Abstract
Sonic hedgehog (Shh) signaling plays important roles in the formation of the auditory epithelium. However, little is known about the detailed expression pattern of Shh and the cell sources from which Shh is secreted. By analyzing ShhCreEGFP/+ mice, we found that Shh was first expressed in all cochlear spiral ganglion neurons by embryonic day 13.5, after which its expression gradually decreased from base to apex. By postnatal day 0, it was not detected in any spiral ganglion neurons. Genetic cell fate mapping results also confirmed that Shh was exclusively expressed in all spiral ganglion neurons and not in surrounding glia cells. The basal-to-apical wave of Shh declining strongly resembles that of hair cell differentiation, supporting the idea that Shh signaling inhibits hair cell differentiation. Furthermore, this ShhCreEGFP/+ mouse is a useful Cre line in which to delete floxed genes specifically in spiral ganglion neurons of the developing cochlea.
Keywords: Sonic Hedgehog (Shh), cochlear duct, hair cell, spiral ganglion neuron, differentiation
INTRODUCTION
The mouse auditory epithelium, the organ of Corti, consists of mechanosensory hair cells (HCs) and surrounding supporting cells (SCs). HCs and SCs extend along the coiled cochlear duct that can be approximately divided into basal, middle, and apical portions, with a total of ~1.75 turns(Kelley, 2006). HCs and SCs are believed to share the same progenitor cells, which are located in the prosensory area of the cochlear duct during embryonic development. These progenitor cells keep proliferating until p27Kip1, a CIP/KIP family cell cycle inhibitor, is turned on in an apical-to-basal gradient (Chen and Segil, 1999; Lowenheim et al., 1999; Kanzaki et al., 2006; Lee et al., 2006). After cell cycle exit, however, these progenitors start differentiation into either HCs or SCs in a basal-to-apical gradient. Previous studies have suggested that Notch signaling (Lanford et al., 1999; Zheng et al., 2000; Zine et al., 2001; Kiernan et al., 2005a; Brooker et al., 2006; Tang et al., 2006; Takebayashi et al., 2007; Li et al., 2008; Doetzlhofer et al., 2009), fibroblast growth factor receptor signaling (Colvin et al., 1996; Mueller et al., 2002; Pirvola et al., 2002; Hayashi et al., 2007; Puligilla et al., 2007; Hayashi et al., 2008), and Sox2 protein (Kiernan et al., 2005b) are necessary to promote the formation of the prosensory area.
Cochlear spiral ganglion neurons are derived from the neuroblasts delaminating at embryonic day 8.5 (E8.5) from the otic placode or otocyst, a thickening ectoderm adjacent to the hindbrain that gives rise to the vestibular end organs and the cochlea (Rubel and Fritzsch, 2002). In contrast, the glia cells are derived from neural crest cells (Noden DM, 1986). These neuroblasts become postmitotic around E11.5 to E15.5 in a basal-to-apical gradient (Ruben, 1967) and gradually differentiate into either Type I or Type II cochlear ganglion neurons. The type I neurons innervate the inner hair cells (IHCs), and type II neurons innervate the outer hair cells (OHCs) in the cochlear sensory epithelium.
Sonic hedgehog (Shh) signaling is a highly conserved signaling pathway that plays critical roles in the development of a variety of organs across different species (Wijgerde et al., 2002; Fuccillo et al., 2006). The importance of Shh signaling in the development of the cochlea was highlighted by the complete absence of cochlea in the Shh knockout mouse (Riccomagno et al., 2002). Shh signaling also can interact with Wnt signaling to pattern the developing inner ear (Bok et al., 2005; Riccomagno et al., 2005). Basically, the dorsal part (the vestibular end organ) of the inner ear requires less Shh signaling than the ventral part (the cochlea). Such a dorsal-ventral Shh signaling gradient is controlled by various Gli activators and repressors (Bok et al., 2007). In addition, Shh signaling is involved in regulating otic capsule chondrogenesis (Liu et al., 2002). Because the complete absence of the cochlear duct in the Shh knockout mouse precludes further analysis of the detailed roles of Shh signaling in cochlear development, a mutant mouse model (Gli3Δ699/Δ699), in which Shh signaling is only partially lost, was analyzed (Bose et al., 2002; Driver et al., 2008). Although the cochlear ducts of Gli3Δ699/Δ699 mutant pups at postnatal day 0 (P0) were approximately half the length of those of their wild-type littermates, the auditory epithelium was wider, with ectopic patches of HCs in Kölliker’s organ, an area medial to the auditory epithelium that normally does not have HCs. Gain-of-function and loss-of-function analyses revealed that Shh signaling represses the prosensory area formation with the consequence of having fewer HCs in the organ of Corti (Driver et al., 2008).
In situ hybridization analysis showed that the cell sources where Shh is secreted are distributed in the cochlear spiral ganglion area (Driver et al., 2008). However, cochlear spiral ganglion neurons and glia cells (Schwann cells) are mixed in this area. It is not clear whether either or both of them express Shh. To date, the detailed expression pattern of Shh remains unknown. Here we analyzed ShhCreEGFP/+ knock-in mutant mice in which the fusion protein Cre/EGFP is controlled by endogenous Shh promoter. There are two apparent advantages to using this ShhCreEGFP/+ mouse line. First, with the high-quality GFP antibody, EGFP can be used to recapitulate the endogenous Shh expression pattern. Second, by crossing the ShhCreEGFP/+ mouse with a Rosa26-EYFPloxp/+ reporter mouse, we can perform in vivo genetic cell fate mapping, an approach widely used for cell lineage analysis in nervous systems(Joyner and Zervas, 2006; Hatch et al., 2009). All cells that express Shh can be historically recorded by the reporter gene EYFP in a Cre-mediated manner, irrespective of their temporal and spatial expression.
In this study, we found that Shh was first expressed in all cochlear spiral ganglion neurons around E13.5, after which the expression gradually declined in a basal-to-apical wave and became undetectable at P0. It strongly resembles the basal-to-apical gradient differentiation pattern of the cochlear prosensory progenitor cells, suggesting that Shh signaling controls the timing of when prosensory progenitor cells start their intrinsic differentiation program. Moreover, genetic cell fate mapping confirmed that Shh expression was exclusive to spiral ganglion neurons, all of which transiently expressed Shh. Therefore, the ShhCreEGFP/+ mouse is an excellent genetic tool for studying functions of genes that are expressed in cochlear spiral ganglion neurons.
RESULTS
Expression pattern of Shh before E12.5
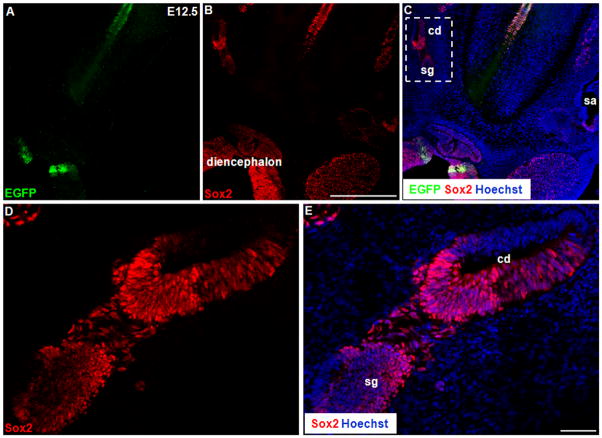
The mouse inner ear derives from a thickened ectodermal region adjacent to the hindbrain, referred as otic placode, at ~E8.0. To show the detailed Shh expression pattern during the development of the inner ear, we analyzed the heterozygous ShhCreEGFP/+ knock-in mutant mouse, in which the fusion protein Cre/EGFP with a nuclear localization signal was inserted to the endogenous Shh locus, resulting in a Shh-null allele (Harfe et al., 2004). Although homozygous ShhCreEGFP/CreEGFP mice die as embryos, heterozygous mice are indistinguishable from their wild-type littermates and display no noticeable phenotypes(Harfe et al., 2004). Therefore, we used nuclear EGFP reporter to recapitulate the Shh expression pattern. We found that the neurosensory progenitor cells, which were competent to develop into both neurons and sensory cells, were Shh-negative at E8.75~E9 (Fig. 1A, B). Similarly, the NeuroD1-positive delaminating neuroblasts at E10.5~E11 had not yet expressed Shh (Fig. 1C). However, Shh was highly expressed in the notochord at both ages (Fig. 1A and inset in Fig. 1C), which served as internal positive controls. Additionally, we analyzed the E12.5 cochleae where the Sox2-positive spiral ganglion neurons were found to be Shh-negative (Fig. 2).
Figure 1.
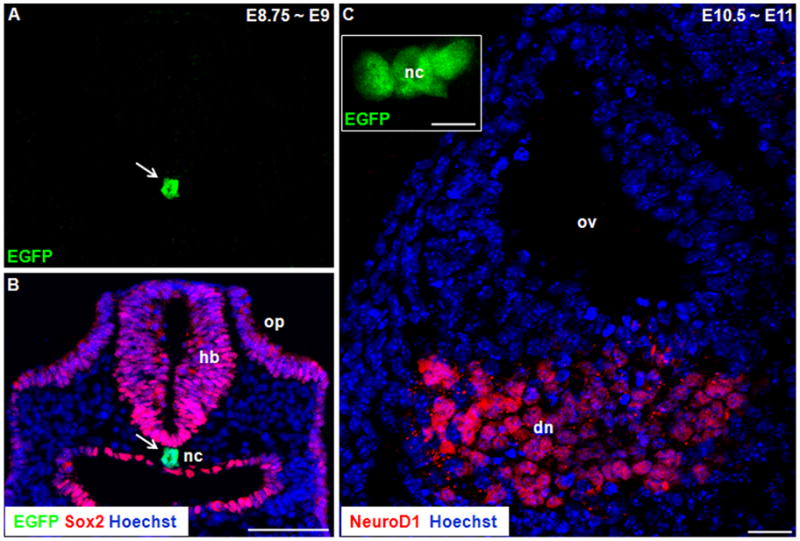
Figure 2.
Expression pattern of Shh at E13.5
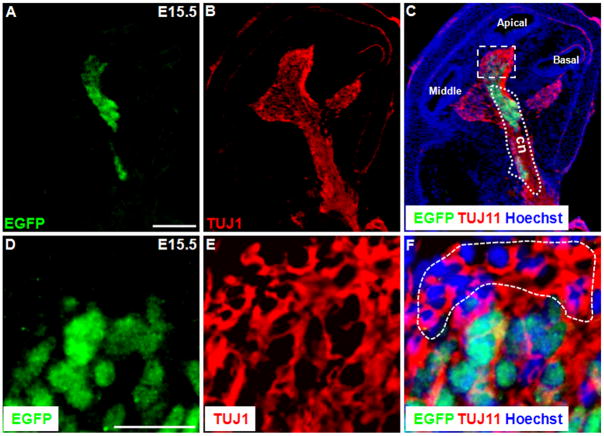
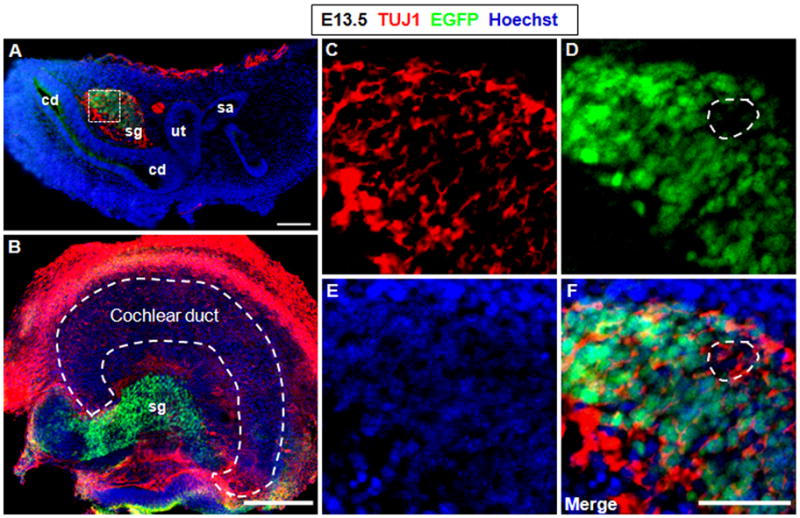
Although we did not find Shh-expressing cells by E12.5, Shh was found to be highly expressed in the spiral ganglion area at E13.5 (Fig. 3). Double immunostaining of EGFP and the widely used neuronal marker TUJ1 confirmed the Shh-expressing cells were spiral ganglion neurons (Fig. 3). Furthermore, both trans-section (Fig. 3A) and whole-mount (Fig. 3B) image analysis showed Shh in spiral ganglion neurons, irrespective of their locations. However, not all the spiral ganglion neurons were Shh-positive (Fig. 3D, F).
Figure 3.
Dynamic expression pattern of Shh at E15.5 and E16.5
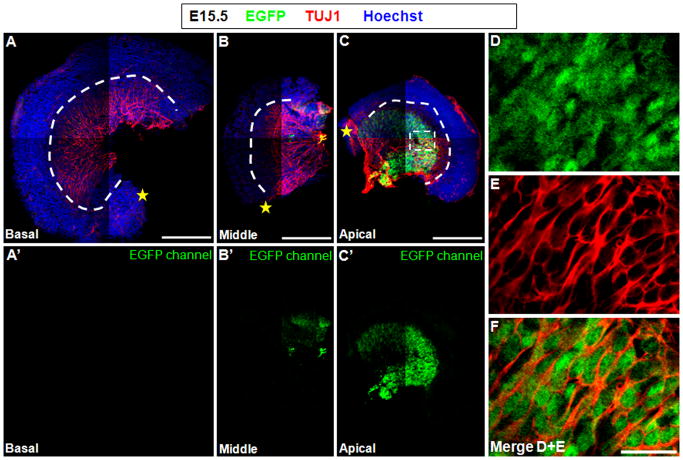
Prosensory progenitor cells start differentiation into either HCs or SCs between E14.5 and E17.5 in a basal-to-apical gradient, with HCs appearing first in basal turns and last in apical turns. We analyzed cochleae at E15.5 and E16.5 to correlate the Shh expression pattern to the differentiation pattern along the whole cochlear turns. Interestingly, at E15.5, both whole-mount (Fig. 4) and trans-section (Fig. 5) image analysis showed no Shh in the spiral ganglion neurons in the entire basal turn and the middle-basal turn, but it was expressed in the apical-middle turn and the entire apical turn. Under the same set-up of the Zeiss META 510 confocal microscope, the fluorescence signal was highest in neurons at the apex and lowest in the apical-middle turn. Additionally, neurons with high EGFP expression were found in the central cochlear nerve area (which is part of cranial nerve VIII) (Fig. 5A–C). The central cochlear nerve area is where axons of the spiral ganglion neurons aggregate, project to the brainstem, and synapse with cochlear nuclei, but that was not the focus of this study.
Figure 4.
Figure 5.
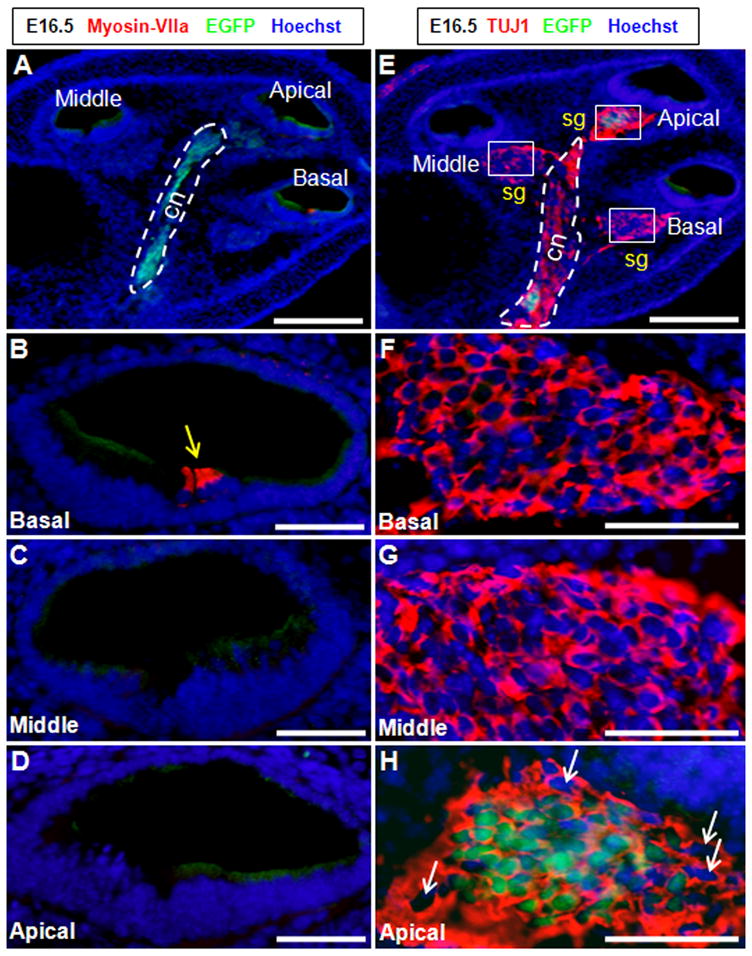
At E16.5, differentiating HCs that were myosin-VIIa-positive were identified exclusively in the basal turns (Fig. 6A–D). Similar to what was seen at E15.5, Shh expression again was found exclusively in the spiral ganglion neurons distributed in the cochlear apical turns (Fig. 6E–H) and cells in the central cochlear nerve (Fig. 6A, E). Again, at both E15.5 and E16.5, not all the spiral ganglion neurons were Shh-positive (Fig. 5F and 6H).
Figure 6.
Expression pattern of Shh at E17.5
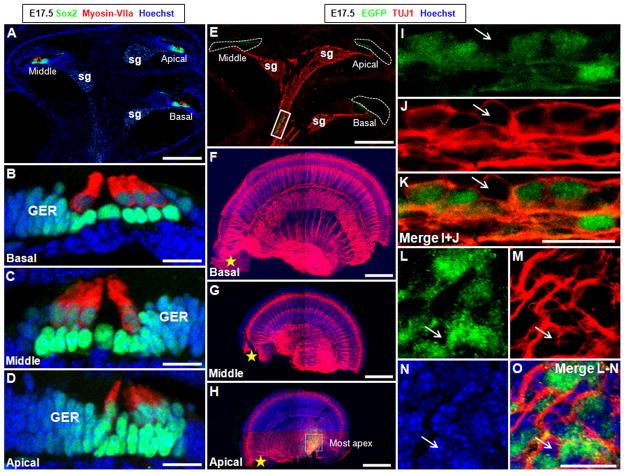
Differentiating HCs and SCs with distinct morphology and molecular markers were easily identified in cochleae at E17.5. In contrast to what was seen at E16.5, myosin-VIIa–positive HCs were found in all cochlear turns at E17.5 (Fig. 7A–D). The auditory epithelium and the greater epithelial ridge were marked with Sox2, a transcription factor indispensable to cochlear development (Kiernan et al., 2005b; Dabdoub et al., 2008). Whole-mount analysis showed Shh only in the spiral ganglion neurons at the most apical cochlear ducts (Fig. 7F–H, L–O). In addition, Shh-expressing cells still were found in the central cochlear nerve area (Fig. 7E, I–K). Taken together, these findings showed that Shh expression declined in the late embryonic ages and was not detected in spiral ganglion neurons except those in the most apical cochlear ducts.
Figure 7.
Genetic fate mapping of Shh-expressing cells
As discussed, during our analysis of the Shh expression pattern at each embryonic age, we found that not all the spiral ganglion neurons were Shh-positive. There are two possibilities we could speculate. One is that there indeed are some portions of spiral ganglion neurons that never express Shh during embryonic development. The other is that rapid dynamic changes in Shh expression occur in all neurons such that different fractions were detected at different times. To clarify this, we took advantage of the fusion protein Cre/EGFP in the heterozygous ShhCreEGFP/+ mouse. Cre-mediated permanent reporter gene expression has been widely used for genetic fate mapping analysis (Joyner and Zervas, 2006). We generated ShhCreEGFP/+; Rosa26-EYFPloxp/+ mice for analysis and ShhCreEGFP/+; Rosa26-EYFP+/+ littermates as controls. In the absence of Cre, EYFP expression is blocked by the floxed stop sequences preceding the EYFP coding frame. When the floxed stop sequences are deleted by Cre, EYFP is expressed permanently as long as the Rosa26 promoter is continuously active (Fig. 8A). Following this scheme, all Shh-expressing cells and their offspring that also express Cre can be traced by EYFP, irrespective of their temporal and spatial expression differences.
Figure 8.
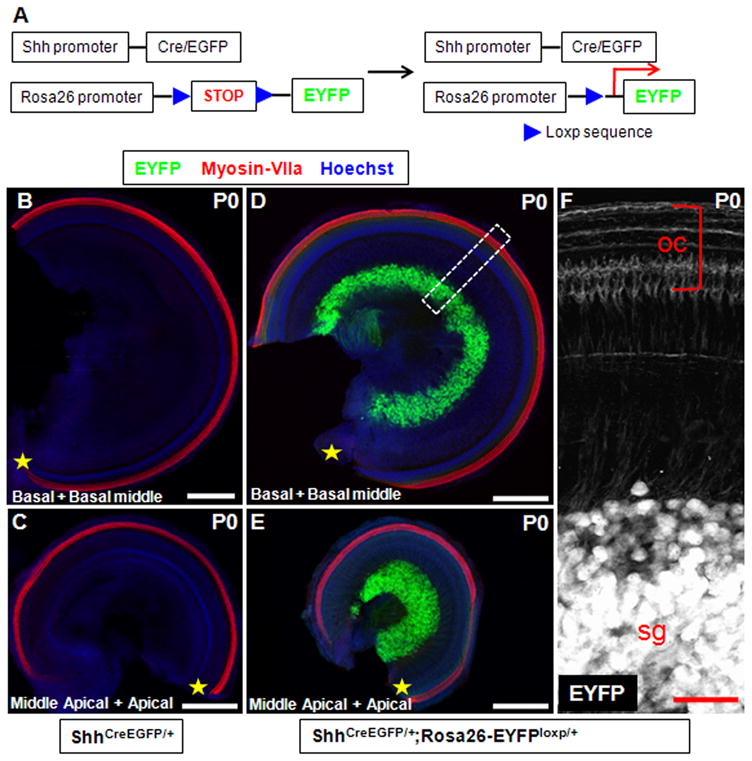
We first analyzed the control ShhCreEGFP/+; Rosa26-EYFP+/+ littermates at P0, and no fluorescent signal was seen in the whole-mount analysis (Fig. 8B, C). This showed that Shh expression was completely undetectable at P0 in spiral ganglion neurons in all cochlear turns, including the most apical. Then we analyzed the ShhCreEGFP/+; Rosa26-EYFPloxp/+ mice at P0. As expected, EYFP was highly expressed in almost 100% of the cochlear spiral ganglion neurons (Fig. 8D–F), and lower expression was detected in the peripheral cochlear nerve fibers surrounding the HCs and SCs (Fig. 8F). This clarified that all spiral ganglion neurons had expressed Shh transiently during embryonic development. Although GFP antibody can recognize both EGFP and EYFP, we concluded that all the fluorescence signals were from EYFP (induced by the Rosa26 promoter) not EGFP (driven by the Shh promoter) because no fluorescent signal was found in ShhCreEGFP/+; Rosa26-EYFP+/+ control cochleae. Moreover, an identical EYFP expression pattern was found in ShhCreEGFP/+; Rosa26-EYFPloxp/+ mice at P6 (Fig. 9A, B), demonstrating that no additional cell types expressed Shh between P0 and P6. Furthermore, double immunostaining of EYFP and TUJ1 confirmed that EYFP was expressed exclusively in the TUJ1-positive spiral ganglion neurons (large round nuclei), while none of the surrounding glia cells (small nuclei) were EYFP-positive (Fig. 9C–F). Additionally, we performed double staining of EYFP and Sox10, a glia cell marker (Davies, 2007; Puligilla et al., 2010), and we did not find any cells that were double-positive for EYFP and Sox10 (Fig. 10A–H), which confirmed that Shh was expressed in neurons but not in glia cells.
Figure 9.
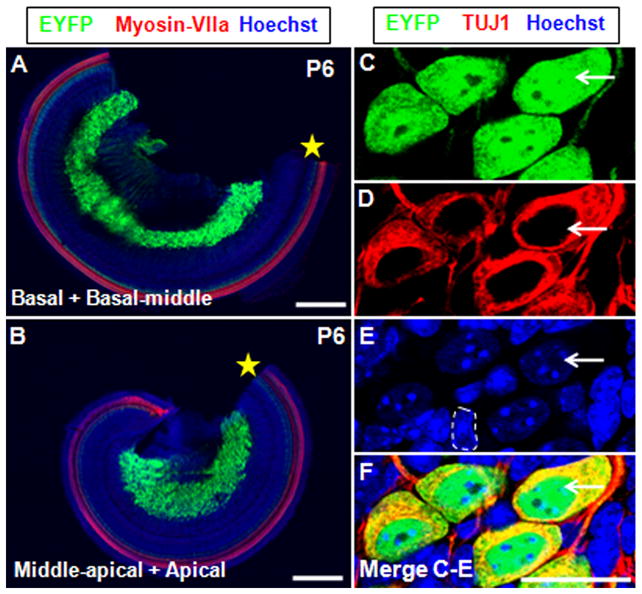
Figure 10.
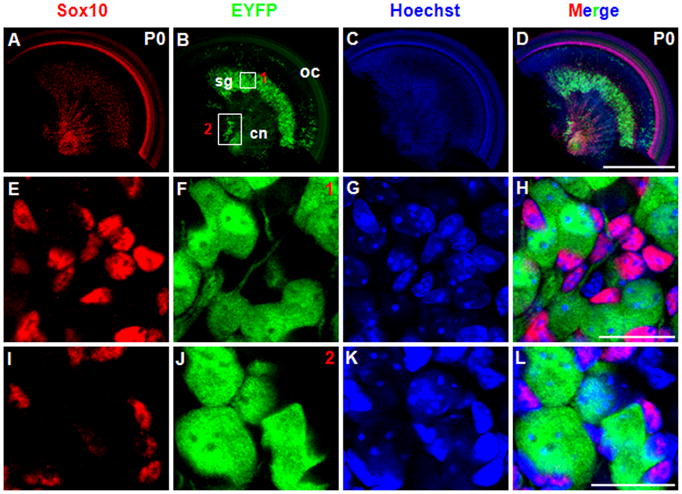
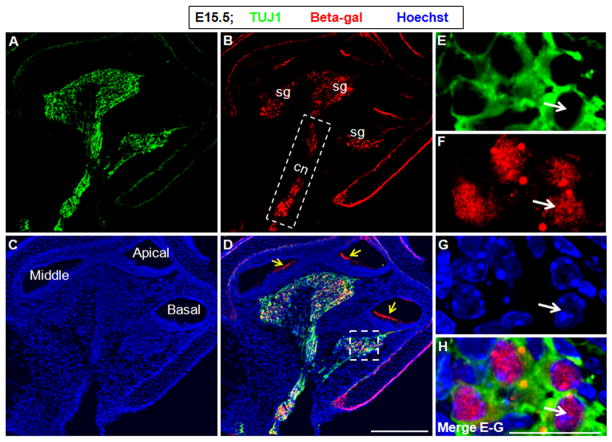
As discussed, neurons distributed in the cochlear central nerve area also expressed Shh. With cell lineage analysis, we also found EYFP-positive neurons in the cochlear central nerve area that were Sox10-negative (Fig. 10I–L). In addition, we determined the onset of Cre activity in spiral ganglion neurons in ShhCreEGFP/+; Rosa26-LacZloxp/+ mice, which can help us to distinguish EGFP and the reporter gene beta-galactosidase. We found that ~E15.5 was the earliest time expression of beta-galactosidase could be detected in TUJ1-positive neurons (Fig. 11).
Figure 11.
DISCUSSION
Shh signaling and cell cycle exit of the prosensory progenitors
Previous studies have shown that cochlear prosensory progenitor cells in apical turns exit the cell cycle first at ~E12.5, but those in the middle and basal turns continue proliferating until ~E14.5. This apical-to-basal gradient cell cycle exit is correlated with a similar apical-to-basal wave of p27Kip1 expression between E12.5 and E14.5 (Lee et al., 2006). The factors regulating p27Kip1 transcription are not clear, but the transcription is thought to be repressed by the Notch-Hes1 pathway (Murata et al., 2009). Here, we wondered whether there was a genetic interaction between Shh signaling and p27Kip1. We found that Shh is present in spiral ganglion neurons at E13.5 along the entire cochlear duct (0.75~1.0 turn). Because only basal turn progenitor cells are proliferating at E13.5, it seems to support the idea that Shh signaling might not promote proliferation of progenitor cells. However, it is also possible that Shh signaling does promote their proliferation in the basal turn cochlear duct, while that signaling is neutralized by other unknown signals present exclusively in the apical and middle cochlear duct at E13.5. This possibility is indirectly supported by the fact that Shh signaling could promote proliferation, in an Atoh1-dependent manner, of external granule cells in the cerebellum in vivo and otic progenitor cells (isolated from E9 mouse otic vesicle) in vitro (Zhao et al., 2006; Flora et al., 2009). The exact roles of Shh signaling can be delineated by targeted deletion of Shh in cochlear spiral ganglion neurons (Doris Wu, NIDCD, personal communication).
Shh signaling and differentiation of the cochlear prosensory progenitors
While cochlear prosensory progenitor cells exit the cell cycle at an apical-to-basal gradient, they start differentiation at an opposite basal-to-apical gradient. How this differentiation pattern occurs is still an enigma. In this study, we comprehensively analyzed the dynamic Shh expression pattern between E8.75 and P0. Its down-regulation in a basal-to-apical wave matches the basal-to-apical gradient differentiation pattern of the cochlear prosensory progenitor cells, highlighting the reverse correlation between Shh signaling and the intrinsic differentiation program inside prosensory progenitor cells (see a proposed working model in Fig. 12). Moreover, this opposite correlation is further supported by the fact that Shh signaling blocked HC formation when E13 cochlear explants were cultured and treated 6 days in vitro with exogenous Shh protein (Driver et al., 2008). Furthermore, the earlier down-regulation of Shh in basal turn spiral ganglion neurons is also supported by the fact that basal turn cochlear explants were less sensitive to Shh treatment than middle and apical turn explants (Driver et al., 2008).
Figure 12.
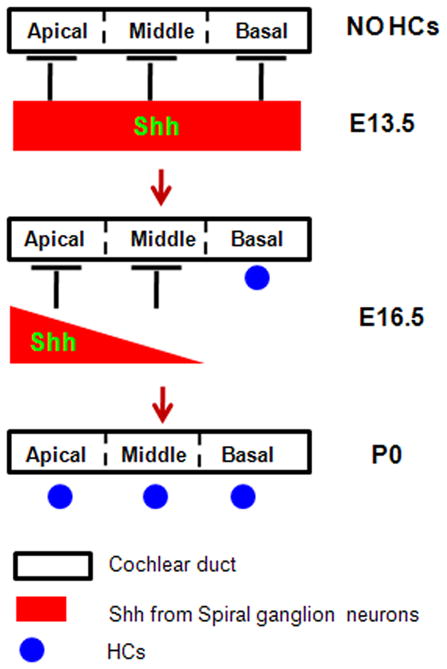
In addition, taking middle turn cochlear ducts at E16.5 as an example, Shh was undetectable in spiral ganglion neurons. However, myosin-VIIa–positive HCs were absent in the prosensory area, indicating the differentiation program had not been initiated yet in the progenitor cells. This shows that Shh signaling should be turned off before progenitors start to differentiate. Nonetheless, there is no direct evidence that Shh signaling does prevent premature differentiation of progenitor cells. It would be strong supportive evidence if Shh signaling could be specifically blocked (such as by deleting the smoothened gene) in the sensory epithelium of the cochlear apical turns between E14.5 and E16.5 and progenitor cells were able to start premature differentiation. Alternatively, the deletion of Shh in spiral ganglion neurons can also test the correlation between Shh signaling and hair cell differentiation.
Potential applications of ShhCreEGFP/+ Cre mouse line in spiral ganglion neurons
Since its first use in studying limb patterning and development (Harfe et al., 2004), the ShhCreEGFP/+ Cre mouse line has been widely used in a variety of biological processes (http://jaxmice.jax.org/strain/005622.html). Our genetic mapping analysis has shown the robust Cre activity of the fusion protein Cre/EGFP, as almost 100% of the cochlear spiral ganglion neurons, but not surrounding glia cells, were traced by EYFP at P0 and P6 in the ShhCreEGFP/+; Rosa26-EYFPloxp/+ mice, and beta-galactosidase at ~E15.5 in the ShhCreEGFP/+; Rosa26-LacZloxp/+ mice. Given the availability of many mutant mouse lines in which the interested genes are floxed, the efficient Cre activity allows ShhCreEGFP/+ to be widely used to study functions of a variety of floxed genes in spiral ganglion neurons, especially for those whose complete loss results in embryonic fatality that precludes further analysis of their functions at later ages.
EXPERIMENTAL PROCEDURES
Mouse strain maintenance
ShhCreEGFP/+ (stock number: 005622), Rosa26-EYFPloxp/+ (stock number: 006148) and Rosa26-LacZloxp/+ mouse (stock number: 003310) strains were purchased from The Jackson Laboratory (Bar Harbor, MN, USA). For genetic fate mapping analysis, male ShhCreEGFP/+ were crossed with female Rosa26-EYFPloxp/+ to get the ShhCreEGFP/+; Rosa26-EYFPloxp/+ mice. This breeding strategy was recommended by The Jackson Laboratory. All animal work conducted during the course of this study was approved by the Institutional Animal Care and Use Committee at St. Jude Children’s Research Hospital and was performed according to NIH guidelines.
Embryonic age determination
Heterozygous male ShhCreEGFP/+ mice were crossed with female Shh+/+ mice, or male ShhCreEGFP/+ mice were crossed with female Rosa26-LacZloxp/+ mice, at 5 pm, and the vaginal plug was checked at 7 am the next day. Given the presence of plugs, that morning was designated as E0.5 for the embryonic age. To guarantee the accuracy of the embryonic age determination and to avoid false-negative outcomes from plug checking, only the females that were plugged after one night of breeding were used in our current study.
Histology and immunofluorescence
The whole embryos at various ages were immersed in 4% paraformaldehyde overnight at 4°C, after which either the whole embryos (prior to E12.5) were analyzed together or the inner ear was carefully dissected out first (after E13.5). The inner ear was then immersed in 4% paraformaldehyde for 3 hours at 4°C. For trans-section analysis, the whole embryo or inner ear was immersed in 30% sucrose overnight at 4°C and then embedded in Optimum Cutting Temperature compound, frozen in dry ice, and cut into sections 12 μm thick. For whole-mount analysis, the whole cochlear duct and corresponding medial spiral ganglion tissues were divided into three parts, the basal, middle, and apical turns, with the exception that the E13.5 cochlear duct was maintained as a whole.
Both whole mounts and trans-sections were permeabilized and blocked at room temperature for 1 hour in solutions containing 1% bovine serum albumin and 1% Triton X-100 in 10 mM phosphate-buffered saline (PBS, pH 7.4). Tissues were then incubated with primary antibodies in blocking solution (1% bovine serum albumin and 0.1% Triton X-100 in 10 mM PBS) overnight at 4°C, followed by 3 washes for 10 minutes each in 10 mM PBS. Then, tissues were incubated with secondary antibodies in the same blocking solution overnight at 4°C, followed by 3 washes for 10 minutes each in 10 mM PBS. Tissues were then incubated for 30 minutes at room temperature in Hoechst 33342 in 10 mM PBS (Invitrogen, H3570, 1:1,000), followed by 3 washes for 10 minutes each in 10 mM PBS, and finally were mounted in ProLong Gold antifade reagent (Invitrogen, P36934). Samples were dried at room temperature for at least 24 hours. All whole-mount samples were analyzed with a Zeiss META 510 confocal microscope. Trans-section samples at E17.5 were analyzed with the Zeiss META 510 confocal microscope, and those at other embryonic ages were analyzed with our regular fluorescence microscope.
The following primary antibodies were used: anti-myosin-VIIa (rabbit, 1:200, Proteus Bioscience, 25-6790), anti-GFP (chicken, 1:1000, Abcam, ab13970), anti-Sox10 (goat, 1:250, Santa Cruz Biotechnology, sc-17342), anti-NeuroD1 (goat, 1:100, Santa Cruz Biotechnology, sc-1084), anti-Sox2 (goat, 1:1000, Santa Cruz Biotechnology, sc-17320), anti-beta-galactosidase (rabbit, 1:500, ICN, 55976), and anti-TUJ1 (mouse, 1:1000, MMS-435P, Covance). The following secondary antibodies from Invitrogen Company were used: donkey anti-rabbit Alexa Fluor 647 (1:1000, A-31573), donkey anti-goat Alexa Fluor 568 (1:1000, A11057), goat anti-chicken Alexa Fluor 488 (1:1000, A11039), goat anti-mouse Alexa Fluor 568 (1:1000, A11031), and goat anti-rabbit Alexa Fluor 568 (1:1000, A11036).
Acknowledgments
Grants: National Institutes of Health: DC06471, DC05168, DC008800, and CA21765; Office of Naval Research: N000140911014; the American Lebanese Syrian Associated Charities (ALSAC) of St. Jude Children’s Research Hospital; The Hartwell Individual Biomedical Research Award.
We thank J. Woods and D. Wash for determining the embryonic ages, S. Connell, L. Zhang, J. Peters, and Y. Ouyang for expertise in confocal imaging and D. Wu at NIDCD for discussion. This work was supported in part by grants from the National Institutes of Health (DC06471, DC05168, DC008800, and CA21765), Office of Naval Research (N000140911014), and the American Lebanese Syrian Associated Charities (ALSAC) of St. Jude Children’s Research Hospital. J. Zuo is a recipient of The Hartwell Individual Biomedical Research Award.
References
- Bok J, Bronner-Fraser M, Wu DK. Role of the hindbrain in dorsoventral but not anteroposterior axial specification of the inner ear. Development. 2005;132:2115–2124. doi: 10.1242/dev.01796. [DOI] [PubMed] [Google Scholar]
- Bok J, Dolson DK, Hill P, Ruther U, Epstein DJ, Wu DK. Opposing gradients of Gli repressor and activators mediate Shh signaling along the dorsoventral axis of the inner ear. Development. 2007;134:1713–1722. doi: 10.1242/dev.000760. [DOI] [PubMed] [Google Scholar]
- Bose J, Grotewold L, Ruther U. Pallister-Hall syndrome phenotype in mice mutant for Gli3. Hum Mol Genet. 2002;11:1129–1135. doi: 10.1093/hmg/11.9.1129. [DOI] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–397. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci U S A. 2008;105:18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. Temporal and spatial regulation of alpha6 integrin expression during the development of the cochlear-vestibular ganglion. J Comp Neurol. 2007;502:673–682. doi: 10.1002/cne.21302. [DOI] [PubMed] [Google Scholar]
- Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell. 2009;16:58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver EC, Pryor SP, Hill P, Turner J, Ruther U, Biesecker LG, Griffith AJ, Kelley MW. Hedgehog signaling regulates sensory cell formation and auditory function in mice and humans. J Neurosci. 2008;28:7350–7358. doi: 10.1523/JNEUROSCI.0312-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora A, Klisch TJ, Schuster G, Zoghbi HY. Deletion of Atoh1 disrupts Sonic Hedgehog signaling in the developing cerebellum and prevents medulloblastoma. Science. 2009;326:1424–1427. doi: 10.1126/science.1181453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Hatch EP, Urness LD, Mansour SL. Fgf16(IRESCre) mice: a tool to inactivate genes expressed in inner ear cristae and spiral prominence epithelium. Dev Dyn. 2009;238:358–366. doi: 10.1002/dvdy.21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Cunningham D, Bermingham-McDonogh O. Loss of Fgfr3 leads to excess hair cell development in the mouse organ of Corti. Dev Dyn. 2007;236:525–533. doi: 10.1002/dvdy.21026. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Ray CA, Bermingham-McDonogh O. Fgf20 is required for sensory epithelial specification in the developing cochlea. J Neurosci. 2008;28:5991–5999. doi: 10.1523/JNEUROSCI.1690-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner AL, Zervas M. Genetic inducible fate mapping in mouse: establishing genetic lineages and defining genetic neuroanatomy in the nervous system. Dev Dyn. 2006;235:2376–2385. doi: 10.1002/dvdy.20884. [DOI] [PubMed] [Google Scholar]
- Kanzaki S, Beyer LA, Swiderski DL, Izumikawa M, Stover T, Kawamoto K, Raphael Y. p27(Kip1) deficiency causes organ of Corti pathology and hearing loss. Hear Res. 2006;214:28–36. doi: 10.1016/j.heares.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–849. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005a;132:4353–4362. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005b;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- Lee YS, Liu F, Segil N. A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development. 2006;133:2817–2826. doi: 10.1242/dev.02453. [DOI] [PubMed] [Google Scholar]
- Li S, Mark S, Radde-Gallwitz K, Schlisner R, Chin MT, Chen P. Hey2 functions in parallel with Hes1 and Hes5 for mammalian auditory sensory organ development. BMC Dev Biol. 2008;8:20. doi: 10.1186/1471-213X-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Li G, Chien JS, Raft S, Zhang H, Chiang C, Frenz DA. Sonic hedgehog regulates otic capsule chondrogenesis and inner ear development in the mouse embryo. Dev Biol. 2002;248:240–250. doi: 10.1006/dbio.2002.0733. [DOI] [PubMed] [Google Scholar]
- Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, Hackney CM, Zenner HP. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc Natl Acad Sci U S A. 1999;96:4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller KL, Jacques BE, Kelley MW. Fibroblast growth factor signaling regulates pillar cell development in the organ of corti. J Neurosci. 2002;22:9368–9377. doi: 10.1523/JNEUROSCI.22-21-09368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata J, Ohtsuka T, Tokunaga A, Nishiike S, Inohara H, Okano H, Kageyama R. Notch-Hes1 pathway contributes to the cochlear prosensory formation potentially through the transcriptional down-regulation of p27Kip1. J Neurosci Res. 2009;87:3521–3534. doi: 10.1002/jnr.22169. [DOI] [PubMed] [Google Scholar]
- Noden DMvdT. The developing ear: tissue origins and interactions. In: Ruben RJ, van de Water TR, Rubel EW, editors. The Biology of Change in Otolaryngology. 1986. pp. 15–46. [Google Scholar]
- Pirvola U, Ylikoski J, Trokovic R, Hebert JM, McConnell SK, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671–680. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Puligilla C, Dabdoub A, Brenowitz SD, Kelley MW. Sox2 induces neuronal formation in the developing mammalian cochlea. J Neurosci. 2010;30:714–722. doi: 10.1523/JNEUROSCI.3852-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puligilla C, Feng F, Ishikawa K, Bertuzzi S, Dabdoub A, Griffith AJ, Fritzsch B, Kelley MW. Disruption of fibroblast growth factor receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Dev Dyn. 2007;236:1905–1917. doi: 10.1002/dvdy.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccomagno MM, Martinu L, Mulheisen M, Wu DK, Epstein DJ. Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 2002;16:2365–2378. doi: 10.1101/gad.1013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccomagno MM, Takada S, Epstein DJ. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 2005;19:1612–1623. doi: 10.1101/gad.1303905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol:Suppl. 1967;220:221–244. [PubMed] [Google Scholar]
- Takebayashi S, Yamamoto N, Yabe D, Fukuda H, Kojima K, Ito J, Honjo T. Multiple roles of Notch signaling in cochlear development. Dev Biol. 2007;307:165–178. doi: 10.1016/j.ydbio.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Tang LS, Alger HM, Pereira FA. COUP-TFI controls Notch regulation of hair cell and support cell differentiation. Development. 2006;133:3683–3693. doi: 10.1242/dev.02536. [DOI] [PubMed] [Google Scholar]
- Wijgerde M, McMahon JA, Rule M, McMahon AP. A direct requirement for Hedgehog signaling for normal specification of all ventral progenitor domains in the presumptive mammalian spinal cord. Genes Dev. 2002;16:2849–2864. doi: 10.1101/gad.1025702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wang Y, Wang Z, Liu H, Shen Y, Li W, Heller S, Li H. Sonic hedgehog promotes mouse inner ear progenitor cell proliferation and hair cell generation in vitro. Neuroreport. 2006;17:121–124. doi: 10.1097/01.wnr.0000198439.44636.49. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Shou J, Guillemot F, Kageyama R, Gao WQ. Hes1 is a negative regulator of inner ear hair cell differentiation. Development. 2000;127:4551–4560. doi: 10.1242/dev.127.21.4551. [DOI] [PubMed] [Google Scholar]
- Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci. 2001;21:4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]