Abstract
Objective
Pentraxin 3 (PTX3) is a soluble pattern recognition receptor that has an important role in immunoregulation and vascular integrity. The aim of this study was to determine if PTX3 is present in amniotic fluid (AF) and if its concentration changes with gestational age, in the presence of labor, and in cases of intra-amniotic infection/inflammation (IAI) associated with spontaneous preterm labor (PTL) or preterm prelabor rupture of membranes (PPROM).
Study design
This was a cross-sectional study which included the following groups: 1) mid-trimester (n=45); 2) uncomplicated pregnancies at term with (n=48) and without (n=40) spontaneous labor; 3) women with PTL and intact membranes: a) who delivered at term (n=44); b) who delivered preterm without IAI (n=40); and c) who delivered preterm with IAI (n=62); 4) women with PPROM with (n=63) and without (n=36) IAI. Pentraxin-3 concentration in AF was determined by ELISA. Non-parametric statistics were used for analyses.
Results
1) Among women in preterm labor with intact membranes, the median AF PTX3 concentration was significantly higher in women with IAI than in those without IAI (7.95 ng/mL vs. 0.38 ng/mL; p<0.001) and than in those who delivered at term (0.55 ng/mL; p<0.001); 2) women with PPROM and IAI had a higher median amniotic fluid PTX3 concentration than those without IAI (9.12 ng/mL vs. 0.76 ng/mL; p<0.001); 3) the median AF PTX3 concentration did not change with gestational age (mid-trimester: 0.79 ng/mL vs. term not in labor: 0.58 ng/mL; p=0.09); and 4) among women at term, no significant differences were observed in the median AF PTX3 concentration between women with spontaneous labor and those not in labor (0.54 ng/mL vs. 0.58 ng/mL, respectively; p=0.9).
Conclusions
PTX3 is a physiologic constituent of the AF, and its concentration is elevated in the presence of IAI, suggesting that PTX3 may play a role in the innate immune response against intra-amniotic infection.
Keywords: preterm labor, preterm delivery, preterm prelabor rupture of membranes, PPROM, pregnancy, amniocentesis, microbial invasion of the amniotic cavity, MIAC, cytokines, pattern recognition receptors
Introduction
Preterm labor (PTL) is a syndrome [119], and one of the most important mechanism of disease is intrauterine infection, the only pathological process for which a causal link with prematurity has been established [18, 36, 46, 47, 49, 61, 75, 77, 88, 91, 128, 129, 131, 134, 135]. Intra-amniotic infection and/or inflammation (IAI) is present in about one third of women with spontaneous PTL with intact membranes [133, 168] and is associated with the development of the fetal inflammatory response syndrome (FIRS) [48, 126], and severe neonatal morbidity [7, 24, 25, 45, 57, 80, 101, 164-167, 169].
Several investigators [5, 40, 87, 152, 155-157] have reported on the antimicrobial activity of components of the amniotic fluid (AF), which are involved in the innate and adaptative immune response against microorganisms. The innate component of the immune system represents the first line of defense against infection and includes a wide range of non-specific mechanisms [32, 35, 55, 59, 60, 73, 125, 150, 153]. One of the mechanisms by which the innate immune system recognizes microorganisms is mediated through pattern recognition receptors (PRRs) [66], which bind to surface markers on microorganisms [58, 100, 124].
Pentraxins are essential components of the humoral arm of the innate immune response and act as soluble PRRs [12, 41] in response to pro-inflammatory signals and Toll-like receptors (TLRs) activation [4, 8, 103, 171]. Pentraxin 3 (PTX3) is produced and released by a variety of cell types such as mononuclear cells, phagocytes, dendritic cells, fibroblasts, and endothelial cells [1, 3, 17, 30, 51, 64, 74, 79, 106]. PTX3 recognizes microbial products, opsonizes fungi, selected Gram-positive and Gram-negative bacteria, viruses, and activates complement [12], and it is considered an acute phase response protein, because its concentrations increase considerably and rapidly in plasma of patients with systemic inflammatory response syndrome, sepsis, or septic shock [89]. Thus, the objective of this study was to determine if PTX3 is present in AF, if its concentration changes with gestational age, spontaneous labor at term, and in the presence of IAI in women with spontaneous PTL with intact membranes and in those with preterm prelabor rupture of the membranes (PPROM).
Materials and Methods
Study design and population
A cross-sectional study was carried out by searching our clinical database and bank of biological samples, and included 378 pregnant women in the following groups: 1) Women at 14-18 weeks gestation whose amniocentesis was conducted for genetic indications (n=45) and who subsequently had an uncomplicated pregnancy; 2) Uncomplicated term pregnancies with (n=48) and without (n=40) spontaneous labor; 3) Women with PTL and intact membranes without IAI who delivered at term (n=44); without IAI who delivered preterm (n=40); and with IAI (n=62); and 4) Women with PPROM with (n=63) and without IAI (n=36).
All women provided written informed consent prior to the collection of AF. The collection and utilization of AF for research purposes was approved by the Institutional Review Boards of the participating institutions and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. Many of these samples have been used previously to study the biology of inflammation, hemostasis, and growth factor concentrations in uncomplicated pregnancies and those with adverse pregnancy outcomes.
Definitions
Women were considered to have an uncomplicated pregnancy if they did not have any medical, obstetrical, or surgical complication, and delivered a normal neonate at term, which was appropriately grown for gestational age [2, 50]. Spontaneous PTL was defined as the presence of regular uterine contractions occurring at a frequency of at least two every 10 minutes associated with cervical change that required hospitalization before 37 completed weeks of gestation. PPROM was diagnosed by sterile speculum examination which confirmed pooling of AF in the vagina in association with nitrazine and ferning tests when necessary, before 37 weeks of gestation and prior to labor. Intra-amniotic infection was defined as a positive AF culture for micro-organisms. Intra-amniotic inflammation was diagnosed by an AF interleukin (IL)-6 concentration ≥2.6 ng/mL[168]. Histologic chorioamnionitis was diagnosed on the basis of inflammatory cells in the chorionic plate and/or chorioamniotic membranes [110]. Acute funisitis was diagnosed by the presence of neutrophils in the wall of the umbilical vessels and/or Wharton's jelly using criteria previously described [99].
Sample collection
The AF samples were obtained by transabdominal amniocentesis for genetic indications, for evaluation of microbial status of the amniotic cavity and/or for assessment of fetal lung maturity in women approaching term in whom the dates were uncertain. For these women to be considered as term, the following criteria had to be fulfilled: 1) analysis of AF consistent with maturity; 2) birthweight >2500g; 3) absence of respiratory distress syndrome or other complications of prematurity; and 4) pediatric neonatal examination consistent with a term neonate. Samples of AF were transported to the laboratory in a sterile capped syringe and cultured for aerobic/anaerobic bacteria and genital mycoplasmas. White blood cell (WBC) count, glucose concentration and Gram-stain were also performed shortly after collection as previously described [122, 127, 132]. The results of these tests were used for clinical management. AF not required for clinical assessment was centrifuged for 10 minutes at 4°C, and the supernatant was aliquoted and stored at −70°C until analysis. The AF IL-6 concentrations were used only for research purposes. Among women with spontaneous preterm labor with intact membranes who delivered within 72 hours of amniocentesis, placenta, umbilical cord, and chorioamniotic membranes were collected, and the presence or absence of histologic chorioamnionitis and/or funisitis was assessed. The 72 hour interval was chosen to preserve a meaningful temporal relationship between AF PTX 3 concentration and placental histopathologic findings.
Determination of human PTX3 concentration in amniotic fluid
Specific and sensitive enzyme-linked immunoassays (Linco Research, St. Charles, MO, USA) were used to determine concentrations of PTX3 in human AF. The PTX3 assays were validated for use in human AF in our laboratory prior to their use in this study. Validation included spike and recovery experiments which produced parallel curves indicating that AF constituents did not interfere with antigen-antibody binding in this assay. Immunoassays were carried out according to the manufacturer's recommendations. The AF samples were incubated in duplicate wells of the micro titer plates, which had been pre-coated with antibodies specific for PTX3. During this incubation, the PTX3 present in the standards or AF samples was bound by the immobilized antibodies in the respective assay plates. After repeated washing and aspiration to remove all unbound substances, an enzyme-linked polyclonal antibody specific for the PTX3 was added to the wells of the assay plates. Unbound enzyme conjugate was removed by repeated washing and a substrate solution was added to the wells of the assay plates, with color developing in proportion to the amount of the PTX3 bound in the initial step. Color development was stopped with the addition of an acid solution, and the intensity of color was read using a programmable spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA). The concentrations of PTX3 in AF samples were determined by interpolation from individual standard curves. The calculated inter-assay and intra-assay coefficients of variation for PTX3 in our laboratory were 2.7% and 3.9% respectively. The sensitivity was 0.126 ng/ml.
The concentrations of PTX3 in AF samples were determined by extrapolation from individual standard curves. The calculated inter-assay and intra-assay coefficients of variation for PTX3 in our laboratory were 2.7% and 3.9% respectively. The sensitivity was 0.126 ng/ml.
Statistical analysis
The normality of the data was tested using the Shapiro-Wilk and Kolmogorov-Smirnov tests. Since AF PTX3 concentrations were not normally distributed, non-parametric tests were used for analyses. Comparisons between proportions were performed with the Chi-square test. Kruskal-Wallis with post-hoc analysis and Mann-Whitney U tests were used for continuous variables. Adjustment for multiple comparisons was performed using the Bonferroni method [11]. Analysis of covariance (ANCOVA) was used to examine the difference of AF PTX3 concentration between the PTL and PPROM subgroup while adjust for storage time. Spearman rank correlation was utilized to assess correlations between AF concentrations of PTX3, IL-6, glucose and WBC count. A p-value of <0.05 was considered statistically significant. The statistical package used was SPSS v.15.0 (SPSS Inc., Chicago, IL, USA).
Results
Demographic and clinical characteristics of the study population
Table I presents the demographic and clinical characteristics of women in the mid-trimester, term not in labor and term in labor groups. Predictably, women in the genetic amniocentesis group had a significantly higher median maternal age and significantly lower median gestational age at amniocentesis than women at term not in labor. Table II and III display the demographic and clinical characteristics of women with spontaneous PTL and intact membranes and those with PPROM, respectively. In women with PTL and intact membranes, those with IAI had a significantly lower median gestational age at amniocentesis than those without IAI who delivered preterm. Women with IAI had also a lower gestational age at delivery compared to women without IAI who delivered preterm and at term. In women with PPROM, the birth weight and gestational age were significantly lower in women with IAI than in those without IAI.
Table I. Demographic and clinical characteristics of women in the mid-trimester and those at term with and without spontaneous labor.
| Mid-trimester (n=61) | pa | Term No labor (n=50) | Term In labor (n=49) | pb | |
|---|---|---|---|---|---|
| Maternal age (years) | 36 (35-38) | <0.001 | 27 (21-32) | 23 (19-30) | NS |
| GA at amniocentesis (weeks) | 16 (16-17) | <0.001 | 39 (38-40) | 39 (37.8-40) | NS |
| GA at delivery (weeks) | 40 (38-40) | NS | 39 (38-40) | 39 (37.8-40) | NS |
| Birthweight (grams) | 3,320 (3,064-3,570) | NS | 3,260 (3,055-3,595) | 3,250 (3,060-3,620) | NS |
Values are expressed as percentage (number) or median (interquartile range).
GA: gestational age; NS: not significant.
pa: comparison between women in the mid-trimester and those at term not in labor
pb: comparison between women at term not in labor and those at term in labor
Table II. Demographic and clinical characteristics of women presenting with spontaneous preterm labor with intact membranes.
| PTL without IAI Term delivery (n=44) | p | PTL without IAI Preterm delivery (n=40) | pa | PTL with IAI Preterm delivery (n=62) | pb | |
|---|---|---|---|---|---|---|
| Maternal age (years) | 23 (20-27) | NS | 21.5 (20-28.8) | NS | 22 (20-26.8) | NS |
| Smoking | 28.6 (6/21) | <0.05 | 3.6 (1/28) | <0.05 | 24.2 (8/33) | NS |
| GA at amniocentesis (weeks) | 30.5 (27.7-33.2) | NS | 31.9 (28.1-33.3) | <0.05 | 28.9 (26.5-32.7) | NS |
| GA at delivery (weeks) | 39.1 (38.1-40) | <0.001 | 34.6 (33.2-35.8) | <0.001 | 30.6 (27.0-32.9) | <0.001 |
| Birthweight (grams) | 3,154 (2,910-3,505) | <0.001 | 2,455 (1,973-2,693) | <0.001 | 1,515 (930-2,112) | <0.001 |
Values expressed as percentage (number) or median (interquartile range)
p: comparison between PTL who delivered at term and PTL without IAI
pa: comparison between PTL who delivered preterm without IAI and PTL with IAI
pb: comparison between PTL who delivered at term and PTL with IAI
PTL: preterm labor; GA: gestational age; IAI: intra-amniotic infection/inflammation; NS: not significant
Table III. Demographic and clinical characteristics of women presenting with preterm prelabor rupture of membranes.
| PPROM without IAI (n=44) | PPROM with IAI (n=47) | p | |
|---|---|---|---|
| Maternal age (years) | 24.5 (20-31) | 26 (22-32) | NS |
| Smoking | 28.6 (4/14) | 29 (9/31) | NS |
| GA at amniocentesis (weeks) | 31.5 (28.1-32.6) | 30 (27-32) | NS |
| GA at delivery (weeks) | 32.7 (30.9-33.8) | 30.7 (28.4-32.6) | <0.05 |
| Birthweight (grams) | 1,837 (1,455-2190) | 1,660 (1,304-1,895) | <0.05 |
Values expressed as percentage (number) or median (interquartile range)
PPROM: preterm prelabor rupture of membranes; GA: gestational age; IAI: intra-amniotic infection/inflammation; NS: not significant.
Amniotic fluid PTX3 concentration did not change with advancing gestational age or in the presence of labor at term
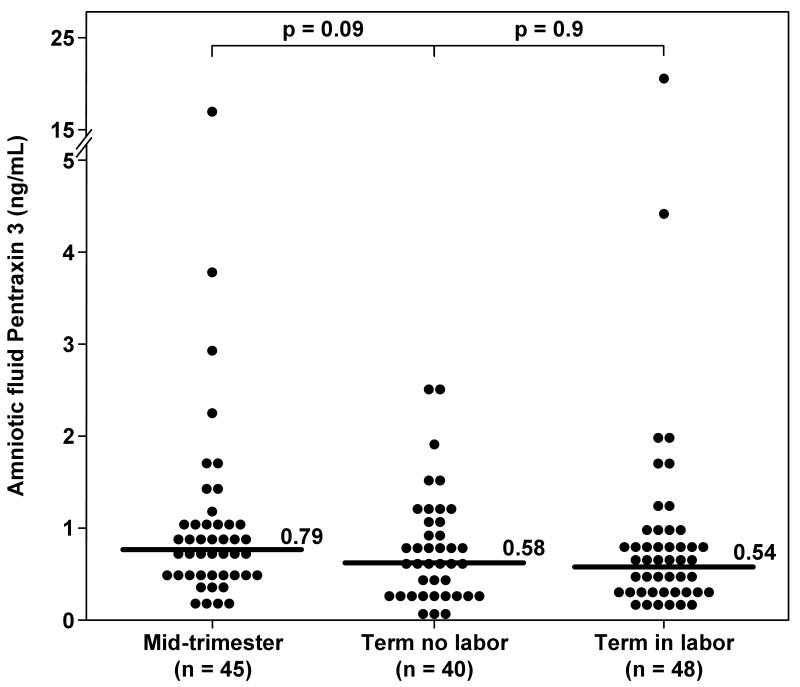
PTX3 was detected in 95.2% (360/378) of all AF samples. There were no significant differences in the median AF PTX3 concentration between women in the mid-trimester and those with an uncomplicated term pregnancy who were not in labor (0.79 ng/mL vs. 0.58 ng/mL, respectively; p=0.09) (Figure 1). Similarly, no significant differences were observed in the median AF PTX3 concentration between women at term in labor and those not in labor (0.54 ng/m vs. 0.58 ng/mL, respectively; p=0.9) (Figure 1).
Figure 1. Amniotic fluid concentrations of Pentraxin 3 (PTX3) in normal pregnancies at mid-trimester and in those at term with and without labor.
There were no differences in the median amniotic fluid PTX3 concentration between women in the mid-trimester and those with a normal pregnancy at term not in labor [0.79 ng/mL, IQR 0.57-1.08 vs. 0.58 ng/mL, IQR 0.27-1.05, respectively; p=0.09]; no significant differences were observed in the median amniotic fluid PTX3 concentration between women with spontaneous labor at term and those at term not in labor (0.58 ng/mL, IQR 0.27-1.05 vs 0.54 ng/mL, IQR 0.34-0.82, respectively; p=0.9).
Amniotic fluid PTX3 concentrations are increased in the presence of intra-amniotic infection/inflammation in women with spontaneous preterm labor and intact membranes
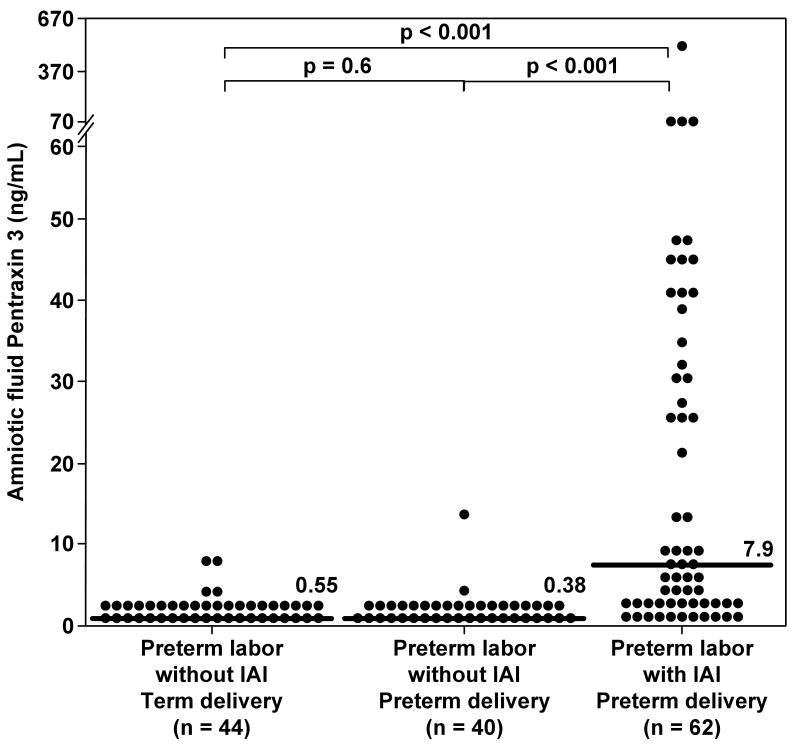
Among women with PTL, those with IAI had a significantly higher median AF concentration of PTX3 compared to those without IAI who delivered preterm (7.95 ng/mL vs. 0.38 ng/mL, respectively; p<0.001) and than those without IAI who delivered at term (0.55 ng/mL; p<0.001) (Figure 2). There were no significant differences in the median AF PTX3 concentration between women with PTL without IAI who delivered preterm and those who delivered at term (p=0.6) (Figure 2). These results did not change after adjusting for gestational age at amniocentesis, and storage time (ANCOVA).
Figure 2. Amniotic fluid concentrations of Pentraxin 3 (PTX3) among women with spontaneous preterm labor (PTL) and intact membranes.
The median amniotic fluid concentration of PTX3 was significantly higher in women with intra-amniotic infection/inflammation (IAI) than in women who delivered preterm without IAI (7.9 ng/mL, IQR 1.7–35.3 vs. 0.38 ng/mL, IQR 0.22–0.82; p<0.001) and in those who delivered at term (0.55 ng/mL, IQR 0.24–1.19; p<0.001). Among women without IAI, there was no significant difference in the median amniotic fluid concentration of PTX3 between those who delivered preterm and those who delivered at term. (0.38 ng/mL, IQR 0.22-0.82 vs 0.55 ng/mL, IQR 0.24-1.19; p= 0.6).
Amniotic fluid PTX3 concentrations are increased in the presence of intra-amniotic infection/inflammation in women with PPROM
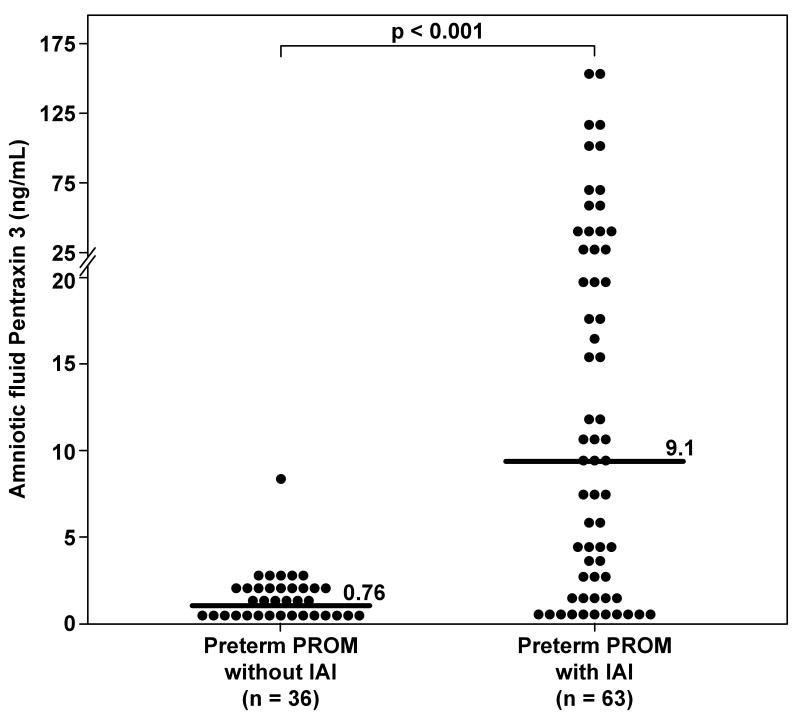
Women with PPROM and IAI had a significantly higher median AF PTX3 concentration than women with PPROM without IAI (9.12 ng/mL, vs. 0.76 ng/mL, respectively; p<0.001) (Figure 3). These results did not change after adjusting for gestational age at amniocentesis, and storage time (ANCOVA).
Figure 3. Amniotic fluid concentrations of Pentraxin 3 (PTX3) in women with preterm prelabor rupture of the membranes (PPROM).
The median amniotic fluid concentration of PTX3 was significantly higher in women with intra-amniotic infection/inflammation (IAI) than in those without IAI (9.1 ng/mL, IQR 1.85-29.6 vs. 0.76 ng/mL, IQR 0.34–1.53; p<0.001).
Correlation of amniotic fluid PTX3 concentration and other indirect markers of intra-amniotic infection/inflammation
A significant correlation was observed between AF PTX3 concentrations and IL-6, WBC count and glucose concentration in women with spontaneous PTL and those with PPROM (Spearman rho coefficient: IL-6 0.74, p<0.001; WBC count 0.49; p<0.001; and glucose -0.3, p<0.001).
Amniotic fluid PTX3 concentrations and histological chorioamnionitis
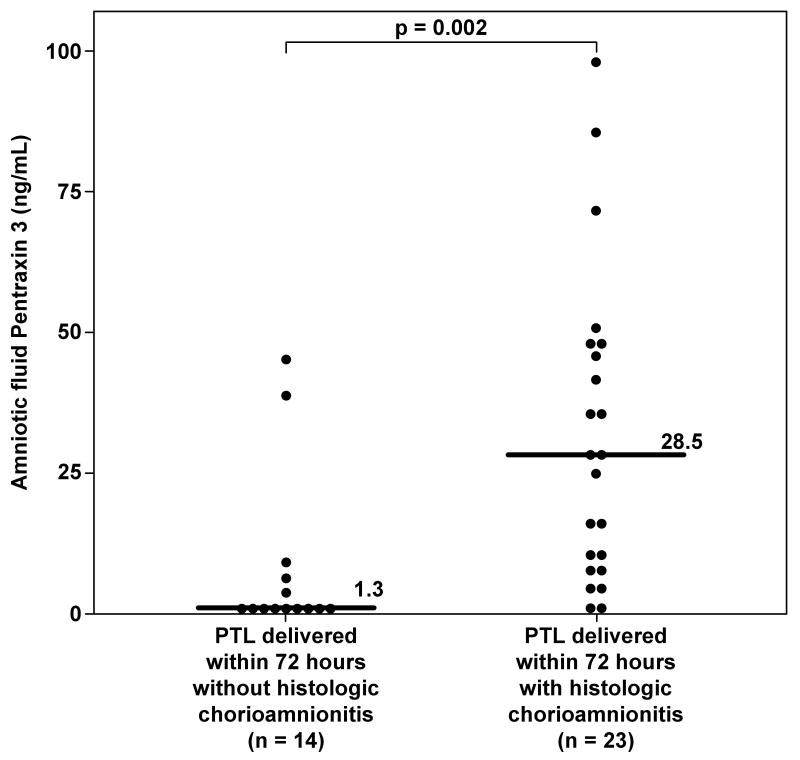
Fifty-two women with spontaneous PTL delivered within 72 hours, and histologic chorioamnionitis was present in 62% (23/37) of the cases with available placental pathologic examination. The median AF PTX3 concentration was significantly higher in women with histologic chorioamnionitis compared to those without placental inflammation (28.5 ng/mL, vs. 1.32 ng/mL, respectively; p=0.002) (Figure 4).
Figure 4. Amniotic fluid concentrations of Pentraxin 3 (PTX3) in women with spontaneous preterm labor with and without histologic chorioamnionitis who delivered within 72 hours from amniocentesis.
Women with histologic chorioamnionitis and/or funisitis had a significantly higher median PTX3 concentration in amniotic fluid than those without histologic inflammation (28.5 ng/mL, IQR 9.25-48.68 vs 1.32 ng/mL, IQR 0.63-7.36; p=0.002).
Discussion
Principal findings of the study
1) Pentraxin 3 is a physiologic constituent of the AF; 2) in women with spontaneous PTL with intact membranes, as well as in those with PPROM, the median AF PTX3 concentration was significantly elevated in the presence of IAI; 3) amniotic fluid PTX3 concentrations correlated significantly with indirect AF markers of IAI, such as IL-6, as well as with histologic chorioamnionitis; and 4) advancing gestational age and spontaneous labor at term were not associated with significant changes in the median AF PTX3 concentrations.
What is Pentraxin 3?
Pentraxins are a group of evolutionarily conserved soluble PRRs and essential components of the humoral arm of the innate immune response, together with other soluble PRRs such as mannose-binding lectin, ficolins and the complement cascade [41]. Pentraxins are characterized by a distinctive cyclic pentameric structure [41, 44] and can be divided into short and long pentraxins, since they share a C-terminal pentraxin-like domain but the long pentraxins hold a unique and unrelated long N-terminal domain [9, 13, 17, 21, 34, 41, 44, 79, 85, 102, 103, 151]. C-reactive protein (CRP) and serum amyloid P-component (SAP) are short pentraxins. CRP was the first described fluid-phase pattern recognition molecule and named after its ability to bind in a calcium-dependent manner the C-polysaccharide of Streptococcus pneumoniae [12]. CRP and SAP are acute-phase proteins that regulate innate resistence to microbes and scavenging of cellular debris [103].
PTX3, also called TNF stimulated gene 14 (TSG14) [78, 79], is a 381 amino acids protein with a molecular weight of 40 kDa, and its gene is located on chromosome 3 [14, 17, 85, 148]. PTX3 was the first long pentraxin identified [17, 78, 79] and other members of this family subsequently discovered are neuronal pentraxin 1, [95, 143] neuronal pentraxin 2, [63, 159] neuronal pentraxin receptor [28, 72], and guinea pig apexin [94, 113]. Similarly to CRP, PTX3 performs as an acute phase response protein in plasma: its physiologic concentration is low (≤2 ng/ml) but increases rapidly (peak at 6-8 hours) and dramatically (200-800 ng/ml) during inflammatory conditions such as autoimmune disease, endotoxic shock, infections, degenerative disorders and sepsis [86, 89, 104].
PTX3 is expressed by human peripheral blood monocytes in response to IL-1β and Tumor Necrosis Factor-α (TNF-α) or after stimulation with microbial components such as lypopolysaccharide (LPS) [13, 41, 67], while IL-6, monocyte chemotactic protein 1 (MCP-1/CCL2), macrophage colony-stimulating factor (M-CSF), granulocyte–macrophage colony-stimulating factor (GM-CSF), or interferon-γ (IFN-γ), are not strong inducers of PTX3 [3, 12]. Interestingly, IL-10 as a mild inducer of PTX3 in monocytes and dendritic cells [105], and it can amplify PTX3 production induced by LPS [12, 41].
PTX3 is also present in neuthrophil granules [65], acting as a reservoir for a rapid release after microbial recognition [12]. Dendritic cells [29, 30] produce high concentrations of PTX3 in response to LPS or TLR agonists such as peptidoglycan (TLR2), double-stranded DNA (TLR3), Candida (TLR4), and flagellin (TLR5) [30]. In contrast to neutrophils, dendritic cells and macrophages produce PTX3 de novo in response to inflammatory signals [12]. Other cell types that produce PTX3 in vitro are endothelial cells [17, 79, 108], smooth muscle cells [74], epithelial cells [93], adipocytes [1], fibroblasts [144, 145], synovial cells [82] and chondrocytes [163].
Pentraxin 3 and normal pregnancy
Only few studies have investigated PTX3 during pregnancy. It has been demonstrated that the maternal blood PTX3 concentration is significantly higher during normal pregnancy compared to non-pregnant women [19, 137], supporting the view that normal pregnancy is a pro-inflammatory state [23, 31, 62, 83, 84, 90, 114, 139, 140, 149]. However, conflicting results have been reported regarding the changes in maternal circulating PTX3 concentration throughout gestation. While Rovere-Querini et al. [137] reported an increase in the maternal serum PTX3 concentrations with advancing gestational age and the highest concentration during labor, Cetin et al. [19] found no change in maternal plasma PTX3 concentrations during pregnancy.
Pentraxin 3 in pregnancy complications
Women with preeclampsia have a significantly higher (6 to 10-fold) median serum/plasma PTX3 concentration than women with uncomplicated pregnancies [19, 137]. Moreover, it has been reported that serum PTX3 concentrations correlate with the severity of preeclampsia [137]. Since PTX3 is expressed in endothelial cells [17, 108], it was proposed [19] that elevated circulating concentrations of PTX3 in women with preeclampsia may represent a state of endothelial dysfunction that characterizes this obstetrical syndrome [16, 20, 43, 68, 107, 111, 112, 115-117, 138, 154]. Indeed, PTX3 has been recently considered to be a marker of vascular bed injury in conditions such as myocardial infarction [76, 104, 142] and disorders associated with autoimmunity such as small-vessel vasculitis [39, 162], rheumatoid arthritis [82], psoriasis [10], and Wegener granulomatosis [161]. Vascular endothelial cells and smooth muscle cells produce high concentrations of PTX3 in response to inflammatory signals, suggesting a role as a regulator of endothelium during thrombogenesis and ischemic vasculature disease [17, 74, 108, 118].
A single study reported on maternal circulating PTX3 concentrations in women with preterm delivery. Assi et al. [6] reported that, regardless of the clinical presentation (PTL or PPROM), women with a preterm delivery (<34 weeks) had a significantly higher maternal plasma PTX3 concentration (but not in vaginal fluid) than normal pregnant women. Moreover, women with placental vasculopathy had significantly higher plasma PTX3 concentrations than those without these placental lesions. In contrast, no differences were found in the peak plasma or peak vaginal concentration of PTX3 between women with clinical and/or histologic chorioamnionitis and those without these conditions. The authors suggested that elevated PTX3 concentrations in maternal plasma of women with PTL or PPROM may be associated to mechanisms other than intra-uterine infection, such as insults related to placental underperfusion [6].
Pentraxin 3 in amniotic fluid in normal pregnancy and term parturition
There is a paucity of information regarding PTX3 concentration in AF. In this study, PTX3 was detected in 95% of AF samples suggesting that this molecule is a physiologic constituent of the AF. In addition, we observed that PTX3 concentrations did not change significantly with advancing gestational age. This finding is in agreement with a report by Greco et al. [54] who compared PTX3 concentrations in AF obtained in mid-trimester amniocenteses and during elective cesarean sections from uncomplicated pregnancies [54].
Spontaneous labor at term is regarded as an inflammatory process [22, 37, 38, 56, 69-71, 81, 96-98, 120, 121, 123, 130, 141, 146, 158]. In the study reported herein, labor at term was not associated with a significant change in the AF concentration of PTX3, whereas Rovere-Querini et al. [137] reported that the maternal serum PTX3 concentrations peaked during labor. These results suggest that PTX3 in AF may have a limited role in the physiologic process of parturition at term.
Pentraxin 3 in amniotic fluid in intra-amniotic infection/inflammation
The findings that IAI is associated with an elevated median AF concentration of PTX3 in women with PTL and in those with PPROM, as well as in women with histologic chorioamnionitis, are novel. Among women with PTL or PPROM, the presence of IAI was associated with a 16-fold and a 12-fold increase in AF PTX3 concentrations, respectively. Similarly, women with histologic chorioamnionitis had a dramatically higher AF PTX3 concentration (22-fold) than those without placental inflammation. Furthermore, a significant correlation was observed between AF PTX3 concentrations and indirect markers of intra-amniotic infection, such as IL-6. Recently, Greco et al. [54] reported an increased concentration of PTX3 in amniotic fluid collected from the vaginal fornix from women with PPROM, and the AF concentration of PTX3 correlated with the presence of histologic chorioamnionitis.
Compelling evidence supports the notion that PTX3 plays an important role against bacterial infection caused by Staphylococcus aureus, Pseudomonas aeroginosa, Salmonella typhimurium, Klebsiella pneumoniae, Streptococcus pneumoniae, Neisseria meningitides [42, 67], fungal infection caused by Aspergillus fumigatus and Paracoccidioides brasiliensis [27, 42], and viral infections, such as cytomegalovirus (CMV) and H3N2 influenza virus [15, 109]. Indeed, PTX3 confers resistance to viral infections by binding both human and murine CMV, limiting viral entry and infectivity in dendritic cells [15], and also by inhibiting influenza viruses [109] through different mechanisms such as inhibition of hemagglutination, neutralization of virus infectivity, and inhibition of viral neuraminidase [12]. In addition, the binding of PTX3 with C1q, which was the first described ligand for PTX3, activates the classical pathway of the complement system and facilitates pathogen recognition by phagocytes [92, 136]. PTX3 also modulates factor H, which is considered the main soluble regulator of the alternative pathway, preventing an exaggerated activation of the complement system [26]. Thus, it has been proposed that PTX3 participates in the crosstalk between the cellular and humoral arms of the innate immunity in response to microbial invasion by facilitating the activity of the cellular arm of the innate immune response and modulating complement activation [12]. This support the concept of activation of the innate immune system and the complement pathway as part of the inflammatory response to microbial invasion of the AF [33, 147, 160].
What is the origin of Pentraxin 3 in amniotic fluid?
The origin of PTX3 in the AF and the main compartment contributing to the higher concentrations in cases with IAI is still unknown. Several potential sources can be suggested: 1) PTX3 was shown to be physiologically expressed in fetal membranes (amniotic epithelium, chorionic mesoderm) from uncomplicated pregnancies [53, 137]. Furthermore, its expression increased in membranes from pregnancies complicated by PPROM and/or with histologic chorioamnionitis [53]. This suggests that the fetal membranes may contribute to the higher AF concentration of PTX3 observed in cases with IAI and histologic chorioamnionitis; 2) the fetus is capable of mounting an inflammatory response to the presence of microbial invasion of the amniotic cavity [48, 52, 126] characterized by systemic activation of the innate immune system. Indeed, it has been reported that CRP, one of the short pentraxins, is significantly higher in preterm neonates from mothers with a positive AF culture than in those with negative culture as well as in neonates with funisitis than in those without funisitis [170]. Although there are no data regarding PTX3 in cord blood, it is possible that AF PTX3 may represent, in part, a fetal inflammatory response to acute intra-amniotic infection; and 3) in maternal circulation, PTX3 concentrations were shown to increase with advancing gestation and to peak during term labor [137]. However, the lack of significant change in AF PTX3 concentrations throughout gestation and during term parturition suggests that maternal blood and amniotic fluid are two independent compartments.
In conclusion, this study demonstrates that PTX3 is a physiologic constituent of the amniotic fluid, and its concentration is significantly elevated in the presence of intra-amniotic infection/inflammation, suggesting that PTX3 may play a role in the innate immune response against intra-amniotic infection.
Acknowledgments
This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Abderrahim-Ferkoune A, Bezy O, Chiellini C, Maffei M, Grimaldi P, Bonino F, et al. Characterization of the long pentraxin PTX3 as a TNFalpha-induced secreted protein of adipose cells. J Lipid Res. 2003;44:994–1000. doi: 10.1194/jlr.M200382-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 3.Alles VV, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994;84:3483–3493. [PubMed] [Google Scholar]
- 4.Altmeyer A, Klampfer L, Goodman AR, Vilcek J. Promoter structure and transcriptional activation of the murine TSG-14 gene encoding a tumor necrosis factor/interleukin-1-inducible pentraxin protein. J Biol Chem. 1995;270:25584–25590. doi: 10.1074/jbc.270.43.25584. [DOI] [PubMed] [Google Scholar]
- 5.Appelbaum PC, Shulman G, Chambers NL, Simon NV, Granados JL, Fairbrother PF, et al. Studies on the growth-inhibiting property of amniotic fluids from two United States population groups. Am J Obstet Gynecol. 1980;137:579–582. doi: 10.1016/0002-9378(80)90699-7. [DOI] [PubMed] [Google Scholar]
- 6.Assi F, Fruscio R, Bonardi C, Ghidini A, Allavena P, Mantovani A, et al. Pentraxin 3 in plasma and vaginal fluid in women with preterm delivery. BJOG. 2007;114:143–147. doi: 10.1111/j.1471-0528.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 7.Bashiri A, Burstein E, Mazor M. Cerebral palsy and fetal inflammatory response syndrome: a review. J Perinat Med. 2006;34:5–12. doi: 10.1515/JPM.2006.001. [DOI] [PubMed] [Google Scholar]
- 8.Basile A, Sica A, d'Aniello E, Breviario F, Garrido G, Castellano M, et al. Characterization of the promoter for the human long pentraxin PTX3. Role of NF-kappaB in tumor necrosis factor-alpha and interleukin-1beta regulation. J Biol Chem. 1997;272:8172–8178. doi: 10.1074/jbc.272.13.8172. [DOI] [PubMed] [Google Scholar]
- 9.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 10.Bevelacqua V, Libra M, Mazzarino MC, Gangemi P, Nicotra G, Curatolo S, et al. Long pentraxin 3: a marker of inflammation in untreated psoriatic patients. Int J Mol Med. 2006;18:415–423. [PubMed] [Google Scholar]
- 11.Bonferroni C. Il calcolo delle assicurazioni su gruppi di teste. 1935:13–60. [Google Scholar]
- 12.Bottazzi B, Garlanda C, Cotena A, Moalli F, Jaillon S, Deban L, et al. The long pentraxin PTX3 as a prototypic humoral pattern recognition receptor: interplay with cellular innate immunity. Immunol Rev. 2009;227:9–18. doi: 10.1111/j.1600-065X.2008.00719.x. [DOI] [PubMed] [Google Scholar]
- 13.Bottazzi B, Garlanda C, Salvatori G, Jeannin P, Manfredi A, Mantovani A. Pentraxins as a key component of innate immunity. Curr Opin Immunol. 2006;18:10–15. doi: 10.1016/j.coi.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Bottazzi B, Vouret-Craviari V, Bastone A, De GL, Matteucci C, Peri G, et al. Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J Biol Chem. 1997;272:32817–32823. doi: 10.1074/jbc.272.52.32817. [DOI] [PubMed] [Google Scholar]
- 15.Bozza S, Bistoni F, Gaziano R, Pitzurra L, Zelante T, Bonifazi P, et al. Pentraxin 3 protects from MCMV infection and reactivation through TLR sensing pathways leading to IRF3 activation. Blood. 2006;108:3387–3396. doi: 10.1182/blood-2006-03-009266. [DOI] [PubMed] [Google Scholar]
- 16.Bretelle F, Sabatier F, Blann A, D'ercole C, Boutiere B, Mutin M, et al. Maternal endothelial soluble cell adhesion molecules with isolated small for gestational age fetuses: comparison with pre-eclampsia. BJOG. 2001;108:1277–1282. doi: 10.1111/j.1471-0528.2001.00259.x. [DOI] [PubMed] [Google Scholar]
- 17.Breviario F, d'Aniello EM, Golay J, Peri G, Bottazzi B, Bairoch A, et al. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267:22190–22197. [PubMed] [Google Scholar]
- 18.Brocklehurst P. Infection and preterm delivery. BMJ. 1999;318:548–549. doi: 10.1136/bmj.318.7183.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cetin I, Cozzi V, Pasqualini F, Nebuloni M, Garlanda C, Vago L, et al. Elevated maternal levels of the long pentraxin 3 (PTX3) in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2006;194:1347–1353. doi: 10.1016/j.ajog.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Clark BA, Halvorson L, Sachs B, Epstein FH. Plasma endothelin levels in preeclampsia: elevation and correlation with uric acid levels and renal impairment. Am J Obstet Gynecol. 1992;166:962–968. doi: 10.1016/0002-9378(92)91372-h. [DOI] [PubMed] [Google Scholar]
- 21.Coe JE, Margossian SS, Slayter HS, Sogn JA. Hamster female protein. A new Pentraxin structurally and functionally similar to C-reactive protein and amyloid P component. J Exp Med. 1981;153:977–991. doi: 10.1084/jem.153.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen J, Ghezzi F, Romero R, Ghidini A, Mazor M, Tolosa JE, et al. GRO alpha in the fetomaternal and amniotic fluid compartments during pregnancy and parturition. Am J Reprod Immunol. 1996;35:23–29. doi: 10.1111/j.1600-0897.1996.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 23.Comeglio P, Fedi S, Liotta AA, Cellai AP, Chiarantini E, Prisco D, et al. Blood clotting activation during normal pregnancy. Thromb Res. 1996;84:199–202. doi: 10.1016/0049-3848(96)00176-4. [DOI] [PubMed] [Google Scholar]
- 24.Dammann O, Leviton A. Role of the fetus in perinatal infection and neonatal brain damage. Curr Opin Pediatr. 2000;12:99–104. doi: 10.1097/00008480-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Deban L, Jarva H, Lehtinen MJ, Bottazzi B, Bastone A, Doni A, et al. Binding of the long pentraxin PTX3 to factor H: interacting domains and function in the regulation of complement activation. J Immunol. 2008;181:8433–8440. doi: 10.4049/jimmunol.181.12.8433. [DOI] [PubMed] [Google Scholar]
- 27.Diniz SN, Nomizo R, Cisalpino PS, Teixeira MM, Brown GD, Mantovani A, et al. PTX3 function as an opsonin for the dectin-1-dependent internalization of zymosan by macrophages. J Leukoc Biol. 2004;75:649–656. doi: 10.1189/jlb.0803371. [DOI] [PubMed] [Google Scholar]
- 28.Dodds DC, Omeis IA, Cushman SJ, Helms JA, Perin MS. Neuronal pentraxin receptor, a novel putative integral membrane pentraxin that interacts with neuronal pentraxin 1 and 2 and taipoxin-associated calcium-binding protein 49. J Biol Chem. 1997;272:21488–21494. doi: 10.1074/jbc.272.34.21488. [DOI] [PubMed] [Google Scholar]
- 29.Doni A, Michela M, Bottazzi B, Peri G, Valentino S, Polentarutti N, et al. Regulation of PTX3, a key component of humoral innate immunity in human dendritic cells: stimulation by IL-10 and inhibition by IFN-gamma. J Leukoc Biol. 2006;79:797–802. doi: 10.1189/jlb.0905493. [DOI] [PubMed] [Google Scholar]
- 30.Doni A, Peri G, Chieppa M, Allavena P, Pasqualini F, Vago L, et al. Production of the soluble pattern recognition receptor PTX3 by myeloid, but not plasmacytoid, dendritic cells. Eur J Immunol. 2003;33:2886–2893. doi: 10.1002/eji.200324390. [DOI] [PubMed] [Google Scholar]
- 31.Efrati P, Presentey B, Margarlith M, Rozenszajn L. Leukocytes of Normal Pregnant Women. Obstet Gynecol. 1964;23:429–432. [PubMed] [Google Scholar]
- 32.Eggert-Kruse W, Botz I, Pohl S, Rohr G, Strowitzki T. Antimicrobial activity of human cervical mucus. Hum Reprod. 2000;15:778–784. doi: 10.1093/humrep/15.4.778. [DOI] [PubMed] [Google Scholar]
- 33.Elimian A, Figueroa R, Canterino J, Verma U, guero-Rosenfeld M, Tejani N. Amniotic fluid complement C3 as a marker of intra-amniotic infection. Obstet Gynecol. 1998;92:72–76. doi: 10.1016/s0029-7844(98)00123-9. [DOI] [PubMed] [Google Scholar]
- 34.Emsley J, White HE, O'Hara BP, Oliva G, Srinivasan N, Tickle IJ, et al. Structure of pentameric human serum amyloid P component. Nature. 1994;367:338–345. doi: 10.1038/367338a0. [DOI] [PubMed] [Google Scholar]
- 35.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13:2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 36.Espinoza J, Goncalves LF, Romero R, Nien JK, Stites S, Kim YM, et al. The prevalence and clinical significance of amniotic fluid ‘sludge’ in patients with preterm labor and intact membranes. Ultrasound Obstet Gynecol. 2005;25:346–352. doi: 10.1002/uog.1871. [DOI] [PubMed] [Google Scholar]
- 37.Esplin MS, Peltier MR, Hamblin S, Smith S, Fausett MB, Dildy GA, et al. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta. 2005;26:661–671. doi: 10.1016/j.placenta.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, et al. Amniotic fluid levels of immunoreactive monocyte chemotactic protein-1 increase during term parturition. J Matern Fetal Neonatal Med. 2003;14:51–56. doi: 10.1080/jmf.14.1.51.56. [DOI] [PubMed] [Google Scholar]
- 39.Fazzini F, Peri G, Doni A, Dell'Antonio G, Dal CE, Bozzolo E, et al. PTX3 in small-vessel vasculitides: an independent indicator of disease activity produced at sites of inflammation. Arthritis Rheum. 2001;44:2841–2850. doi: 10.1002/1529-0131(200112)44:12<2841::aid-art472>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 40.Galask RP, Snyder IS. Antimicrobial factors in amniotic fluid. Am J Obstet Gynecol. 1970;106:59–65. doi: 10.1016/0002-9378(70)90126-2. [DOI] [PubMed] [Google Scholar]
- 41.Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–366. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- 42.Garlanda C, Hirsch E, Bozza S, Salustri A, De AM, Nota R, et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–186. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- 43.Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001;185:792–797. doi: 10.1067/mob.2001.117311. [DOI] [PubMed] [Google Scholar]
- 44.Gewurz H, Zhang XH, Lint TF. Structure and function of the pentraxins. Curr Opin Immunol. 1995;7:54–64. doi: 10.1016/0952-7915(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 45.Gibbs RS. The relationship between infections and adverse pregnancy outcomes: an overview. Ann Periodontol. 2001;6:153–163. doi: 10.1902/annals.2001.6.1.153. [DOI] [PubMed] [Google Scholar]
- 46.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 47.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 48.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 49.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, et al. A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000. Rev Med Chil. 2004;132:1155–1165. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- 51.Goodman AR, Levy DE, Reis LF, Vilcek J. Differential regulation of TSG-14 expression in murine fibroblasts and peritoneal macrophages. J Leukoc Biol. 2000;67:387–395. doi: 10.1002/jlb.67.3.387. [DOI] [PubMed] [Google Scholar]
- 52.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 53.Greco M, Nebuloni M, Garlanda C, Consonni S, Lauri E, Locatelli A. PTX3 Histological Espression in Preterm Premature Rupture of Membranes. Reproductive Sciences. 2009;16:200A–200A. [Google Scholar]
- 54.Greco M, Garlanda C, Consonni S, Maina V, Locatelli A. PTX3 in amniotic fluid: a novel marker of inflammation in pPROM. Reproductive Sciences. 2009;16:201A–201A. [Google Scholar]
- 55.Guleria I, Pollard JW. The trophoblast is a component of the innate immune system during pregnancy. Nat Med. 2000;6:589–593. doi: 10.1038/75074. [DOI] [PubMed] [Google Scholar]
- 56.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195:394–24. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG. 2005;112 1:16–18. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 58.Hargreaves DC, Medzhitov R. Innate sensors of microbial infection. J Clin Immunol. 2005;25:503–510. doi: 10.1007/s10875-005-8065-4. [DOI] [PubMed] [Google Scholar]
- 59.Hein M, Helmig RB, Schonheyder HC, Ganz T, Uldbjerg N. An in vitro study of antibacterial properties of the cervical mucus plug in pregnancy. Am J Obstet Gynecol. 2001;185:586–592. doi: 10.1067/mob.2001.116685. [DOI] [PubMed] [Google Scholar]
- 60.Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol. 2002;187:137–144. doi: 10.1067/mob.2002.123034. [DOI] [PubMed] [Google Scholar]
- 61.Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Investig. 2005;12:145–155. doi: 10.1016/j.jsgi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 62.Hopkinson ND, Powell RJ. Classical complement activation induced by pregnancy: Implications for management of connective tissue diseases. J Clin Pathol. 1992;45:66–67. doi: 10.1136/jcp.45.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsu YC, Perin MS. Human neuronal pentraxin II (NPTX2): conservation, genomic structure, and chromosomal localization. Genomics. 1995;28:220–227. doi: 10.1006/geno.1995.1134. [DOI] [PubMed] [Google Scholar]
- 64.Introna M, Alles VV, Castellano M, Picardi G, De GL, Bottazzai B, et al. Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood. 1996;87:1862–1872. [PubMed] [Google Scholar]
- 65.Jaillon S, Peri G, Delneste Y, Fremaux I, Doni A, Moalli F, et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med. 2007;204:793–804. doi: 10.1084/jem.20061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Janeway C, Travers P, Walport M, Schlmochik M. Innate immunity. 2005:37–102. [Google Scholar]
- 67.Jeannin P, Bottazzi B, Sironi M, Doni A, Rusnati M, Presta M, et al. Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity. 2005;22:551–560. doi: 10.1016/j.immuni.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 68.Johnson MR, nim-Nyame N, Johnson P, Sooranna SR, Steer PJ. Does endothelial cell activation occur with intrauterine growth restriction? BJOG. 2002;109:836–839. doi: 10.1111/j.1471-0528.2002.01045.x. [DOI] [PubMed] [Google Scholar]
- 69.Junqueira LC, Zugaib M, Montes GS, Toledo OM, Krisztan RM, Shigihara KM. Morphologic and histochemical evidence for the occurrence of collagenolysis and for the role of neutrophilic polymorphonuclear leukocytes during cervical dilation. Am J Obstet Gynecol. 1980;138:273–281. doi: 10.1016/0002-9378(80)90248-3. [DOI] [PubMed] [Google Scholar]
- 70.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta. 2003;24 A:S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 71.Keski-Nisula L, Aalto ML, Katila ML, Kirkinen P. Intrauterine inflammation at term: a histopathologic study. Hum Pathol. 2000;31:841–846. doi: 10.1053/hupa.2000.8449. [DOI] [PubMed] [Google Scholar]
- 72.Kirkpatrick LL, Matzuk MM, Dodds DC, Perin MS. Biochemical interactions of the neuronal pentraxins. Neuronal pentraxin (NP) receptor binds to taipoxin and taipoxin-associated calcium-binding protein 49 via NP1 and NP2. J Biol Chem. 2000;275:17786–17792. doi: 10.1074/jbc.M002254200. [DOI] [PubMed] [Google Scholar]
- 73.Kjaergaard N, Hein M, Hyttel L, Helmig RB, Schonheyder HC, Uldbjerg N, et al. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol. 2001;94:224–229. doi: 10.1016/s0301-2115(00)00345-6. [DOI] [PubMed] [Google Scholar]
- 74.Klouche M, Peri G, Knabbe C, Eckstein HH, Schmid FX, Schmitz G, et al. Modified atherogenic lipoproteins induce expression of pentraxin-3 by human vascular smooth muscle cells. Atherosclerosis. 2004;175:221–228. doi: 10.1016/j.atherosclerosis.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 75.Kusanovic JP, Espinoza J, Romero R, Goncalves LF, Nien JK, Soto E, et al. Clinical significance of the presence of amniotic fluid ‘sludge’ in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30:706–714. doi: 10.1002/uog.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110:2349–2354. doi: 10.1161/01.CIR.0000145167.30987.2E. [DOI] [PubMed] [Google Scholar]
- 77.Ledger WJ. Infection and premature labor. Am J Perinatol. 1989;6:234–236. doi: 10.1055/s-2007-999583. [DOI] [PubMed] [Google Scholar]
- 78.Lee GW, Goodman AR, Lee TH, Vilcek J. Relationship of TSG-14 protein to the pentraxin family of major acute phase proteins. J Immunol. 1994;153:3700–3707. [PubMed] [Google Scholar]
- 79.Lee GW, Lee TH, Vilcek J. TSG-14, a tumor necrosis factor- and IL-1-inducible protein, is a novel member of the pentaxin family of acute phase proteins. J Immunol. 1993;150:1804–1812. [PubMed] [Google Scholar]
- 80.Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr Res. 1999;46:566–575. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 81.Liggins GC. Cervical ripening as an inflammatory reaction. 1981:1–9. [Google Scholar]
- 82.Luchetti MM, Piccinini G, Mantovani A, Peri G, Matteucci C, Pomponio G, et al. Expression and production of the long pentraxin PTX3 in rheumatoid arthritis (RA) Clin Exp Immunol. 2000;119:196–202. doi: 10.1046/j.1365-2249.2000.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luppi P, Haluszczak C, Betters D, Richard CA, Trucco M, DeLoia JA. Monocytes are progressively activated in the circulation of pregnant women. J Leukoc Biol. 2002;72:874–884. [PubMed] [Google Scholar]
- 84.Luppi P, Haluszczak C, Trucco M, DeLoia JA. Normal pregnancy is associated with peripheral leukocyte activation. Am J Reprod Immunol. 2002;47:72–81. doi: 10.1034/j.1600-0897.2002.1o041.x. [DOI] [PubMed] [Google Scholar]
- 85.Mantovani A, Garlanda C, Bottazzi B. Pentraxin 3, a non-redundant soluble pattern recognition receptor involved in innate immunity. Vaccine. 2003;21 2:S43–S47. doi: 10.1016/s0264-410x(03)00199-3. [DOI] [PubMed] [Google Scholar]
- 86.Mantovani A, Garlanda C, Doni A, Bottazzi B. Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J Clin Immunol. 2008;28:1–13. doi: 10.1007/s10875-007-9126-7. [DOI] [PubMed] [Google Scholar]
- 87.Miller J, Michel J, Bercovici B, Argaman M, Sacks T. Studies on the antimicrobial activity of amniotic fluid. Am J Obstet Gynecol. 1976;125:212–214. doi: 10.1016/0002-9378(76)90595-0. [DOI] [PubMed] [Google Scholar]
- 88.Minkoff H. Prematurity: infection as an etiologic factor. Obstet Gynecol. 1983;62:137–144. [PubMed] [Google Scholar]
- 89.Muller B, Peri G, Doni A, Torri V, Landmann R, Bottazzi B, et al. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med. 2001;29:1404–1407. doi: 10.1097/00003246-200107000-00017. [DOI] [PubMed] [Google Scholar]
- 90.Naccasha N, Gervasi MT, Chaiworapongsa T, Berman S, Yoon BH, Maymon E, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol. 2001;185:1118–1123. doi: 10.1067/mob.2001.117682. [DOI] [PubMed] [Google Scholar]
- 91.Naeye RL, Ross SM. Amniotic fluid infection syndrome. Clin Obstet Gynaecol. 1982;9:593–607. [PubMed] [Google Scholar]
- 92.Nauta AJ, Bottazzi B, Mantovani A, Salvatori G, Kishore U, Schwaeble WJ, et al. Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur J Immunol. 2003;33:465–473. doi: 10.1002/immu.200310022. [DOI] [PubMed] [Google Scholar]
- 93.Nauta AJ, de H S, Bottazzi B, Mantovani A, Borrias MC, Aten J, et al. Human renal epithelial cells produce the long pentraxin PTX3. Kidney Int. 2005;67:543–553. doi: 10.1111/j.1523-1755.2005.67111.x. [DOI] [PubMed] [Google Scholar]
- 94.Noland TD, Friday BB, Maulit MT, Gerton GL. The sperm acrosomal matrix contains a novel member of the pentaxin family of calcium-dependent binding proteins. J Biol Chem. 1994;269:32607–32614. [PubMed] [Google Scholar]
- 95.Omeis IA, Hsu YC, Perin MS. Mouse and human neuronal pentraxin 1 (NPTX1): conservation, genomic structure, and chromosomal localization. Genomics. 1996;36:543–545. doi: 10.1006/geno.1996.0503. [DOI] [PubMed] [Google Scholar]
- 96.Opsjln SL, Wathen NC, Tingulstad S, Wiedswang G, Sundan A, Waage A, et al. Tumor necrosis factor, interleukin-1, and interleukin-6 in normal human pregnancy. Am J Obstet Gynecol. 1993;169:397–404. doi: 10.1016/0002-9378(93)90096-2. [DOI] [PubMed] [Google Scholar]
- 97.Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9:41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 98.Osmers RG, Blaser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol. 1995;86:223–229. doi: 10.1016/0029-7844(95)93704-4. [DOI] [PubMed] [Google Scholar]
- 99.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 100.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 101.Patrick LA, Smith GN. Proinflammatory cytokines: a link between chorioamnionitis and fetal brain injury. J Obstet Gynaecol Can. 2002;24:705–709. doi: 10.1016/s1701-2163(16)30325-5. [DOI] [PubMed] [Google Scholar]
- 102.Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- 103.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peri G, Introna M, Corradi D, Iacuitti G, Signorini S, Avanzini F, et al. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–641. doi: 10.1161/01.cir.102.6.636. [DOI] [PubMed] [Google Scholar]
- 105.Perrier P, Martinez FO, Locati M, Bianchi G, Nebuloni M, Vago G, et al. Distinct transcriptional programs activated by interleukin-10 with or without lipopolysaccharide in dendritic cells: induction of the B cell-activating chemokine, CXC chemokine ligand 13. J Immunol. 2004;172:7031–7042. doi: 10.4049/jimmunol.172.11.7031. [DOI] [PubMed] [Google Scholar]
- 106.Polentarutti N, Bottazzi B, Di SE, Blasi E, Agnello D, Ghezzi P, et al. Inducible expression of the long pentraxin PTX3 in the central nervous system. J Neuroimmunol. 2000;106:87–94. doi: 10.1016/s0165-5728(00)00214-9. [DOI] [PubMed] [Google Scholar]
- 107.Poston L, Chappell LC. Is oxidative stress involved in the aetiology of pre-eclampsia? Acta Paediatr Suppl. 2001;90:3–5. doi: 10.1111/j.1651-2227.2001.tb01619.x. [DOI] [PubMed] [Google Scholar]
- 108.Presta M, Camozzi M, Salvatori G, Rusnati M. Role of the soluble pattern recognition receptor PTX3 in vascular biology. J Cell Mol Med. 2007;11:723–738. doi: 10.1111/j.1582-4934.2007.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reading PC, Bozza S, Gilbertson B, Tate M, Moretti S, Job ER, et al. Antiviral activity of the long chain pentraxin PTX3 against influenza viruses. J Immunol. 2008;180:3391–3398. doi: 10.4049/jimmunol.180.5.3391. [DOI] [PubMed] [Google Scholar]
- 110.Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med. 2006;11:296–301. doi: 10.1016/j.siny.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 111.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 112.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 113.Reid MS, Blobel CP. Apexin, an acrosomal pentaxin. J Biol Chem. 1994;269:32615–32620. [PubMed] [Google Scholar]
- 114.Richani K, Soto E, Romero R, Espinoza J, Chaiworapongsa T, Nien JK, et al. Normal pregnancy is characterized by systemic activation of the complement system. J Matern Fetal Neonatal Med. 2005;17:239–245. doi: 10.1080/14767050500072722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 116.Roberts JM, Lain KY. Recent Insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23:359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 117.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 118.Rolph MS, Zimmer S, Bottazzi B, Garlanda C, Mantovani A, Hansson GK. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:e10–e14. doi: 10.1161/01.atv.0000015595.95497.2f. [DOI] [PubMed] [Google Scholar]
- 119.Romero R. The child is the father of the man. Prenat Neonat Med. 1996;1:8–11. [Google Scholar]
- 120.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–1123. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 121.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–820. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 122.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159:114–119. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 123.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113 3:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Romero R, Gomez R, Araneda H, Ramirez M, Cotton DB. Cervical mucus inhibits microbial growth: a host defense mechanism to prevent ascending infection in pregnant and non-pregnant women. Am J Obstet Gynecol. 1993;168:312–312. [Google Scholar]
- 126.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 127.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163:968–974. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 128.Romero R, Kusanovic JP, Espinoza J, Gotsch F, Nhan-Chang CL, Erez O, et al. What is amniotic fluid ‘sludge’? Ultrasound Obstet. Gynecol. 2007;30:793–798. doi: 10.1002/uog.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 130.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166:1576–1587. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- 131.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–279. [PubMed] [Google Scholar]
- 132.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165:821–830. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 133.Romero R, Salafia CM, Athanassiadis AP, Hanaoka S, Mazor M, Sepulveda W, et al. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol. 1992;166:1382–1388. doi: 10.1016/0002-9378(92)91609-e. [DOI] [PubMed] [Google Scholar]
- 134.Romero R, Schaudinn C, Kusanovic JP, Gorur A, Gotsch F, Webster P, et al. Detection of a microbial biofilm in intraamniotic infection. Am J Obstet Gynecol. 2008;198:135–135. doi: 10.1016/j.ajog.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 136.Roumenina LT, Ruseva MM, Zlatarova A, Ghai R, Kolev M, Olova N, et al. Interaction of C1q with IgG1, C-reactive protein and pentraxin 3: mutational studies using recombinant globular head modules of human C1q A, B, and C chains. Biochemistry. 2006;45:4093–4104. doi: 10.1021/bi052646f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rovere-Querini P, Antonacci S, Dell'Antonio G, Angeli A, Almirante G, Cin ED, et al. Plasma and tissue expression of the long pentraxin 3 during normal pregnancy and preeclampsia. Obstet Gynecol. 2006;108:148–155. doi: 10.1097/01.AOG.0000224607.46622.bc. [DOI] [PubMed] [Google Scholar]
- 138.Sabatier F, Bretelle F, D'ercole C, Boubli L, Sampol J, gnat-George F. Neutrophil activation in preeclampsia and isolated intrauterine growth restriction. Am J Obstet Gynecol. 2000;183:1558–1563. doi: 10.1067/mob.2000.108082. [DOI] [PubMed] [Google Scholar]
- 139.Sacks GP, Redman CW, Sargent IL. Monocytes are primed to produce the Th1 type cytokine IL-12 in normal human pregnancy: an intracellular flow cytometric analysis of peripheral blood mononuclear cells. Clin Exp Immunol. 2003;131:490–497. doi: 10.1046/j.1365-2249.2003.02082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 141.Saito S, Kasahara T, Kato Y, Ishihara Y, Ichijo M. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine. 1993;5:81–88. doi: 10.1016/1043-4666(93)90027-3. [DOI] [PubMed] [Google Scholar]
- 142.Salio M, Chimenti S, De A N, Molla F, Maina V, Nebuloni M, et al. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117:1055–1064. doi: 10.1161/CIRCULATIONAHA.107.749234. [DOI] [PubMed] [Google Scholar]
- 143.Schlimgen AK, Helms JA, Vogel H, Perin MS. Neuronal pentraxin, a secreted protein with homology to acute phase proteins of the immune system. Neuron. 1995;14:519–526. doi: 10.1016/0896-6273(95)90308-9. [DOI] [PubMed] [Google Scholar]
- 144.Semov A, Semova N, Lacelle C, Marcotte R, Petroulakis E, Proestou G, et al. Alterations in TNF- and IL-related gene expression in space-flown WI38 human fibroblasts. FASEB J. 2002;16:899–901. doi: 10.1096/fj.01-1002fje. [DOI] [PubMed] [Google Scholar]
- 145.Shelton RC, Liang S, Liang P, Chakrabarti A, Manier DH, Sulser F. Differential expression of pentraxin 3 in fibroblasts from patients with major depression. Neuropsychopharmacology. 2004;29:126–132. doi: 10.1038/sj.npp.1300307. [DOI] [PubMed] [Google Scholar]
- 146.Shynlova O, Tsui P, Dorogin A, Lye SJ. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol. 2008;181:1470–1479. doi: 10.4049/jimmunol.181.2.1470. [DOI] [PubMed] [Google Scholar]
- 147.Soto E, Romero R, Richani K, Yoon BH, Chaiworapongsa T, Vaisbuch E, et al. Evidence for Complement Activation in the Amniotic Fluid of Women with Spontaneous Preterm Labor and Intra-amniotic Infection. J Matern Fetal Neonatal Med. 2009 doi: 10.3109/14767050902994747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Srinivasan N, White HE, Emsley J, Wood SP, Pepys MB, Blundell TL. Comparative analyses of pentraxins: implications for protomer assembly and ligand binding. Structure. 1994;2:1017–1027. doi: 10.1016/S0969-2126(94)00105-7. [DOI] [PubMed] [Google Scholar]
- 149.Stirling Y, Woolf L, North WR, Seghatchian MJ, Meade TW. Haemostasis in normal pregnancy. Thromb Haemost. 1984;52:176–182. [PubMed] [Google Scholar]
- 150.Svinarich DM, Wolf NA, Gomez R, Gonik B, Romero R. Detection of human defensin 5 in reproductive tissues. Am J Obstet Gynecol. 1997;176:470–475. doi: 10.1016/s0002-9378(97)70517-9. [DOI] [PubMed] [Google Scholar]
- 151.Szalai AJ, Agrawal A, Greenhough TJ, Volanakis JE. C-reactive protein: structural biology and host defense function. Clin Chem Lab Med. 1999;37:265–270. doi: 10.1515/CCLM.1999.046. [DOI] [PubMed] [Google Scholar]
- 152.Tafari N, Ross SM, Naeye RL, Galask RP, Zaar B. Failure of bacterial growth inhibition by amniotic fluid. Am J Obstet Gynecol. 1977;128:187–189. doi: 10.1016/0002-9378(77)90685-8. [DOI] [PubMed] [Google Scholar]
- 153.Talmi YP, Sigler L, Inge E, Finkelstein Y, Zohar Y. Antibacterial properties of human amniotic membranes. Placenta. 1991;12:285–288. doi: 10.1016/0143-4004(91)90010-d. [DOI] [PubMed] [Google Scholar]
- 154.Taylor RN, de Groot CJ, Cho YK, Lim KH. Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:17–31. doi: 10.1055/s-2007-1016249. [DOI] [PubMed] [Google Scholar]
- 155.Thadepalli H, Appleman MD, Maidman JE, Arce JJ, Davidson EC., Jr Antimicrobial effect of amniotic fluid against anaerobic bacteria. Am J Obstet Gynecol. 1977;127:250–254. doi: 10.1016/0002-9378(77)90463-x. [DOI] [PubMed] [Google Scholar]
- 156.Thadepalli H, Bach VT, Davidson EC., Jr Antimicrobial effect of amniotic fluid. Obstet Gynecol. 1978;52:198–204. [PubMed] [Google Scholar]
- 157.Thadepalli H, Gangopadhyay PK, Maidman JE. Amniotic fluid analysis for antimicrobial factors. Int J Gynaecol Obstet. 1982;20:65–72. doi: 10.1016/0020-7292(82)90047-9. [DOI] [PubMed] [Google Scholar]
- 158.Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–236. [PubMed] [Google Scholar]
- 159.Tsui CC, Copeland NG, Gilbert DJ, Jenkins NA, Barnes C, Worley PF. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J Neurosci. 1996;16:2463–2478. doi: 10.1523/JNEUROSCI.16-08-02463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vaisbuch E, Romero R, Erez O, Mazaki-Tovi S, Kusanovic JP, Soto E, et al. Fragment Bb in amniotic fluid: Evidence for complement activation by the alternative pathway in women with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2009 doi: 10.1080/14767050902994663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.van Rossum AP, Limburg PC, Kallenberg CG. Activation, apoptosis, and clearance of neutrophils in Wegener's granulomatosis. Ann N Y Acad Sci. 2005;1051:1–11. doi: 10.1196/annals.1361.041. [DOI] [PubMed] [Google Scholar]
- 162.van Rossum AP, Pas HH, Fazzini F, Huitema MG, Limburg PC, Jonkman MF, et al. Abundance of the long pentraxin PTX3 at sites of leukocytoclastic lesions in patients with small-vessel vasculitis. Arthritis Rheum. 2006;54:986–991. doi: 10.1002/art.21669. [DOI] [PubMed] [Google Scholar]
- 163.Wisniewski HG, Vilcek J. Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Rev. 2004;15:129–146. doi: 10.1016/j.cytogfr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 164.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 165.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1997;177:825–830. doi: 10.1016/s0002-9378(97)70276-x. [DOI] [PubMed] [Google Scholar]
- 166.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 167.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999;181:773–779. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 168.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 169.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–681. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 170.Yoon BH, Romero R, Shim JY, Shim SS, Kim CJ, Jun JK. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med. 2003;14:85–90. doi: 10.1080/jmf.14.2.85.90. [DOI] [PubMed] [Google Scholar]
- 171.Zhang D, Jiang SL, Rzewnicki D, Samols D, Kushner I. The effect of interleukin-1 on C-reactive protein expression in Hep3B cells is exerted at the transcriptional level. Biochem J. 1995;310(Pt 1):143–148. doi: 10.1042/bj3100143. [DOI] [PMC free article] [PubMed] [Google Scholar]