of cell death. J Clin Invest. 2005;115:2665–2672. doi: 10.1172/JCI26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YI, Cho JY, Kim MH, Kim KB, Lee DJ, Lee KS. Effects of exercise training on pathological cardiac hypertrophy related gene expression and apoptosis. Eur J Appl Physiol. 2006;97:216–224. doi: 10.1007/s00421-006-0161-5. [DOI] [PubMed] [Google Scholar]
- Liu NK, Wang XF, Lu QB, Xu XM. Altered microRNA expression following traumatic spinal cord injury. Exp Neurol. 2009;219:424–429. doi: 10.1016/j.expneurol.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27:4373–4379. doi: 10.1038/onc.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T. PTEN: its deregulation and tumorigenesis. Biol Pharm Bull. 2007;30:1624–1627. doi: 10.1248/bpb.30.1624. [DOI] [PubMed] [Google Scholar]
- Mudduluru G, Medved F, Grobholz R, Jost C, Gruber A, Leupold JH, Post S, Jansen A, Colburn NH, Allgayer H. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer. 2007;110:1697–1707. doi: 10.1002/cncr.22983. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Kanter JL, He Z. PTEN/mTOR and axon regeneration. Exp Neurol. 2010;223:45–50. doi: 10.1016/j.expneurol.2009.12.032. [DOI] [PubMed] [Google Scholar]
- Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B, Müller P, Spring J, Srinivasan A, Fishman M, Finnerty J, Corbo J, Levine M, Leahy P, Davidson E, Ruvkun G. Conservation of the sequence and temporal expression of Let-7 heterochronic regulatory RNA. Nature. 2000;408:86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- Reese NB, Skinner RD, Mitchell D, Yates C, Barnes CN, Kiser TS, Garcia-Rill E. Restoration of frequency-dependent depression of the H-reflex by passive exercise in spinal rats. Spinal Cord. 2006;44:28–34. doi: 10.1038/sj.sc.3101810. [DOI] [PubMed] [Google Scholar]
- Sampson VB, Rong NH, Han J, Yang Q, Aris V, Soteropoulos P, Petrelli NJ, Dunn SP, Krueger LJ. MicroRNA Let-7a downregulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 2007;67:9762–9770. doi: 10.1158/0008-5472.CAN-07-2462. [DOI] [PubMed] [Google Scholar]
- Sayed D, He M, Hong C, Gao S, Rane S, Yang Z, Abdellatif M. MicroRna-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of fas ligand. J Biol Chem. 2010;285:20281–20290. doi: 10.1074/jbc.M110.109207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- Secombe J, Pierce SB, Eisenman RN. Myc: a weapon of mass destruction. Cell. 2004;117:153–156. doi: 10.1016/s0092-8674(04)00336-8. [DOI] [PubMed] [Google Scholar]
- Shields JM, Pruitt K, McFall A, Shaub A, Der CJ. Understanding RAS: it ain t over til it s over. Trends Cell Biol. 2000;10:147–154. doi: 10.1016/s0962-8924(00)01740-2. [DOI] [PubMed] [Google Scholar]
- Shuman SL, Bresnahan JC, Beattie MS. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J Neurosci Res. 1997;50:798–808. doi: 10.1002/(SICI)1097-4547(19971201)50:5<798::AID-JNR16>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- Siegel RM. Caspases at the crossroads of immune-cell life and death. Nat Rev Immunol. 2006;6:308–317. doi: 10.1038/nri1809. [DOI] [PubMed] [Google Scholar]
- Siniscalco D, Giordano C, Fuccio C, Luongo L, Ferraraccio F, Rossi F, de Novellis V, Roth KA, Maione S. Involvement of subtype 1 metabotropic glutamate receptors in apoptosis and caspase-7 over-expression in spinal cord of neuropathic rats. Pharmacol Res. 2008;57:223–233. doi: 10.1016/j.phrs.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner RD, Houle JD, Reese NB, Berry CL, Garcia-Rill E. Effects of exercise and fetal spinal cord implants on the H-reflex in chronically spinalized adult rats. Brain Res. 1996;29:127–131. [PubMed] [Google Scholar]
- Tillakaratne NJ, Mouria M, Ziv NB, Roy RR, Edgerton VR, Tobin AJ. Increased expression of glutamine decarboxylase (GAD67) in feline lumbar spinal cord after complete thoracic spinal cord transection. J Neurosci Res. 2000;60:219–230. doi: 10.1002/(SICI)1097-4547(20000415)60:2<219::AID-JNR11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:34.1–34.12. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek AB, Der CJ. Increasing complexity of the ras signaling pathway. J Biol Chem. 1998;273:19925–19928. doi: 10.1074/jbc.273.32.19925. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lee CG. MicroRNA and cancer – focus on apoptosis. J Cell Mol Med. 2009;13:12–23. doi: 10.1111/j.1582-4934.2008.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ozaki I, Mizuta T, Hamajima H, Yasutake T, Eguchi Y, Ideguchi H, Yamamoto K, Matsuhashi S. Involvement of programmed cell death 4 in transforming growth factor-beta1-induced apoptosis in human hepatocellular carcinoma. Oncogene. 2006;25:6101–6112. doi: 10.1038/sj.onc.1209634. [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005;193:411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.