Abstract
HIV infection, once established, is never cleared. Rare individuals do, however, control viral replication to low levels. These successful immune responses are primarily linked to certain class I MHC alleles (MHC-I). Because of this association, many AIDS vaccines in development are designed to generate virus-specific CD8+ T cells. The Merck STEP phase 2b efficacy trial of one such vaccine was recently halted, and declared a failure. Thus, basic questions regarding what constitutes an effective T cell response and how such responses could be elicited by vaccination remain open. The best animal model available to explore such issues is simian immunodeficiency virus infection of rhesus macaques, which serves as the primary proving ground for AIDS vaccines.
Introduction
Twenty-five years after the discovery of HIV as the infectious agent that causes AIDS, a tremendous amount has been learned about the biology of this virus. Despite this knowledge, efforts to vaccinate against HIV have been dismal failures. Most vaccines currently in use against other pathogens rely largely on generating neutralizing antibodies [1]. These antibodies either prevent infection outright (referred to as sterilizing immunity) or sufficiently blunt pathogen replication to allow clearance by other arms of the immune system. The HIV envelope glycoprotein, which is accessible to neutralizing antibodies, is extremely variable. It is therefore unusually difficult to elicit antibodies that are broadly effective against multiple HIV isolates. In this context, the HIV vaccine field expanded its efforts to inducing T cell-mediated immunity, while research pertaining to antibodies continues to be pursued.
Focus on T cell-mediated immunity necessarily shifts the definition of success for a vaccine. T cells recognize virally derived peptides presented by the major histocompatibility complex (MHC) proteins of an infected cell – therefore they can be effective only after a virion has gained entry into a cell; T cells cannot attack free virions before they infect target cells, as neutralizing antibodies do. It is therefore unlikely that T cell-based vaccines will be able to prevent detectable infection. Instead, the more realistic outcome would be containment of viral replication to low levels, mimicking rare examples of natural control. Containing viral replication can have two positive consequences: preservation of the infected individual’s immune system and greatly reduced likelihood that the infected person could transmit the virus through sexual contact [2].
Unfortunately, what constitutes a protective T cell response, much less an effective vaccination strategy to generate such, is yet unknown. Vaccines have typically been evaluated based on neutralizing antibody assays, with a certain titer affording protection. No such validated assays exist for T cells, and although many studies of HIV-infected individuals have claimed to identify correlates of protection, it is often difficult to determine whether such correlates are a cause or effect of HIV containment. Thus, simian immunodeficiency virus (SIV) infected rhesus macaques provide a much-needed complement to HIV studies. Scientists have greater control over variables important in AIDS pathogenesis and immunology in this animal model, both before and after infection.
SIV history
SIVs are endemic to a variety of African non-human primates. The pandemic strains of HIV-1 are derived from SIVs that naturally infect chimpanzees, apparently without causing disease [3]. The most common SIVs used in animal studies originate from viruses that benignly infect sooty mangabeys. These viruses were initially isolated from captive Asian macaques that had been co-housed with sooty mangabeys and developed immunodeficiencies similar to AIDS [4,5]. When Indian rhesus macaques (Macaca mulatta), the most widely used animal model, are infected with these viruses they experience CD4+ T cell loss similar to that which is seen in HIV-infected humans [6] and typically develop AIDS-like immunodeficiency within 1–2 years.
There are a limited number of SIV strains used for AIDS studies in macaques. SIVmac239 is a cloned virus with the broadest usage. SIVmac239 (and the closely related SIV-mac251) is a CCR5-tropic virus, meaning that, like HIV, its primary targets during acute infection are activated CD4+ T cells and macrophages. Derived from SIVmac239 is a chimeric virus with an HIV envelope protein cloned into it, SHIV89.6. This SHIV was initially developed for use in experiments designed to elicit neutralizing antibodies against the HIV envelope glycoprotein. This virus is CXCR4-tropic, meaning that it infects naïve CD4+ T cells during acute infection, and so manifests an altered disease course, making it inappropriate for most T cell-based vaccine studies [7]. However, new SIV-HIV chimeras that have different regions of the genome exchanged and have a more normal disease course could prove useful in the future. SIVsmE660 is an uncloned or swarm viral stock derived from a different sooty mangabey isolate [8].
One pertinent difference between HIV in humans and SIV studies in macaques is that SIV sequence evolution selected for by the macaque immune system was effectively halted 20 years ago. This allows in-depth studies of a standard virus with defined properties. It does not, however, provide a good model of the global sequence diversity of HIV, which has been selected for over decades in large populations of infected humans. The major similarities and differences between the SIV-infected macaque model and HIV are summarized in Box 1.
Box 1. Major similarities and differences between the SIV-infected macaque model and HIV.
Similarities
Infects CD4+ T cells and causes a decline in their numbers over time, leading to AIDS-like immunodeficiency in macaques
Genetically similar to HIV-1
CD8+ T cells select for the majority of sequence variation outside of the envelope protein
Natural control of viral replication is associated with particular MHC-I alleles
Differences
SIV-infected macaques typically have a higher viral set-point during chronic infection
Disease progression is faster than that of HIV-infected humans
HIV sequence diversity has been selected for over decades of replication in humans, whereas most widely used SIV isolates have undergone limited passaging in macaques
MHC-I and control of SIV
HIV-infected humans experience a range of clinical outcomes. A few, although chronically infected, do not have high levels of viral replication, and have very slow disease progression. A substantial proportion, although not all, of these elite controllers have particular MHC-I alleles, namely HLA-B57 and HLA-B27 [9]. Elite controllers are studied in the hopes that their successful control can be mimicked by vaccination, possibly by refocusing the immune system towards particular parts of the viral proteome, or by altering qualitative properties of vaccine-induced T cells. The association with MHC-I implicates ‘good’ CD8+ T cell responses, although it must be remembered that MHC-I proteins are also important ligands for natural killer (NK) cells.
MHC-I genes, which encode proteins that dictate which virally derived peptides can be targeted by CD8+ T cells, are highly polymorphic. Each MHC-I molecule binds to different sets of peptides, typically requiring the presence of a particular anchor amino acid at a certain position within the peptide. Rhesus macaque MHC-I alleles are divergent from human genes. Macaques express a varying number of MHC-I genes, and do not have an MHC-C locus [10]. Variation among individual animals in number of MHC-I genes, combined with incomplete knowledge of possible alleles, makes comprehensive genotyping of outbred macaques more difficult than genotyping of human subjects, who have a fixed number of MHC-I alleles. Thus, researchers typically do not know all of the MHC-I alleles a given animal expresses. Despite this uncertainty, use of macaques still provides clear advantages – namely, the sequence of the infecting virus is known. For SIV-mac239, the epitopes in SIV proteins restricted by several alleles have been exhaustively mapped, affording predictability about the CD8+ T cell responses that will be made by SIV-infected animals expressing those alleles [11–14].
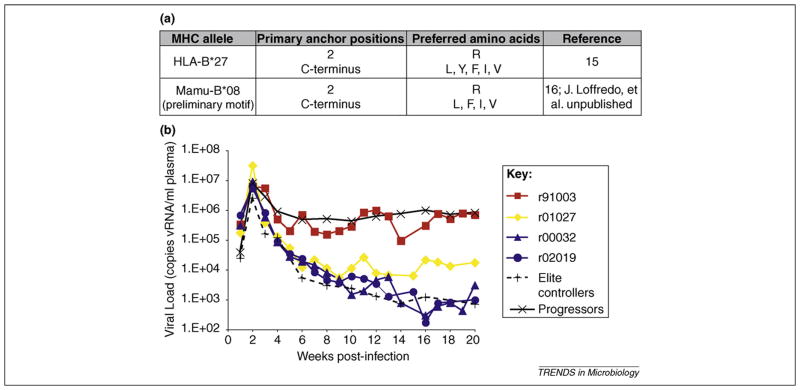
Some SIVmac239-infected macaques also become elite controllers, and the recently described parallels with human elite controllers are striking. HLA-B27 presents peptides with an arginine at position two, which anchors the peptide to the MHC-I molecule [15]. Though not a homologous protein, the macaque MHC-I molecule most strongly associated with elite control, Mamu-B*08, also presents peptides that are anchored by a position 2 arginine (J. Loffredo et al., unpublished data) [16,17] (Figure 1a). The other macaque MHC-I allele enriched in cohorts of elite controllers, Mamu-B*17, uses a C-terminal tryptophan as an anchor residue [11,18], as does HLA-B57, although this is a fairly common binding motif.
Figure 1.
Mamu-B*08+ Indian rhesus macaques as a model for elite control of HIV. (a) HLA-B27, a class I MHC allele associated with elite control of HIV, has a very similar peptide-binding motif to Mamu-B*08, a macaque class I MHC allele associated with elite control of SIVmac239 [16]. (b) Acute-phase viral loads of four Mamu-B*08+ macaques infected with SIVmac239. Longitudinal SIVmac239 plasma virus concentrations were plotted for four Mamu-B*08-positive macaques and the geometric mean of viral loads from 10 Mamu-B*08-negative elite controllers and 175 Mamu-B*08-negative animals that progressed to AIDS (black lines). Only one of the four animals had normal viral loads (91003, red line), while two animals (02019 and 00032, blue lines) became elite controllers, maintaining viral loads <1000 copies vRNA/ml plasma for longer than 1 year. One animal (01027, yellow line), had a set-point viral load tenfold lower than typically seen for SIVmac239-infected progressors, and was considered a ‘controller.’ Figure originally published in [19].
Not all macaques with these alleles become elite controllers. An acute-phase study of four Mamu-B*08+ animals found that control is mediated (or not) very early in acute infection, as viremia is brought down from its initial peak (Figure 1b). This study also observed that the non-controller macaque’s CD8+ T cells were focused largely on one epitope, rather than multiple Mamu-B*08-restricted epitopes [19]. Whether a broad acute-phase immune response proves to be determinative of elite control in a large cohort of animals has yet to be investigated.
What do similarities between humans and the animal model tell us? This concordance might indicate that something is special about how these [MHC-I + peptide] complexes on professional antigen-presenting cells initially prime responses, resulting in a qualitatively different CD8+ T-cell response. It is also possible that virally infected CD4+ T cells and macrophages present epitopes restricted by these alleles such that they are more effectively recognized by already-primed effector CD8+ T cells. Alternatively, it could be difficult for SIV/HIV to escape from responses restricted by these alleles, making the T cell responses more durable. Indeed, studies of HIV variation driven by the immunodominant HLA-B27 and –B57 responses found that viruses with escape mutations within these Gag epitopes had in vitro fitness defects [20–22]. Preliminary studies of SIVmac239 engineered to have the escape variants selected for by Mamu-B*08 or B*17-directed T-cell responses have not found fitness defects (D. Watkins and L. Valentine, unpublished data). Regardless, when it is determined what distinguishes responses restricted by these particular alleles, the primary challenge will be in making such lessons applicable to individuals who lack these protective MHC-I genes, and so cannot make immune responses to exactly the same T cell epitopes.
Functional defects of SIV/HIV-specific T cells
Depletion of CD8+ lymphocytes (which includes CD8+ T cells and NK cells) from SIV-infected macaques results in failure to bring down peak viremia during acute infection, or in a spike of viremia in elite controller animals [23–26]. Depletion of CD16+ lymphocytes (which includes most NK cells) during acute SIV infection does not alter disease course [27]. These data point to CD8+ T cells as the primary mediators of viral control.
This then raises the question of why control is generally so poor, with the exceptions of elite controllers. Untreated, chronically infected humans typically have a viral load of ~30 000 copies of HIV RNA per milliliter plasma [28], whereas SIVmac239+ macaques have ~450 000 copies of SIV per milliliter [18]. SIV virions have short half-lives [29], so viral loads are indicative of ongoing viral replication. At least early in chronic infection, this replication occurs in the presence of readily detectable SIV or HIV-specific T cells –indeed, as measured by frequency in the blood, there are typically robust SIV/HIV-specific responses [30,31].
In the past few years it has become clear that dysfunctional T cells are the norm in immunodeficiency virus infection [32]. HIV-specific CD4+ and CD8+ T cells from individuals with normal-to-high viral loads fail to proliferate in response to antigen, a defect also seen in SIV-infected animals. Meanwhile, T cells from individuals with very low viral loads proliferate normally when exposed to antigen in vitro [33–38]. A high frequency of HIV-specific T cells are negative for the costimulatory molecule CD28 [39], a phenotype typical of fully differentiated effector memory T cells. This lack of CD28 might be a cause of the failure to proliferate, because CD28− cells do not produce their own interleukin 2 (IL-2) [40], a cytokine required for survival and expansion upon T cell receptor engagement. Indeed, addition of IL-2 to in vitro assays restores proliferation to some extent [41,42].
The failure to produce IL-2 and to proliferate is not the only functional defect of T cells. HIV-specific and SIV-specific cells do not produce a full array of other cytokines when stimulated. Instead, T cells from individuals with high viral loads secrete perhaps one or two cytokines, as measured by flow-cytometry assays, whereas T cells from individuals with little viral replication can secrete upwards of five cytokines [43–45]. Overall, poor T cell responsiveness to antigen correlates with high viral loads, whereas polyfunctional responsiveness to SIV/HIV antigen correlates with limited viral replication. Data in a recent longitudinal study indicate that polyfunctionality is a consequence, rather than a cause of, low antigen exposure, which calls into question its utility for evaluating the quality of vaccine-induced responses [46].
So what is the origin of these defects? An immunological mechanism underlying the apparent exhaustion of SIV/HIV-specific T cells is their upregulation of the cell-surface molecule programmed death 1 (PD-1). The role of PD-1 in chronic viral infections was first clearly delineated in studies of mice. This molecule is upregulated during acute infections, and downregulated upon clearance of antigen. Blocking PD-1’s engagement with its ligands restores functions such as proliferation and cytokine secretion in otherwise ‘exhausted’ CD8+ T cells [47]. Subsequent studies of HIV-infected persons have also found high levels of PD-1 expression on virus-specific T cells, and have shown that, at least in vitro, PD-1 expression caused greater susceptibility to apoptosis [48,49].
In the SIV-infected macaque, studies of PD-1 show similar results [50]. Petrovas et al. [37] demonstrated the dependence of PD-1 expression on antigen exposure by following its expression on CD8+ T cells that select for viral escape mutations during acute infection. Escape was followed by a decrease in PD-1 expression on these antigen-specific cells, presumably because cells were no longer able to engage with their cognate [MHC + peptide] complex and no longer recognized high amounts of antigen. High levels of PD-1 expression and susceptibility to antigen-induced cell death were maintained by SIV-specific cells that did not select for escape mutations, although in contrast to some findings in humans, they could still secrete multiple cytokines [37]. Another group working on SIV has suggested a valuable future use of macaques in this area, creating antibodies to be used for in vivo studies of the effects of blocking this pathway – such antibodies have the potential to restore T-cell function and perhaps lower viral loads [51]. These therapies could have serious side effects if they cause generalized immune activation, which makes extensive characterization and testing in non-human primates important.
Escape and progression
Accurate knowledge of the sequence of the infecting virus has meant that SIV-infected macaques have proved particularly useful in studying the dynamics and effects of viral sequence evolution. CD8+ T cells were first definitively shown to select for escape variants with targeted epitopes in SIV-infected macaques [52,53]. SIV-specific CD8+ T cells select for escape mutations in many, although not all epitopes targeted by the immune response. Indeed, this selection accounts for the majority of amino acid replacements in SIV or HIV, outside of the envelope glycoprotein (which is under selective pressure from antibodies) [54,55]. It is not clear why specific epitopes targeted by the immune response consistently acquire escape variation while others do not. Lack of escape might indicate that variation in a targeted region exacts a substantial fitness cost from the virus. For a well-characterized immunodominant SIV-Gag epitope that escapes only very late in infection, this appears to be the case, with compensatory mutations being required [56,57] and reversion occurring on transmission of the escape-mutant virus to another macaque [58]. These observations suggest that such a region of the virus would be useful to target by vaccination. However, another explanation for lack of variation within an epitope is that the selective pressure exerted by CD8+ T cells targeting it is weak, due to inherent differences in CTL effectiveness, or exhaustion of the epitope-specific cells.
The ultimate determinants of rate of progression to AIDS following a period of asymptomatic chronic infection are still somewhat unclear. Gradual decline of the ability of central-memory CD4+ T cells to replace the rapidly infected and short-lived effector-memory CD4+ T cells leads to immunological collapse in SIV-infected macaques [59]. CD8+ T cells, by controlling viral replication and slowing CD4+ T cell turnover, might retard this process. Some data suggest that escape from CD8+ T cell responses correlates with an increase in viral loads in individuals who had been controllers. For HLA-B27+ HIV-infected humans, one CD8+ T cell response directed against an epitope in Gag may be enough to control viral loads to very low levels. In one long-term nonprogressor patient, an escape mutation in this epitope directly preceded a sharp increase in HIV viremia [60]. Similarly, in Gag-vaccinated macaques that had controlled SIV replication to below the limits of detection, the acquisition of mutations in multiple Gag epitopes was associated with increasing viral loads and concomitant declines in CD4 counts [61].
The degree to which escape affects progression in animals that already have fairly normal levels of viremia is less well-studied. In a different study of Gag-vaccinated Indian rhesus macaques, a transient lowering of set-point viremia was seen after infection, but subsequent increases in viral load could not be correlated with escape in defined Gag epitopes [62]. Documentation of escape during chronic infection, however, does indicate that at least some CD8+ T cells are actively suppressing viral replication during this time. Additional evidence for this is that depletion of CD8+ lymphocytes during chronic infection in macaques leads to a spike in viremia [24]. Thus, experiments from SIV-infected macaques suggest that CD8+ T cells likely play a major role in determining rapidity of disease progression.
Vaccine evaluation
The greatest value of the SIV-infected macaque model for AIDS is its use in testing vaccines. The recent failure of the T cell-based STEP clinical trial was predicted by preclinical testing of a similar regimen in SIVmac239-challenged macaques [63]. The most effective vaccinations of macaques have involved live-attenuated SIV, but safety concerns make the use of a live-attenuated HIV vaccine improbable. Live-attenuated vaccination of macaques does, however, provide a model for what protective immune responses look like, and could be useful for establishing parameters for measuring effectiveness of vaccine-induced immune responses [64].
Currently, vaccine-elicited T cell responses are quantitated by the percentage of CD8+ T cells in the blood that secrete cytokines – generally interferon-gamma – in response to SIV/HIV-derived peptides. The limitations of this approach are increasingly apparent [65]. There are a variety of viral vectors that can elicit robust T cell responses by these measures. However, the magnitude of such responses does not generally correlate with protection upon SIV challenge. For example, following a DNA-prime/Sendai virus boost Gag vaccine, five of eight Burmese macaques controlled SIVmac239 to undetectable levels. However, the vaccine-induced CD8+ and CD4+ T cell responses of the protected animals were indistinguishable from those animals that failed to control viremia [66]. A recent study using a live-attenuated SIV vaccine also failed to find a correlation between quantity of vaccine-induced T cell responses (or neutralizing antibodies) and protection after infection [67]. Therefore, at this time vaccines can be tested in macaques for efficacy at curbing viral replication, but the model does not provide definitive guidance regarding assays that would be useful in assessing whether vaccination has induced a protective response in an individual.
Finding predictors of protection would be invaluable. Such predictors might be defined in the setting of successful vaccination of macaques – that is by characterizing responses in animals that, upon challenge, go on to have viral loads at least 1.5 logs lower than the average set-point. Data on this front are limited. It could be important for such assays to take into account the ability of vaccine-induced T cells to recognize infected cells, rather than high concentrations of purified peptides that are typically used [68,69]. Also needed is a way to evaluate whether cells can traffic to sites of viral replication in the body.
Other than live-attenuated viruses, there is a dearth of successful vaccines. Delivering SIV proteins in adenovirus-vector vaccines has shown promise, and has illustrated that, in principle, T cell-based vaccines (those that do not prime an antibody response) can exert some control over a homologous challenge virus [70,71]. Improvements in such vectored vaccines will likely be empirical. Various viral vectors for stimulating CD8+ T cell responses have been produced, but these have not been carefully tested in a standardized manner to determine which are most promising. Relatively little is known about the impact of immune responses directed against vaccine vectors themselves, but such responses could complicate vaccination plans and possibly preclude repeated immunization with the same viral vector.
In addition, the macaque model will be useful for evaluating adjuvants, crucial components of vaccines that can be used to manipulate the quality of T cell responses induced. For example, in at least one study, adding a Toll-like receptor 9 ligand during the DNA prime phase of vaccination dramatically improved the protective effect of the vaccine, while Toll-like receptor 7/8 stimulation had much less of an effect [72].
Beyond determining the best way to deliver and adjuvant immunogens, it will be important to find which HIV proteins, or parts of proteins, are most useful to include in a vaccine. Several studies have correlated Gag-directed CD8+ T cell responses with lower HIV viral loads, and suggested that responses to other HIV proteins might even be detrimental [30,73,74]. However, vaccinating macaques with Gag, Tat, Rev and Nef SIV proteins afforded stronger control than vaccination with Gag alone, suggesting that a broadening of the immune response to target more epitopes, not necessarily in the Gag protein, is beneficial [71].
A caveat regarding the above examples of relatively successful vaccines is that they all were tested with homologous challenge viruses. Homologous challenges – in which the amino acid sequence of the infecting virus matches that of the immunogens – are unrealistic approximations of what will be faced in the setting of HIV vaccination, due to the high degree of sequence variation between HIV isolates, even within the same viral clade. Thus, a more meaningful evaluation of promising vaccine candidates in macaques should also involve tests against heterologous viruses. Ideally, such viruses would have been passaged in rhesus macaques that express relevant class I MHC alleles. Such passaging would yield viruses with sequence variation in epitopes targeted by CD8+ T cells. Mutations that then revert upon transmission to hosts lacking the restricting allele (i.e. those mutations that incur at a fitness cost) would be preserved, whereas mutations that failed to revert would be lost, as has been the case with HIV sequence evolution.
Research and progress on these vaccine-related topics –vector, adjuvant, and immunogen selection – has, to date, not been systematic. These basic issues will likely determine success or failure of a vaccine. Their effects on outcome following pathogenic SIV challenge will need to be evaluated in the non-human primates. After the recent failure of the Merck STEP phase 2b trial, and the subsequent cancellation of a similar proposed study [75], it is unlikely that vaccines will progress to expensive human clinical trials without some demonstrated success in non-human primates providing scientific rationale to move forward.
Concluding remarks and future directions
CD8+ T cell-mediated immunity will likely be a component of a successful AIDS vaccine. Potential vaccines need to be evaluated in some way before use in large-scale clinical trials, but relevant in vitro assays to test efficacy of vaccine-elicited T cells are nonexistent. SIV-infected rhesus macaques make CD8+ T cell responses that are similar to those of HIV-infected humans, and vaccine trials in macaques can provide a rational method for selecting promising vaccine candidates. Important future avenues of research using SIV-infected rhesus macaques will include (1) developing new assays for CD8+ T cell function, (2) understanding the immunological mechanisms that make live-attenuated vaccines effective, and (3) evaluating vaccine efficacy in the face of more realistic, non-homologous challenge viruses (Box 2).
Box 2. Future important areas of research using the macaque model.
Dissect basic immunological mechanisms underlying MHC-I associations with elite control, which is mediated early in acute infection
Determine the immune responses generated by live-attenuated vaccines that contribute to their success, and attempt to replicate using alternative vaccination strategies
Develop viruses that more closely reflect the diversity selected for in HIV, so as to provide a more realistic tests of vaccines in non-human primates
Acknowledgments
We thank Shari Piaskowski for help in editing this article. The authors of this article are supported by US N.I.H. grants R01 AI049120, R01 AI052056, R24 RR015371 and R24 RR016038 (to D.I.W.).
References
- 1.Burton DR. Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;2:706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- 2.Gray RH, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 3.Keele BF, et al. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313:523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner MB. The history of simian AIDS. J Med Primatol. 1996;25:148–157. doi: 10.1111/j.1600-0684.1996.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 5.Daniel MD, et al. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985;228:1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- 6.Letvin NL, et al. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230:71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- 7.Feinberg MB, Moore JP. AIDS vaccine models: challenging challenge viruses. Nat Med. 2002;8:207–210. doi: 10.1038/nm0302-207. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein S, et al. Immunization with whole inactivated vaccine protects from infection by SIV grown in human but not macaque cells. J Med Primatol. 1994;23:75–82. doi: 10.1111/j.1600-0684.1994.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 9.Pereyra F, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197:563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 10.Otting N, et al. Unparalleled complexity of the MHC class I region in rhesus macaques. Proc Natl Acad Sci U S A. 2005;102:1626–1631. doi: 10.1073/pnas.0409084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mothe BR, et al. Characterization of the peptide-binding specificity of Mamu-B*17 and identification of Mamu-B*17-restricted epitopes derived from simian immunodeficiency virus proteins. J Immunol. 2002;169:210–219. doi: 10.4049/jimmunol.169.1.210. [DOI] [PubMed] [Google Scholar]
- 12.Allen TM, et al. CD8(+) lymphocytes from simian immunodeficiency virus-infected rhesus macaques recognize 14 different epitopes bound by the major histocompatibility complex class I molecule mamu-A*01: implications for vaccine design and testing. J Virol. 2001;75:738–749. doi: 10.1128/JVI.75.2.738-749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loffredo JT, et al. Identification of seventeen new simian immunodeficiency virus-derived CD8+ T cell epitopes restricted by the high frequency molecule, Mamu-A*02, and potential escape from CTL recognition. J Immunol. 2004;173:5064–5076. doi: 10.4049/jimmunol.173.8.5064. [DOI] [PubMed] [Google Scholar]
- 14.Sette A, et al. Characterization of the peptide-binding specificity of Mamu-A*11 results in the identification of SIV-derived epitopes and interspecies cross-reactivity. Immunogenetics. 2005;57:53–68. doi: 10.1007/s00251-004-0749-z. [DOI] [PubMed] [Google Scholar]
- 15.Marsh SGE, et al. The HLA Facts Book. Academic Press; 2000. [Google Scholar]
- 16.Loffredo JT, et al. CD8 T cells from SIV elite controller macaques recognize mamu-B*08-bound epitopes and select for widespread viral variation. PLoS One. 2007;2:e1152. doi: 10.1371/journal.pone.0001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loffredo JT, et al. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J Virol. 2007;81:8827–8832. doi: 10.1128/JVI.00895-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yant LJ, et al. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J Virol. 2006;80:5074–5077. doi: 10.1128/JVI.80.10.5074-5077.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loffredo JT, et al. Patterns of CD8+ immunodominance may influence the ability of Mamu-B*08-positive macaques to naturally control simian immunodeficiency virus SIVmac239 replication. J Virol. 2008;82:1723–1738. doi: 10.1128/JVI.02084-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Picado J, et al. Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J Virol. 2006;80:3617–3623. doi: 10.1128/JVI.80.7.3617-3623.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brockman MA, et al. Escape and compensation from early HLA-B57-mediated cytotoxic T-lymphocyte pressure on human immunodeficiency virus type 1 Gag alter capsid interactions with cyclophilin A. J Virol. 2007;81:12608–12618. doi: 10.1128/JVI.01369-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneidewind A, et al. Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication. J Virol. 2007;81:12382–12393. doi: 10.1128/JVI.01543-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao H, et al. CD8+ and CD20+ lymphocytes cooperate to control acute simian immunodeficiency virus/human immunodeficiency virus chimeric virus infections in rhesus monkeys: modulation by major histocompatibility complex genotype. J Virol. 2005;79:14887–14898. doi: 10.1128/JVI.79.23.14887-14898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitz JE, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 25.Matano T, et al. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998;72:164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedrich TC, et al. Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. J Virol. 2007;81:3465–3476. doi: 10.1128/JVI.02392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi EI, et al. In vivo natural killer cell depletion during primary simian immunodeficiency virus infection in rhesus monkeys. J Virol. 2008;82:6758–6761. doi: 10.1128/JVI.02277-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wawer MJ, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 29.Brandin E, et al. Rapid viral decay in simian immunodeficiency virus-infected macaques receiving quadruple antiretroviral therapy. J Virol. 2006;80:9861–9864. doi: 10.1128/JVI.00394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiepiela P, et al. CD8(+) T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 31.Maness NJ, et al. Comprehensive immunological evaluation reveals surprisingly few differences between elite controller and progressor Mamu-B*17-positive Simian immunodeficiency virus-infected rhesus macaques. J Virol. 2008;82:5245–5254. doi: 10.1128/JVI.00292-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Boritz E, et al. Human immunodeficiency virus type 1 (HIV-1)-specific CD4+ T cells that proliferate in vitro detected in samples from most viremic subjects and inversely associated with plasma HIV-1 levels. J Virol. 2004;78:12638–12646. doi: 10.1128/JVI.78.22.12638-12646.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Younes SA, et al. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J Exp Med. 2003;198:1909–1922. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lichterfeld M, et al. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J Exp Med. 2004;200:701–712. doi: 10.1084/jem.20041270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Migueles SA, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3:1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 37.Petrovas C, et al. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007;110:928–936. doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horton H, et al. Preservation of T cell proliferation restricted by protective HLA alleles is critical for immune control of HIV-1 infection. J Immunol. 2006;177:7406–7415. doi: 10.4049/jimmunol.177.10.7406. [DOI] [PubMed] [Google Scholar]
- 39.Gamberg J, et al. Lack of CD28 expression on HIV-specific cytotoxic T lymphocytes is associated with disease progression. Immunol Cell Biol. 2004;82:38–46. doi: 10.1111/j.1440-1711.2004.01204.x. [DOI] [PubMed] [Google Scholar]
- 40.Topp MS, et al. Restoration of CD28 expression in CD28− CD8+ memory effector T cells reconstitutes antigen-induced IL-2 production. J Exp Med. 2003;198:947–955. doi: 10.1084/jem.20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iyasere C, et al. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J Virol. 2003;77:10900–10909. doi: 10.1128/JVI.77.20.10900-10909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmerli SC, et al. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc Natl Acad Sci U S A. 2005;102:7239–7244. doi: 10.1073/pnas.0502393102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Almeida JR, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duvall MG, et al. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol. 2008;38:350–363. doi: 10.1002/eji.200737768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harari A, et al. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol Rev. 2006;211:236–254. doi: 10.1111/j.0105-2896.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 46.Streeck H, et al. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8(+) T cells. PLoS Med. 2008;5:e100. doi: 10.1371/journal.pmed.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 48.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 49.Petrovas C, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Velu V, et al. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol. 2007;81:5819–5828. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Onlamoon N, et al. Soluble PD-1 rescues the proliferative response of simian immunodeficiency virus-specific CD4 and CD8 T cells during chronic infection. Immunology. 2008;124:277–293. doi: 10.1111/j.1365-2567.2007.02766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allen TM, et al. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature. 2000;407:386–390. doi: 10.1038/35030124. [DOI] [PubMed] [Google Scholar]
- 53.Evans DT, et al. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat Med. 1999;5:1270–1276. doi: 10.1038/15224. [DOI] [PubMed] [Google Scholar]
- 54.Allen TM, et al. Selective escape from CD8+ T-cell responses represents a major driving force of human immunodeficiency virus type 1 (HIV-1) sequence diversity and reveals constraints on HIV-1 evolution. J Virol. 2005;79:13239–13249. doi: 10.1128/JVI.79.21.13239-13249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Connor DH, et al. A dominant role for CD8+-T-lymphocyte selection in simian immunodeficiency virus sequence variation. J Virol. 2004;78:14012–14022. doi: 10.1128/JVI.78.24.14012-14022.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Friedrich TC, et al. Extraepitopic compensatory substitutions partially restore fitness to simian immunodeficiency virus variants that escape from an immunodominant cytotoxic-T-lymphocyte response. J Virol. 2004;78:2581–2585. doi: 10.1128/JVI.78.5.2581-2585.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeh WW, et al. Compensatory substitutions restore normal core assembly in simian immunodeficiency virus isolates with Gag epitope cytotoxic T-lymphocyte escape mutations. J Virol. 2006;80:8168–8177. doi: 10.1128/JVI.00068-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedrich TC, et al. Reversion of CTL escape-variant immunodeficiency viruses in vivo. Nat Med. 2004;10:275–281. doi: 10.1038/nm998. [DOI] [PubMed] [Google Scholar]
- 59.Okoye A, et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feeney ME, et al. Immune escape precedes breakthrough human immunodeficiency virus type 1 viremia and broadening of the cytotoxic T-lymphocyte response in an HLA-B27-positive long-term-nonprogressing child. J Virol. 2004;78:8927–8930. doi: 10.1128/JVI.78.16.8927-8930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawada M, et al. Involvement of multiple epitope-specific cytotoxic T-lymphocyte responses in vaccine-based control of simian immunodeficiency virus replication in rhesus macaques. J Virol. 2006;80:1949–1958. doi: 10.1128/JVI.80.4.1949-1958.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McDermott AB, et al. Cytotoxic T-lymphocyte escape does not always explain the transient control of simian immunodeficiency virus SIVmac239 viremia in adenovirus-boosted and DNA-primed Mamu-A*01-positive rhesus macaques. J Virol. 2005;79:15556–15566. doi: 10.1128/JVI.79.24.15556-15566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watkins DI, et al. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat Med. 2008;14:617–621. doi: 10.1038/nm.f.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koff WC, et al. HIV vaccine design: insights from live attenuated SIV vaccines. Nat Immunol. 2006;7:19–23. doi: 10.1038/ni1296. [DOI] [PubMed] [Google Scholar]
- 65.Yang OO. Retracing our STEP towards a successful CTL-based HIV-1 vaccine. Vaccine. 2008;26:3138–3141. doi: 10.1016/j.vaccine.2008.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matano T, et al. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J Exp Med. 2004;199:1709–1718. doi: 10.1084/jem.20040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mansfield K, et al. Vaccine protection by live, attenuated simian immunodeficiency virus in the absence of high-titer antibody responses and high-frequency cellular immune responses measurable in the periphery. J Virol. 2008;82:4135–4148. doi: 10.1128/JVI.00015-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bennett MS, et al. Cross-clade detection of HIV-1-specific cytotoxic T lymphocytes does not reflect cross-clade antiviral activity. J Infect Dis. 2008;197:390–397. doi: 10.1086/525281. [DOI] [PubMed] [Google Scholar]
- 69.Valentine LE, et al. Recognition of escape variants in ELISPOT does not always predict CD8+ T-cell recognition of simian immunodeficiency virus-infected cells expressing the same variant sequences. J Virol. 2008;82:575–581. doi: 10.1128/JVI.00275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Letvin NL, et al. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312:1530–1533. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson NA, et al. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J Virol. 2006;80:5875–5885. doi: 10.1128/JVI.00171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwissa M, et al. Adjuvanting a DNA vaccine with a TLR9 ligand plus Flt3 ligand results in enhanced cellular immunity against the simian immunodeficiency virus. J Exp Med. 2007;204:2733–2746. doi: 10.1084/jem.20071211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peut V, Kent SJ. Utility of human immunodeficiency virus type 1 envelope as a T-cell immunogen. J Virol. 2007;81:13125–13134. doi: 10.1128/JVI.01408-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rolland M, et al. Broad and Gag-biased HIV-1 epitope repertoires are associated with lower viral loads. PLoS One. 2008;3:e1424. doi: 10.1371/journal.pone.0001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cohen J. AIDS vaccine research. Thumbs down on expensive, hotly debated trial of NIH AIDS vaccine. Science. 2008;321:472. doi: 10.1126/science.321.5888.472. [DOI] [PubMed] [Google Scholar]