The purpose of this report is to describe some unusual manifestations that followed a very extensive hepatectomy (85 to 90 per cent) and to comment about the less than complete degree of regeneration that occurred postoperatively.
Case Report
A nineteen year old girl had undergone exploration one week prior to admission because of a huge mass that occupied all of the upper abdomen and which, as indicated by liver scan (Figure 1, left), replaced much of the liver. Tissue diagnosis of a well differentiated hepatoma was made from the previous exploration. Measurement of alpha fetoprotein was negative. For some months she had had severe abdominal pain that was eventually demonstrated to be due to hemorrhage into the tumor. A selective celiac arteriogram demonstrated a single hepatic artery. (Figure 1, right.) Although the entire arterial blood supply appeared to be involved in tumor, an attempt at resection was decided on.
Figure 1.
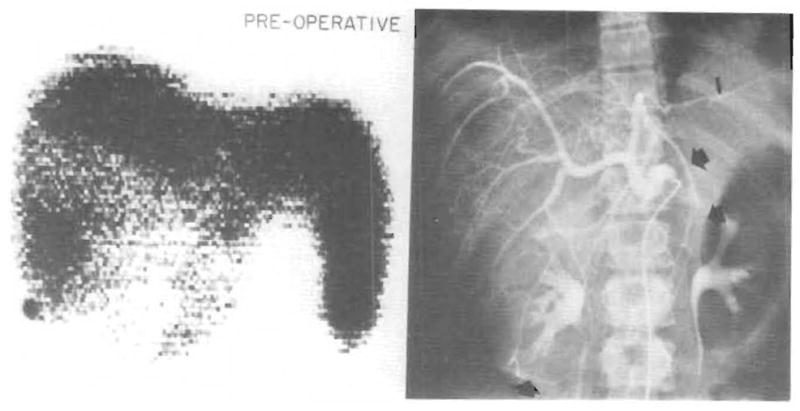
Preoperative radiographic studies. Left, 99m technetium liver scan (anteroposterior projection) showing the very large filling detect. Right, selective common hepatlc arteriogram. The broad arrows Indicate the extent of the tumor as outlined by the abnormal configuration of vessels. The thin arrow points to the dorsolateral branch of the left hepatic artery. This vessel, supplying the lateral segment of the left lobe, was the only hepatic arterial branch preserved.
The procedure was carried out May 10, 1972, through a bilateral subcostal incision. The right hepatic artery, portal vein, and hepatic duct were ligated and divided. Branches of the left main hilar structures passing to the medial segment of the left lobe were cut, leaving only the lateral segment connected. The right and middle hepatic veins and all the small hepatic veins entering the inferior vena cava from the caudate lobe were sacrificed so that only the left hepatic vein remained. The lateral segment of the left lobe was smaller than usual, constituting approximately 15 per cent of the normal liver mass. Part of this segment had to be excised to remove the tumor, which extended to the left of the falciform ligament. The remaining hepatic fragment was thought to represent between 10 and 15 per cent of a normal liver. The excised specimen weighed 2,100 gm and had areas of hemorrhage within the tumor. It was examined histologically in five university departments of pathology noted for their special interest in liver disease. All consultants described the well-differentiated features of the neoplasm. Two diagnoses of hepatocellular adenoma were made, but three of the consultants classified the lesion as a hepatoma (hepatocarcinoma).
After resection was completed, the residual fragment felt tense, a condition that was aggravated by efforts to cover the raw liver edge with the falciform ligament or omentum. Consequently, the raw surface was left open. The large right subphrenic cavity was extensively drained. The operation required nine hours and 5,500 ml of blood. Postoperative ventilatory support was provided for the first five hours. The patient was completely disoriented for three days but then suddenly became lucid.
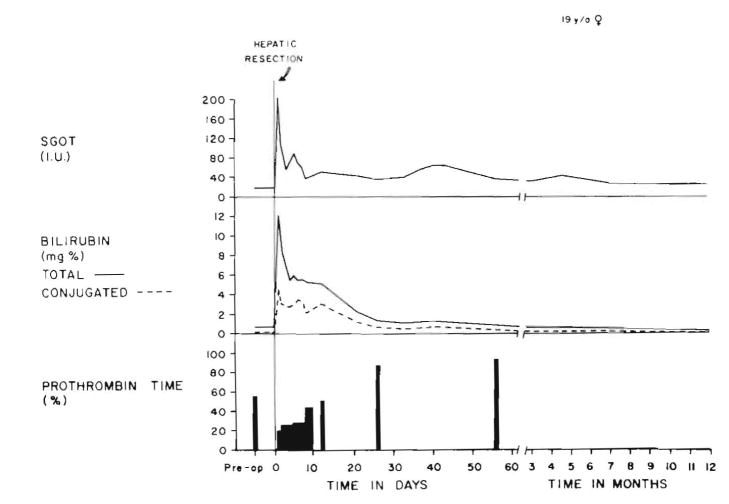
Serious abnormalities in liver function, including jaundice, did not clear until the third week. (Figure 2) Albumin, totaling 250 gm, was administered during the first five days, which maintained the serum albumin concentration at greater than 3.0 gm/100 ml. The patient had a continuous leak of ascitic fluid from the drain sites. Measurements of abdominal girth also indicated retained ascitic material. Within a few days, the patient’s hair began to fall out, and she soon became nearly bald. Chemotherapy, never administered to the patient, was not an explanation for the alopecia. The patient had daily fevers, usually low grade, that lasted two months with or without antibiotics.
Figure 2.
Postoperative course. The hyperbilirubinemia and the depressed prothrombin time returned to normal in about a month.
The 99m technetium liver scans taken postoperatively are shown in Figure 3. At first, obvious splenomegaly indicated that the tiny liver fragment permitted only a borderline state of portal venous drainage. At one and six months postoperatively, substantial but incomplete regeneration had occurred. (Figure 3) Further regeneration was not evident at thirteen months postoperatively.
Figure 3.
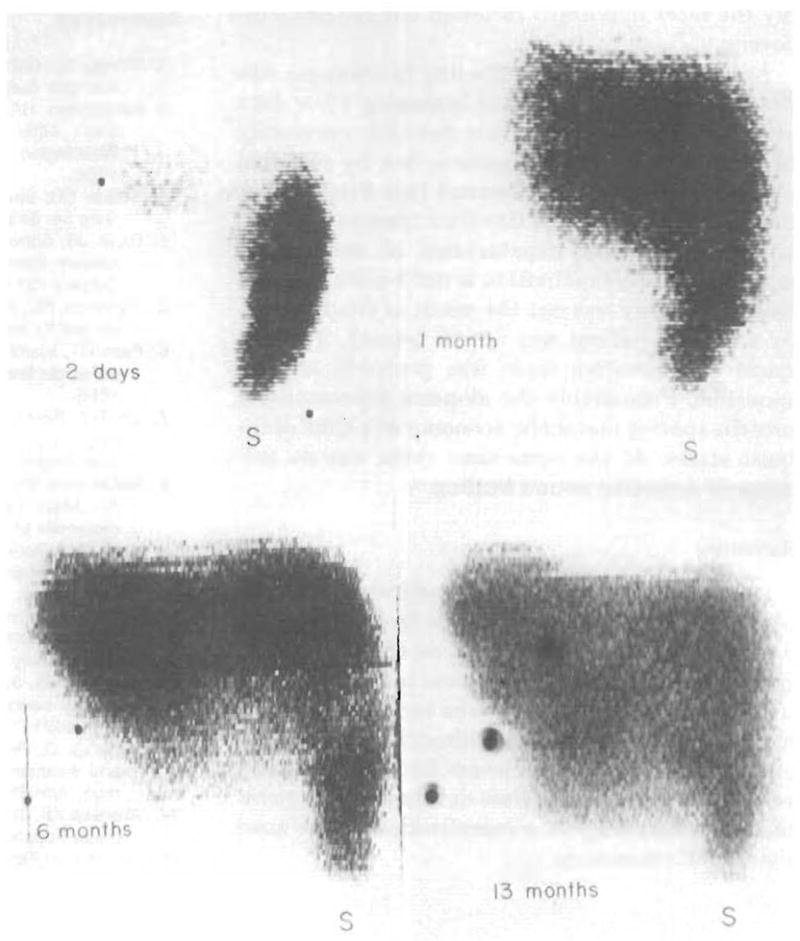
Serial postoperative 99m technetium liver scans. Two days, only a tiny fragment of the liver is visualized; the spleen (S) has enlarged in comparison with the pre-operative study. One month, regeneration has begun. Six months, further regeneration has occurred. Thirteen months, the hepatic mass has increased slightly in the intervening seven months, but regeneration remains incomplete.
The ascites was treated with hydrochlorothiazide (HydroDiuril®) and spironolactone (Aldactone®) for eight months and did not recur. Nine months were required for regrowth of hair. After a follow-up period of twenty months, the patient had quite normal liver function and no evidence of recurrent tumor. Her weight was 125 pounds, 12 pounds greater than her preoperative weight. Her menstrual periods, which had stopped for a long time after operation, returned.
Comments
Among hepatic neoplasms, the type of tumor found in this patient is recognized for its occasional huge size, slow natural history, and tendency not to metastasize. These characteristics have led some authorities [1,2] to call it benign hepatoma or adenoma. Hemorrhage into the tumor as occurred in this patient has been reported [3–5].
Appreciation of these favorable prognostic features prompted our aggressive attitude toward resection. The extent of hepatic resection in this patient apparently reached nearly the absolute limit compatible with survival. The diminutive residual liver fragment could barely transmit the portal flow, resulting in splenomegaly and probably contributing to the ascites that persisted for months afterward. The degree of regeneration that followed was not complete more than a year later and probably never will be.
To our knowledge, only a few patients with such extensive hepatic resection have survived. The surviving patients are usually reported as having undergone “extended right hepatic lobectomy,” a term introduced by Pack et al [6]. As summarized by Lin [7], most reported series of partial hepatectomies have included very few or no such procedures; Lin reported none in his personal series of 107 hepatic resections.
The reported amount of tissue remaining after extended right hepatic lobectomy is not easily quantifiable and is almost exclusively dependent upon the estimate of the surgeon, which could vary from surgeon to surgeon. In most patients, the residual tissue in a full lateral segment would be approximately 20 per cent. However, there seems little doubt that the remnants left in the cases of McDermott et al [8], Stahl [9], and Smith [10] were smaller than this. Whatever the size of the fragment, almost all authorities claim that full restitution of liver mass occurs by regeneration. Only Stahl [9] has described the kind of incomplete recovery observed in our patient.
Despite the hazardous metabolic state of our patient during the first postoperative days and weeks, recovery was not marred by any serious extrahepatic complications. The cyclic changes in basic liver function were similar to those described by others after major hepatic resection [8,9,11–14]. Prompt resumption of a healthful diet was probably the most important factor in our patient’s recovery.
A unique postoperative finding in this case was the development of baldness beginning a few days after hepatic resection. This has not previously been described in the literature, but by personal communication we have learned that Fitts [15] at the University of South Carolina observed a similar condition after hepatectomy of comparable magnitude. The explanation is not known, but the baldness clearly was not the result of chemotherapy since the patient was not so treated. The low grade postoperative fever was probably not responsible. Presumably the alopecia represented a protein-sparing metabolic economy at a time of intense stress. At the same time there was no evidence of defective wound healing.
Summary
A nineteen year old female underwent 85 to 90 per cent partial hepatectomy to treat a minimal deviation hepatoma. Observations afterwards suggested that the limit of resection compatible with survival had been reached. She recovered perfect health after many months, although liver regeneration was not complete. Severe but eventually reversible alopecia and ascites developed postoperatively, undoubtedly as a complication of the massive hepatic resection.
Acknowledgments
This work was supported by the Veterans Administration Hospital. Denver. Colorado; by grants AI–AM-08898 and AM–01772 from the National Institutes of Health; and by grants RR–00051 and RR–00069 from the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health.
References
- 1.Motsay GJ, Gamble WG. Clinical experience with hepatic adenomas. Surg Gynecol Obstet. 1972;134:415. [PubMed] [Google Scholar]
- 2.Edmonston HA. Atlas of Tumor Pathology. fascicle 25. section VII. Washington, DC: Armed Forces Institute of Pathology; 1958. Tumors of the liver and intrahepatic bile ducts. [Google Scholar]
- 3.Scorer CG. Spontaneous rupture of hepatic adenoma. Br J Surg. 1969;56:633. doi: 10.1002/bjs.1800560818. [DOI] [PubMed] [Google Scholar]
- 4.Davis JB, Schenken JR, Zimmerman O. Massive hemoperitoneum from rupture of benign hepatocellular adenoma. Surgery. 1973;73:181. [PubMed] [Google Scholar]
- 5.Hermann RE, David TE. Spontaneous rupture of the liver caused by hepatomas. Surgery. 1973;74:715. [PubMed] [Google Scholar]
- 6.Pack GT, Islami AH, Hubbard JC, Brasfield RD. Regeneration of human liver after major hepatectomy. Surgery. 1962;52:617. [PubMed] [Google Scholar]
- 7.Lin T-Y. Results in 107 hepatic lobectomies with a preliminary report on the use of a clamp to reduce blood loss. Ann Surg. 1973;177:413. doi: 10.1097/00000658-197304000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott WV, Jr, Greenberger NJ, Isselbacher KJ, Weber AL. Major hepatic resection: diagnostic techniques and metabolic problems. Surgery. 1963;54:56. [Google Scholar]
- 9.Stahl WM. Radical right hepatic lobectomy for hepatoblastoma in infancy. Five year survival. NY State J Med. 1972;72:2083. [PubMed] [Google Scholar]
- 10.Smith R. Liver resection. J R Coll Surg Edinb. 1972;17:154. [PubMed] [Google Scholar]
- 11.Pack GT, Molander DW. Metabolism before and after hepatic lobectomy for cancer. Arch Surg. 1960;80:685. doi: 10.1001/archsurg.1960.01290210153030. [DOI] [PubMed] [Google Scholar]
- 12.Pinkerton JA, Sawyers JL, Foster JH. A study of the postoperative course after hepatic lobectomy. Ann Surg. 1971;173:800. doi: 10.1097/00000658-197105000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almersjö O, Bengmark S, Hafstrom LO, Olsson R. Enzyme and function changes after extensive liver resection in man. Ann Surg. 1969;169:111. doi: 10.1097/00000658-196901000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aronsen KF, Ericsson B, Pihl B. Metabolic changes following major hepatic resection. Ann Surg. 1969;169:102. doi: 10.1097/00000658-196901000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitts WT., Jr Personal communication. Nov 3, 1972.