Abstract
Purpose
The prevalence of bladder dysfunctions increases with age. In humans it is difficult to separate changes related to exogenous factors from those directly related to the aging process. Some confounding variables can be avoided by studying age related changes in an animal model. We evaluated the impact of age on bladder function in vivo and in vitro, and characterized the corresponding morphological changes.
Materials and Methods
Young (4 to 6 months old) and old (older than 28 to 30 months) male Fischer/Brown Norway rats were used in the study. Cystometric studies were done in conscious, freely moving rats. After cystometry tissue strips from the bladder body were used in in vitro studies of muscarinic receptor activation and electrical field stimulation, and histological examination.
Results
Old rats had higher bladder weight than young rats but the bladder-to-body weight ratio did not change. We noted significant age related differences in 8 of 10 cystometric parameters. Old rats had increased bladder capacity, post-void residual volume, micturition volume and frequency, baseline and intermicturition pressure, and spontaneous activity but decreased micturition pressure. Bladder strip responses to carbachol and electrical field stimulation were significantly lower in old than in young rats. Histological examination revealed urothelial thinning, lower muscle mass and higher collagen content in the bladders of old vs young rats.
Conclusions
Physiological aging alters bladder function in male rats even when external factors remain constant. Thus, in old rats bladder capacity, post-void residual urine and spontaneous activity are higher, and responses to muscarinic receptor stimulation and electrical field stimulation are lower than in young rats. Such changes correspond to findings in aging human bladders, supporting the view that the Fischer/Brown Norway rat is a useful model in which to study age related bladder function changes.
Keywords: urinary bladder, physiology, aging, urodynamics, male
Recent reviews in the clinical literature document a clear age related decrease in the ability of the bladder to fill, store and empty.1–3 However, the relationship of aging to other external influences on the detrusor, such as nervous system disease, vascular supply or lower urinary tract smooth muscle, remains poorly understood.4–6 More insight into the impact of these pathophysiological conditions on bladder function is needed but equally important is the development of relevant preclinical models in which these external influences can be reduced.
Several groups have evaluated age related changes in rodent bladder function but only a few used in vivo cystometry to characterize the changes.7,8 In vitro studies in old vs young rat bladder tissue have yielded contradictory results that may be attributable at least partly to strain specific differences. For example, in Fischer 344 rats muscarinic receptor mediated detrusor contraction was increased,9 unchanged10,11 or decreased.12 In Wistar rats muscarinic receptor mediated bladder contraction was unaltered.8,13 Studies of Sprague-Dawley ® rats showed decreased muscarinic receptor mediated detrusor contraction.14–16
Thus, despite the number of studies of age related changes in bladder function few reports have assessed in vivo and in vitro parameters in the same rat. To this end we evaluated the impact of aging on bladder function in vivo and in vitro in male Fischer/Brown Norway rats (Harlan™). The Fischer/Brown Norway rat strain is often used in aging studies17,18 since these rats have an increased life span compared to that of other rodent strains, and a decrease in age related pituitary, testicular and kidney pathology compared to Sprague-Dawley rats of similar ages. We report our initial observations on age related changes in urodynamic parameters, detrusor contractile responses to muscarinic receptor and EFS, and bladder wall histological characteristics in young and old Fischer/Brown Norway rats.
MATERIALS AND METHODS
Animals
In this study we used 15 young (4 to 6 months old) and 15 old (28 to 30 months old) male Fischer/Brown Norway rats from the National Institute on Aging colony, National Institutes of Health. All experimental protocols were approved by the Wake Forest University Health Sciences animal care and use committee. Animals were maintained on a 12-hour light/12-hour dark cycle in polycarbonate cages with free access to Purina™ rat chow and water. After surgery rats were kept 1 per cage to prevent damage to implanted catheters. Animal well-being was supervised daily during the entire experimental period. Animals with large tumors or decreased body weight indicating underlying disease were not used in these studies.
Urodynamics
Bladder catheter implantation
Rats were anesthetized by intraperitoneal injection of 50 mg/kg pentobarbital. The abdomen was opened through a lower midline incision and a PE-50 polyethylene catheter (Clay-Adams, Parsippany, New Jersey) was implanted into the bladder through the dome. The indwelling catheter was tunneled subcutaneously and exited through an orifice in the back of the neck.
Cystometric recording
Cystometric recording was done without anesthesia 3 days after bladder catheterization, as previously described.19 The bladder catheter was connected to a pressure transducer, an ETH 400 transducer amplifier (CB Sciences, Dover, New Hampshire) and a PowerLab®/8e data acquisition board. Bladder pressure and MV real-time display and recording were done on a computer using PowerLab software, version 5.1. The conscious rat was placed without restraint in a metabolic cage, which also enabled urine volume measurement by a fluid collector connected to a force displacement transducer. Room temperature saline was infused into the bladder at 10 ml per hour.
Recorded or calculated cystometric parameters were 1) BC—RV at the previous micturition plus the volume of infused saline at micturition, 2) MV—expelled urine volume, 3) RV—BC – MV, 4) BP—lowest average pressure recorded between voids, generally recorded shortly after voiding, 5) TP—pressure at which the micturition contraction is initiated, 6) MP—maximum bladder pressure during voiding, 7) IMP—mean pressure between 2 micturitions, 8) SA—IMP – BP, 9) Bcom—(BC/(TP – BP) and 10) MF—number of voids per hour (see table).
Rat demographics, and estimated cystometric and logistic CRC parameters
| Old | Young | |
|---|---|---|
| Mean ± SEM wt: | ||
| Bladder (gm) | 0.33 ± 0.01 | 0.16 ± 0.01* |
| Body (gm) | 616.7 ± 16.62 | 314.5 ± 8.8 |
| Bladder/body | 0.53 × 10−3 ± 0.1 × 10−4 | 0.52 ± 10−3 ± 0.2 × 10−4 |
| Mean ± SD cystometric parameters: | ||
| BC (ml) | 1.78 ± 0.16 | 1.169 ± 0.114* |
| RV (ml) | 0.29 ± 0.06 | 0.12 ± 0.03* |
| MV (ml) | 1.49 ± 0.12 | 1.05 ± 0.12* |
| SA (cm H2O) | 8.94 ± 1.20 | 3.68 ± 0.89* |
| MF (No. voids/hr) | 9.65 ± 1.08 | 6.16 ± 0.43* |
| BP (cm H2O) | 10.99 ± 1.32 | 7.37 ± 1.13* |
| MP (cm H2O) | 50.32 ± 3.13 | 69.59 ± 3.16* |
| IMP (cm H2O) | 19.92 ± 1.33 | 11.05 ± 1.25* |
| TP (cm H2O) | 23.19 ± 1.9 | 22.15 ± 2.12 |
| Bcom | 0.18 ± 0.03 | 0.11 ± 0.03 |
| Mean ± SD logistic parameters: | ||
| Emax | 60.8 ± 11.9 | 110.7 ± 17.6* |
| pEC50 | 5.43 ± 0.09 | 5.38 ± 0.17 |
| Slope | 1.06 ± 0.13 | 1.30 ± 0.09 |
Significantly different (Student’s t test).
Organ Bath Studies
Tissue preparation
After cystometry the rats were sacrificed and the bladders were carefully removed. Full-thickness strips of the lateral part of the bladder wall were saved for histology. After removing the urothelium/suburothelium the bladder body was cut longitudinally into 4, 4 × 10 mm strips, which were placed in 15 ml tissue bath chambers. The chamber was filled with Krebs solution maintained at 37C and bubbled continuously with a mixture of 95% O2 and 5% CO2, resulting in pH 7.4. The strips were suspended between 2 L-shaped hooks by silk ligatures. One hook was connected to a movable unit, allowing adjustment of passive tension, and the other was connected to an FT03C force transducer (Grass Instruments, Rockland, Massachusetts). Isometric tension was recorded using the same digital PowerLab system as for cystometric measurement. After mounting the strips were stretched to 2 gm passive tension and allowed to equilibrate for 60 minutes before further experiments.
Electrical field stimulation
EFS was done using 2 platinum electrodes placed on each side of the strips and controlled by an S88 stimulator (Grass Instruments) delivering single square wave pulses at select frequencies. Train duration was 5 seconds, pulse duration was 0.8 milliseconds and the stimulation interval was 2 minutes. Electrode polarity was shifted after each pulse by a polarity changing unit. The strips were continuously stimulated from low to high selected frequencies (1 to 32 Hz).
Carbachol response curve
Cumulative steady-state CRCs were constructed on isolated bladder strip preparations by adding carbachol at ½ log increments at concentrations of 3 × 10−9 to 10−4 M.
Histology
Bladder wall histological data were analyzed in 4 µm sections stained with hematoxylin and eosin or Masson’s trichrome stain. With the latter method in formalin fixed, paraffin embedded sections collagen fibers stain blue, nuclei stain black and cellular material (muscle and cytoplasm) stain red. Urothelial thickness and collagen content (blue areas) were analyzed using Image-Pro® AMS 6.0. For all preparations data on 2 old and 6 young rats were pooled to determine the mean ± SD for statistical analysis, as described.
Data Analysis
Unless otherwise noted, eg pEC50, all results are expressed as the arithmetic mean ± SEM. Student’s 2-tailed t test for unpaired samples was used to compare the old and young groups, and the paired t test was used to assess effects before and after treatment, ie atropine, in the same strip. CRC data were computer fit to a 4 parameter logistic equation using Prism®, version 5.00 for Windows® to derive Emax and EC50, expressed as pEC50, and the slope factor. For histological evaluation data were pooled to determine the mean ± SD. Statistical analysis was done using Student’s t test for collagen content but the Mann-Whitney test for urothelial thickness since the data were not normally distributed.
RESULTS
In Vivo Studies
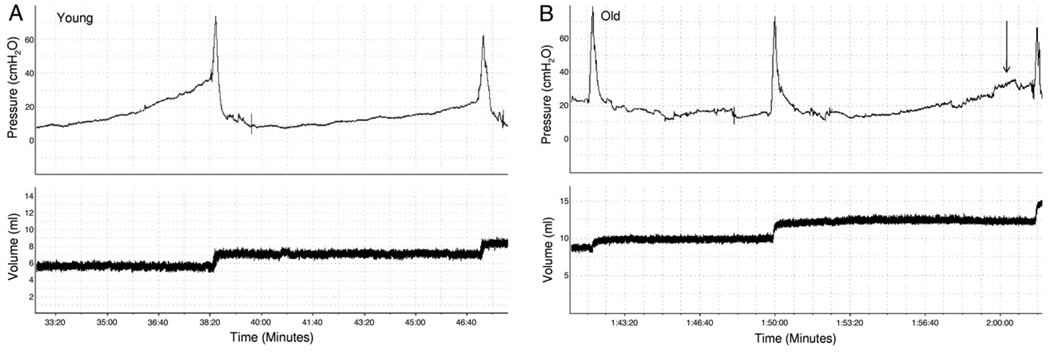
The table lists rat population demographics, and mean bladder and body weight. Bladder and body weight in old rats was significantly higher than in young rats but the bladder-to-body weight ratio was not significantly different between the groups. Urodynamics were done in all 15 young and 15 old rats (fig. 1). The table also lists mean cystometric parameter estimates. Significant age related differences were observed for 8 of the 10 cystometric parameters, including BC, RV, MV, SA, MF, BP, MP and IMP, showing the robust impact of age on bladder function in vivo. No significant differences were found in TP or Bcom.
Figure 1.
Representative cystometric tracings show micturition cycle and volume. A, young rat. B, typical old rat results were characterized by nonvoiding contractions (arrows), larger BC and lower MF.
In Vitro Studies
Carbachol induced contraction pharmacological studies
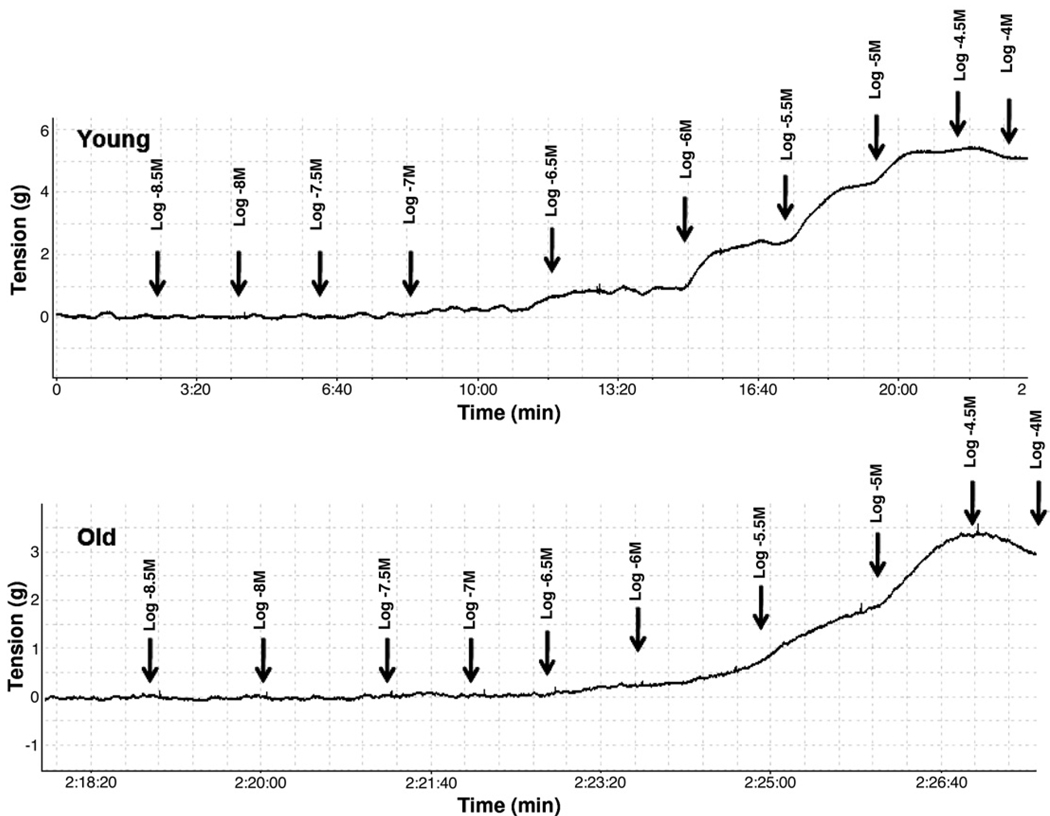
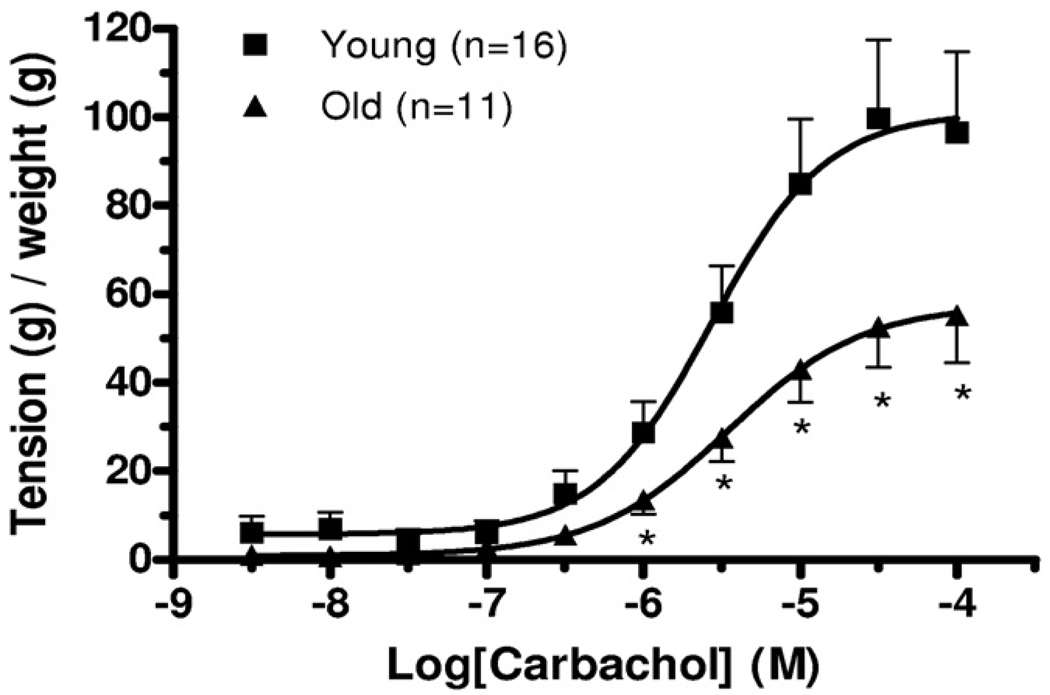
To evaluate the impact of age on agonist mediated contractile responses in isolated detrusor smooth muscle strips steady-state carbachol CRCs were determined in tissue from a subpopulation of 16 young and 11 old animals. Figures 2 and 3 show representative carbachol CRC data in detrusor strips from young and old rats as well as mean CRC data in all such experiments. Computer fits of CRC data to the described logistic equation revealed that the contractile Emax in old rats was significantly decreased vs that in young rats (see table). However, there was no detectable effect of age on pEC50 or the slope factor.
Figure 2.
Representative curves show CRC response in young and old rat bladder strips. In small subset in each age group desensitization was noted at highest carbachol concentration. g, gm.
Figure 3.
Steady-state CRC in bladder strips from old and young rats. g, gm. Asterisk indicates Student’s t test p <0.05.
EFS induced contraction physiological studies
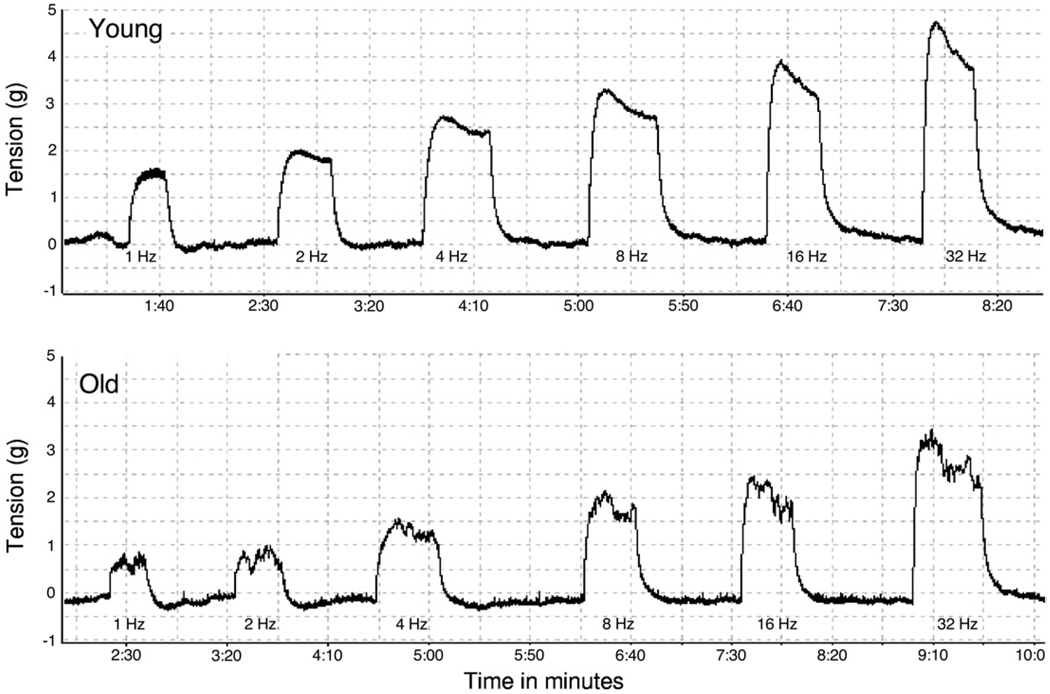
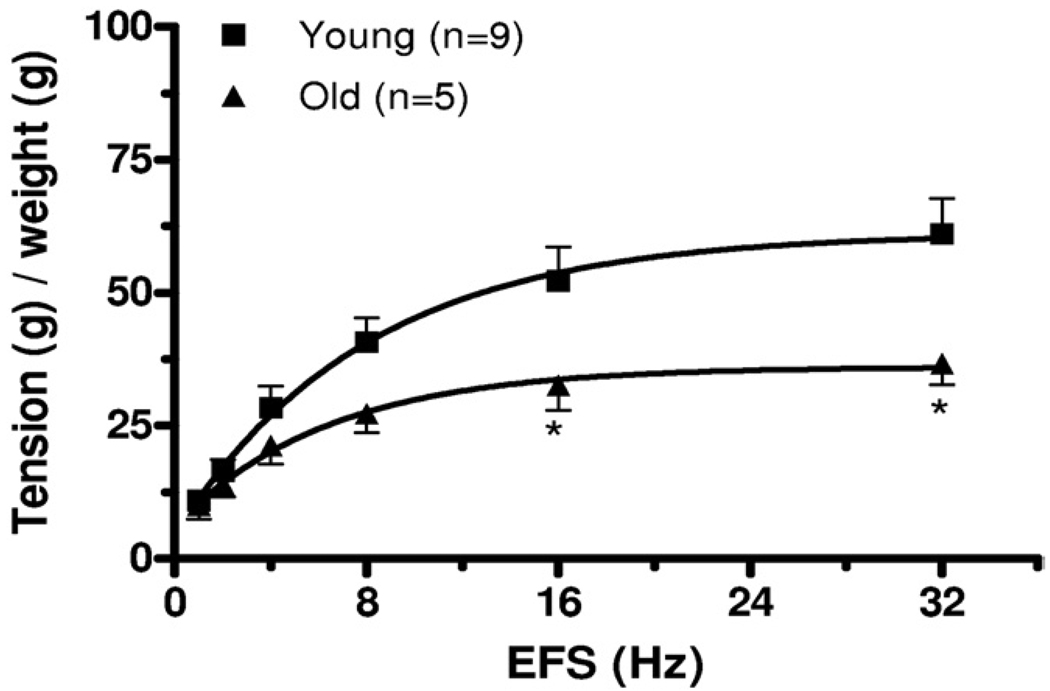
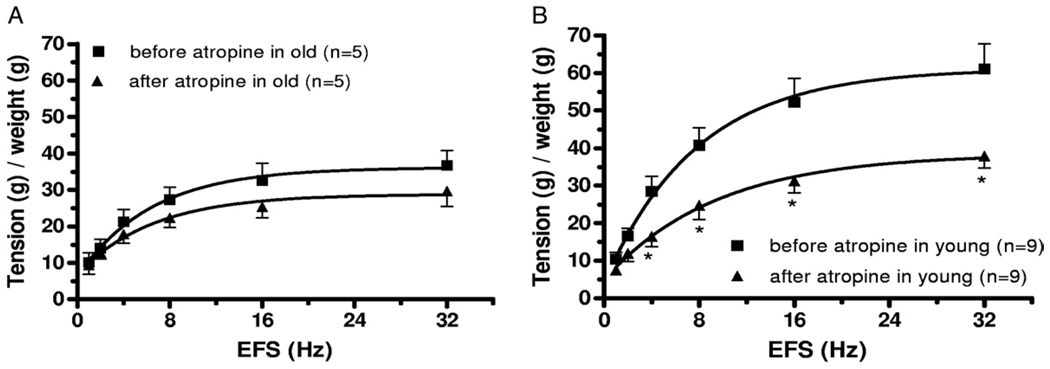
To evaluate the potential impact of age on nerve mediated contractile responses in isolated detrusor smooth muscle strips EFS responses were determined in a distinct subpopulation of detrusor strips from young and old animals. Figures 4 and 5 show representative EFS data in detrusor strips from young and old rats as well as mean CRC data from all such experiments. Maximal contractile responses to EFS were also significantly lower in old than in young rats. Initial studies to determine the nature of the nerve mediated contractile response were also done in these detrusor strips. Pre-incubation with 1 µM atropine significantly decreased EFS induced contraction in young but not in old rats (fig. 6), consistent with apparent loss of an atropine resistant component to neurostimulation in older rats.
Figure 4.
Representative curves show EFS response in young and old rat bladder strips. g, gm.
Figure 5.
EFS frequency-response curves in bladder strips from old and young rats. g, gm. Asterisk indicates Student’s t test p <0.05.
Figure 6.
Frequency-response curves in bladder strips before and after 1 µM atropine pretreatment show age related effects on atropine resistant component of EFS. A, old rats. B, young rats. g, gm. Asterisk indicates Student’s t test p <0.05.
Histology
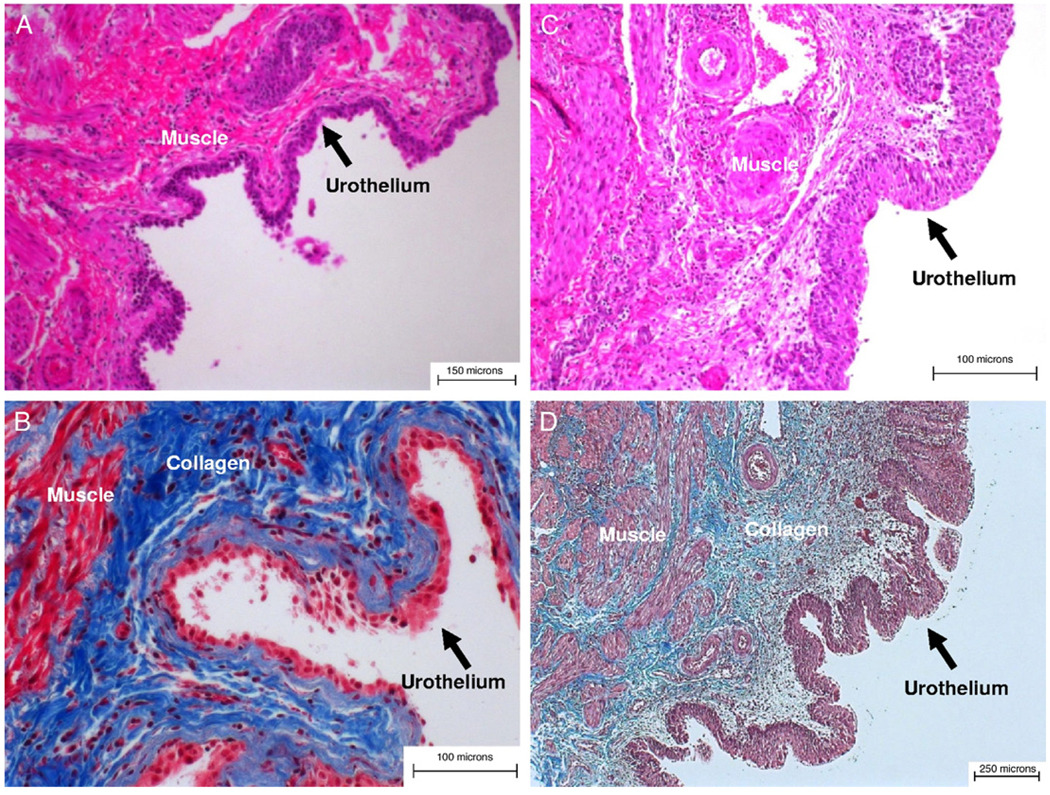
Figure 7 shows representative examples of overall bladder wall cellular architecture and composition in young and old rats. Histological examination revealed evidence of urothelial thinning, decreased muscle mass and increased collagen deposition in the bladders of old vs young rats. To quantify these observations we performed detailed image analysis of a series of sections derived from the bladder of 2 old and 6 young rats, as described.
Figure 7.
Bladder strips. A and B, young rat. C and D, old rat. A and C, H & E, reduced from ×100. B and D, trichrome stain, reduced from ×200.
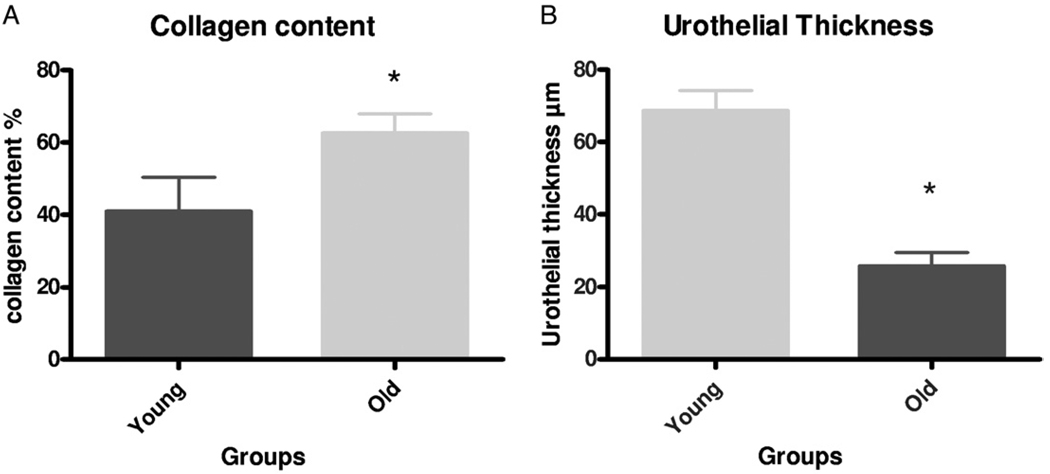
Figure 8 shows mean urothelial thickness and collagen content data. Urothelial thickness data were not normally distributed. Also, from 2 old rats a total of 13 sections were analyzed for collagen content and another 13 observations were made of urothelial thickness, including 3 or 4 high power fields from an additional 4 sections from the same 2 bladders. From 6 young rats a total of 13 sections were analyzed for collagen content and a total of 44 observations were made of urothelial thickness, including 3 or 4 high power fields from an additional 13 sections from the same 6 bladders. In all cases statistics were based on the number of sections studied per high power field. There was an approximately 60% age related increase in collagen content and urothelial thickness in old rats was only approximately 40% that in young rats. These histological changes were consistent with the observed age related urodynamic alterations as well as the decreased responses to carbachol and EFS mediated contractions (see table and figs. 1 to 5).
Figure 8.
Age related effects on collagen content and urothelial thickness. A, statistically significant increase in percent of collagen in bladder wall of old vs young rats. Asterisk indicates Student’s t test p <0.001. B, significant decrease in urothelial thickness was noted in bladders of old vs young rats. Asterisk indicates Mann-Whitney test p <0.001.
DISCUSSION
In vivo studies comparing cystometric parameters in young and old rats are scarce.7,8 Chun et al studied the effects of age on micturition in 5 to 7, 16 to 18 and 22 to 24-month-old male Fischer 344 rats.7 MP was 100% greater at ages 22 to 24 and 16 to 18 vs 5 to 7 months with no age related difference in bladder volume at micturition. This is in contrast to our findings in male rats of significant decreases in MP and significant increases in BC between the 4 to 6 (young) and 28 to 30-month-old (old) groups (see table and fig. 1). We also documented significant age related increases in MV, RV, MF, SA, BP and IMP. Correspondingly old rats had higher bladder weight (larger bladders) than young rats (see table). Although the bladder-to-body weight ratio did not change (see table), together these data imply that bladders in old rats were apparently decompensating at that time. Briefly, these findings are similar to those in humans, in whom decreased detrusor contractility and detrusor overactivity are among the most conspicuous age related changes in bladder function.4,5
There are differences among rat strains in bladder changes induced by aging. There may also be gender differences but to our knowledge this has not been explored in the same strain in a single study. Lluel et al investigated 10 and 30-month-old female Wistar rats using conscious cystometry.8 Of conscious senescent rats 60% but only 25% of young adult rats had spontaneous contractions during the bladder filling phase, consistent with our findings in male Fischer/Brown Norway rats. In contrast to our observations, MP and micturition duration were significantly higher (40% to 50%) in old than in young rats. Also in contrast to our findings, they noted no BC or RV changes but reported a significant increase in mean muscularis layer thickness with age while collagen density significantly decreased in the muscularis and in the lamina propria layers. Again, this is in contrast to our initial findings of urothelial thinning, decreased muscle mass and increased collagen deposition in the bladders of old vs young rats (figs. 7 and 8). The reasons for these differences are not clear but may reflect strain dependent variations in the onset, development and progression of age related alterations in bladder function.
Most in vitro studies of aging have focused on muscarinic receptors since they are physiologically the most important for bladder contraction, at least in humans,20 and muscarinic receptor antagonists are the main form of overactive bladder treatment.21 However, in rats a significant part of bladder contraction is mediated by adenosine triphosphate.22 Since old patients with overactive bladder seem to have an increased adenosine triphosphate mediated contractile component,23 this may also be expected in rats. We found a larger atropine resistance component of EFS induced bladder contraction in old vs young rats (fig. 6, B).
In vitro studies in bladder tissue from aged vs young rats have also yielded contradictory results that again may be strain specific. For example, studies in Fischer 344 rats showed an age related increase9 or no change in muscarinic receptor mediated detrusor contraction.10,11 Chun et al postulated that micturition changes would be related primarily to alterations in peripheral innervation and central control of micturition rather than to alterations in bladder contractility.10 In Wistar rat8 and human23,24 bladder strips unchanged muscarinic receptor mediated bladder contractions were also noted with increasing age. However, many studies in old Sprague-Dawley rats showed decreased muscarinic receptor mediated detrusor contraction.14–16 Consistent with the latter studies we found that contractile responses of bladder strips to carbachol and EFS were significantly decreased in old rats, which was not unexpected considering the observed histological changes (see table, and figs. 2 to 5, 7 and 8).
As pointed out, it may be difficult to separate the influence of aging vs the impact of various other external influences on the detrusor from different diseases that may involve lower urinary tract smooth muscle. In our series the external environment was constant during the study period and the rats had no obvious comorbidity. These facts support the supposition that the aging process was the main factor behind the changes observed.
CONCLUSIONS
Physiological aging alters bladder function in this rodent model. In our study in male rats BC, RV, MV, MF, SA, BP and IMP were significantly increased with age while responses to muscarinic receptor stimulation and EFS were decreased in old vs young rats. These changes were associated with urothelial thinning, decreased muscle mass and increased collagen deposition in the bladder of old rats. Together this constellation of findings is remarkably similar to that reported in the aging human bladder, eg the overactive hypocontractile bladder in many men with benign prostatic hyperplasia. These data suggest that the Fischer/Brown Norway rat model may be useful for translational research into the mechanistic basis of age related changes in bladder function.
Acknowledgments
Study received Wake Forest University Health Sciences animal care and use committee approval.
Abbreviations and Acronyms
- BC
bladder capacity
- Bcom
bladder compliance
- BP
baseline pressure
- CRC
carbachol response curve
- EC50
drug concentration needed to produce 50% of calculated maximum
- EFS
electrical field stimulation
- Emax
calculated maximum effect
- IMP
interMP
- MF
micturition frequency
- MP
micturition pressure
- MV
micturition volume
- pEC50
negative logarithm of EC50
- RV
post-void residual volume
- SA
spontaneous activity
- TP
threshold pressure
Footnotes
Financial interest and/or other relationship with Ion Channel Innovations and Tengion.
REFERENCES
- 1.Hashim H, Abrams P. Overactive bladder: an update. Curr Opin Urol. 2007;17:231. doi: 10.1097/MOU.0b013e32819ed7f9. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan SA, Roehrborn CG, Chancellor M, et al. Extended-release tolterodine with or without tamsulosin in men with lower urinary tract symptoms and overactive bladder: effects on urinary symptoms assessed by the International Prostate Symptom Score. BJU Int. 2008;102:1133. doi: 10.1111/j.1464-410X.2008.07761.x. [DOI] [PubMed] [Google Scholar]
- 3.Wein AJ, Khullar V, Wang JT, et al. Achieving continence with antimuscarinic therapy for overactive bladder: effects of baseline incontinence severity and bladder diary duration. BJU Int. 2007;99:360. doi: 10.1111/j.1464-410X.2006.06621.x. [DOI] [PubMed] [Google Scholar]
- 4.Elbadawi A, Diokno AC, Millard RJ. The aging bladder: morphology and urodynamics. World J Urol, suppl. 1998;16:S10. doi: 10.1007/pl00014134. [DOI] [PubMed] [Google Scholar]
- 5.Hald T, Horn T. The human urinary bladder in ageing. Br J Urol, suppl. 1998;82:59. doi: 10.1046/j.1464-410x.1998.0820s1059.x. [DOI] [PubMed] [Google Scholar]
- 6.Nordling J. The aging bladder—a significant but underestimated role in the development of lower urinary tract symptoms. Exp Gerontol. 2002;37:991. doi: 10.1016/s0531-5565(02)00094-3. [DOI] [PubMed] [Google Scholar]
- 7.Chun AL, Wallace LJ, Gerald MC, et al. Effect of age on in vivo urinary bladder function in the rat. J Urol. 1988;139:625. doi: 10.1016/s0022-5347(17)42546-8. [DOI] [PubMed] [Google Scholar]
- 8.Lluel P, Palea S, Barras M, et al. Functional and morphological modifications of the urinary bladder in aging female rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R964. doi: 10.1152/ajpregu.2000.278.4.R964. [DOI] [PubMed] [Google Scholar]
- 9.Kolta MG, Wallace LJ, Gerald MC. Age-related changes in sensitivity of rat urinary bladder to autonomic agents. Mech Ageing Dev. 1984;27:183. doi: 10.1016/0047-6374(84)90043-5. [DOI] [PubMed] [Google Scholar]
- 10.Chun AL, Wallace LJ, Gerald MC, et al. Effects of age on urinary bladder function in the male rat. J Urol. 1989;141:170. doi: 10.1016/s0022-5347(17)40634-3. [DOI] [PubMed] [Google Scholar]
- 11.Ordway GA, Esbenshade TA, Kolta MG, et al. Effect of age on cholinergic muscarinic responsiveness and receptors in the rat urinary bladder. J Urol. 1986;136:492. doi: 10.1016/s0022-5347(17)44928-7. [DOI] [PubMed] [Google Scholar]
- 12.Pagala MK, Tetsoti L, Nagpal D, et al. Aging effects on contractility of longitudinal and circular detrusor and trigone of rat bladder. J Urol. 2001;166:721. [PubMed] [Google Scholar]
- 13.Schneider T, Hein P, Michel-Reher MB, et al. Effects of ageing on muscarinic receptor subtypes and function in rat urinary bladder. Naunyn Schmiedeb Arch Pharmacol. 2005;372:71. doi: 10.1007/s00210-005-1084-0. [DOI] [PubMed] [Google Scholar]
- 14.Hegde SS, Mandel DA, Wilford MR, et al. Evidence for purinergic neurotransmission in the urinary bladder of pithed rats. Eur J Pharmacol. 1998;349:75. doi: 10.1016/s0014-2999(98)00173-3. [DOI] [PubMed] [Google Scholar]
- 15.Lluel P, Deplanne V, Heudes D, et al. Age-related changes in urethrovesical coordination in male rats: relationship with bladder instability? Am J Physiol Regul Integr Comp Physiol. 2003;284:R1287. doi: 10.1152/ajpregu.00499.2001. [DOI] [PubMed] [Google Scholar]
- 16.Yu HI, Wein AJ, Levin RM. Contractile responses and calcium mobilization induced by muscarinic agonists in the rat urinary bladder: effects of age. Gen Pharmacol. 1997;28:623. doi: 10.1016/s0306-3623(96)00400-4. [DOI] [PubMed] [Google Scholar]
- 17.Renganathan M, Sonntag WE, Delbono O. L-type Ca2+ channel-insulin-like growth factor-1 receptor signaling impairment in aging rat skeletal muscle. Biochem Biophys Res Commun. 1997;235:784. doi: 10.1006/bbrc.1997.6881. [DOI] [PubMed] [Google Scholar]
- 18.Sonntag WE, Lynch CD, Cooney PT, et al. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- 19.Christ GJ, Day NS, Day M, et al. Bladder injection of "naked" hSlo/pcDNA3 ameliorates detrusor hyperactivity in obstructed rats in vivo. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1699. doi: 10.1152/ajpregu.2001.281.5.R1699. [DOI] [PubMed] [Google Scholar]
- 20.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004;84:935. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 21.Andersson KE. Antimuscarinics for treatment of overactive bladder. Lancet Neurol. 2004;3:46. doi: 10.1016/s1474-4422(03)00622-7. [DOI] [PubMed] [Google Scholar]
- 22.Streng T, Talo A, Andersson KE. Transmitters contributing to the voiding contraction in female rats. BJU Int. 2004;94:910. doi: 10.1111/j.1464-410X.2004.05058.x. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida M, Homma Y, Inadome A, et al. Age-related changes in cholinergic and purinergic neurotransmission in human isolated bladder smooth muscles. Exp Gerontol. 2001;36:99. doi: 10.1016/s0531-5565(00)00175-3. [DOI] [PubMed] [Google Scholar]
- 24.Wuest M, Morgenstern K, Graf EM, et al. Cholinergic and purinergic responses in isolated human detrusor in relation to age. J Urol. 2005;173:2182. doi: 10.1097/01.ju.0000158126.53702.e4. [DOI] [PubMed] [Google Scholar]