Abstract
Objective
In this study, we investigated whether dyslipidemia-associated perturbed invariant natural killer T (iNKT) cell function is due to intrinsic changes in iNKT cells or defects in the ability of antigen-presenting cells to activate iNKT cells.
Methods and Results
We compared iNKT cell expansion and cytokine production in C57BL/6J (B6) and apolipoprotein E-deficient (apoE−/−) mice. In response to in vivo stimulation with α-galactosylceramide, a prototypic iNKT cell glycolipid antigen, apoE−/− mice showed significantly decreased splenic iNKT cell expansion at 3 days after injection, a profile associated with iNKT cell anergy due to chronic stimulation. This decrease in expansion and cytokine production was accompanied by a 2-fold increase in percentage of iNKT cells expressing the inhibitory marker programmed death-1 in apoE−/− mice compared with controls. However, in vivo and in vitro blockade of programmed death-1 using monoclonal antibody was not able to restore functions of iNKT cells from apoE−/− mice to B6 levels. iNKT cells from apoE−/− mice also had increased intracellular T cell receptor and Ly49 expression, a phenotype associated with previous activation. Changes in iNKT cell functions were cell autonomous, because dendritic cells from apoE−/− mice were able to activate B6 iNKT cells, but iNKT cells from apoE−/− mice were not able to respond to B6 dendritic cells.
Conclusion
These data suggest that chronic dyslipidemia induces an iNKT cell phenotype that is unresponsive to further simulation by exogenous glycolipid and that sustained unresponsiveness is iNKT cell intrinsic.
Keywords: hypercholesterolemia, lymphocytes, immunology, lipid, antigen
Natural killer T (NKT) cells are a unique subset of T lymphocytes that share common surface receptors with both conventional T cells (T cell receptor [TCR] and cluster of differentiation [CD]4) and NK cells (NK1.1 and Ly49) and are found in both humans and mice. A major subset of NKT cells, type I NKT cells or invariant (i)NKT cells, have restricted T cell receptor expression (Vα14-Jα18/V/β8 in mice, Vα24-Jα18/Vβ11 in humans).1 iNKT cells recognize glycolipid antigen presented by the major histocompatibility complex class I-like molecule CD1d on antigen-presenting cells (APCs), such as dendritic cells (DCs), B cells, and to a lesser extent, macrophages.2
iNKT cells play an important role in regulating innate and adaptive immunity. On activation, iNKT cells rapidly secrete large amounts of antiinflammatory cytokines, such as inter-leukin 4 (IL-4), IL-10, and IL-13, and proinflammatory cytokines, such as interferon (IFN)-γ, which allows for a wide range of regulatory potential.3 Activated iNKT cells can also promote DC maturation and monocyte activation by signaling through CD1d4 and are capable of promoting tolerance by communicating with regulatory T cells.5
Although few “natural” ligands of iNKT cells have been identified, it is well established that iNKT cells respond strongly to, and are specifically activated by, α-galactosylceramide (α-GalCer).6 Animal studies have demonstrated that activation of iNKT cells by in vivo administration of α-GalCer can mediate both protective and pathological immune responses. For example, α-GalCer has been shown to suppress inflammation in mouse models of autoimmune diseases, such as type 1 diabetes and multiple sclerosis.7,8 Our laboratory,9 as well as others, 10–12 has shown that activation of iNKT cells with α-GalCer is proatherogenic. Because of the strong immunoregulatory potential of iNKT cells, α-GalCer has been proposed as a viable human therapy for autoimmune and inflammatory diseases, as well as cancer.13 Therefore, understanding how environmental factors can influence or skew the iNKT cell response to glycolipids is extremely important.
Interestingly, Nakai et al11 demonstrated that C57BL/B6 mice fed a high-fat diet have decreased iNKT cell numbers and functions, as measured by cytokine secretion. Our laboratory has reported similar changes in iNKT cell dynamics in spontaneously dyslipidemic apolipoprotein E-deficient (apoE−/−) mice.9 These studies demonstrate, perhaps not surprisingly, that iNKT cells are extremely sensitive to their lipid environment. It is not known, however, whether diet and lipid-mediated changes in iNKT cell functions are due to intrinsic changes in iNKT cells or defects in the ability of APCs, such as DCs, to activate iNKT cells.
In the current study, we investigated the mechanism(s) by which spontaneous dyslipidemia decreases the responsiveness of iNKT cells to exogenous glycolipid antigen. We demonstrate that hyperlipidemia chronically activates iNKT cells, placing them in an irreversible anergic-like state and that these changes in the iNKT cell phenotype are irreversible once established and independent of the source of APC.
Methods
Animals
Male B6 and B6.129P2-Apoetm1Unc/J (apoE−/−) mice were originally purchased from The Jackson Laboratories and maintained in our colonies under pathogen-free conditions. ApoE−/− mice have been backcrossed to B6 mice for more than 10 generations. Animals were fed standard chow diet ad libitum. All animal studies were approved by the Institutional Animal Care and Use Committee of Vanderbilt University.
Flow Cytometry
Single-cell suspensions of the spleen and liver were prepared and stained with fluorescently labeled monoclonal antibodies as described previously.14 Flow cytometry was performed using a FAC-SCalibur instrument (BD Pharmingen), and the data were analyzed using FCS Express software (De Novo Software). The iNKT cell population was defined as B220− TCRβint tetramer+.
Intracellular Cytokine Staining
For intracellular cytokine staining, splenocytes were stimulated with 25 to 100 ng/mL α-GalCer in the presence of GolgiStop for 4 hours. Cells were fixed and permeabilized with Cytofix/Cytoperm (BD Pharmingen) reagents according to the manufacturer’s protocol.
ELISA
Mouse IL-4 and IFN-γ were measured by standard sandwich ELISA according to the manufacturer’s protocol (BD Pharmingen).
Measurement of In Vitro and In Vivo Responses to α-GalCer
Four-microgram α-GalCer reconstituted in 0.5% polysorbate (vehicle) was IP injected into mice, splenocytes stained and analyzed by flow cytometry, and serum IL-4 and IFN-γ measured by ELISA.
In vitro, splenocytes were plated in 96-well plates at 2.5×105 cells per well in RPMI medium 1640 (Hyclone) containing 10% FBS (Sigma), penicillin-streptomycin with 50 µmol/L l-glutamine (Gibco), and 50 µmol/L β-mercaptoethanol (Sigma) in the presence of varying concentrations of α-GalCer. Supernatants were collected after 72 hours of culture, and cytokine levels were determined by ELISA. For proliferation studies, splenocytes were labeled with carboxyfluorescein succinimidyl ester (CFSE) and cultured in the presence of 50 U/mL recombinant mouse IL-2 (BD Biosciences) as described previously.15
Isolation of Splenic DCs
Splenic DCs were isolated as described previously.14 Briefly, spleens were digested with 1 mg/mL collagenase type II (Sigma) in Hanks’ balanced salt solution (Mediatech) for 30 minutes. DCs were enriched by magnetic sorting using anti-CD11c microbeads (Miltenyi Biotech) according to the manufacturer’s protocol. The purity of the enriched CD11c+ population was 80 to 85% (data not shown). Purified DC were pulsed with 50 ng/mL α-GalCer for 30 minutes, washed twice with RPMI medium 1640, and then cultured (5×104 cell per well of a 96-well plate) with iNKT cells. Bone marrow-derived DCs were isolated and cultured as previously described.16
Enrichment of iNKT Cells
Liver iNKT cells were enriched as previously described.14 Briefly, livers were perfused with PBS, digested with type II collagenase (Sigma), and then pressed through a 70-µm cell strainer. To remove adherent APCs, 2 rounds of panning, 1 hour each, were performed. The enriched iNKT cells were cocultured for 48 hours with α-GalCer-loaded DCs as described above.
Concanavalin A (ConA)-Induced Hepatitis
Mice were injected IV with 350 µg ConA (Sigma) in 200 µL PBS and killed 24 hours after injection. Hematoxylin and eosin staining of paraffin-embedded livers sections was performed as previously described.17
Serum Lipid and Free Fatty Acid Analyses
Total serum cholesterol and triglyceride were measured in fasting mice as previously described.18 Free fatty analysis was conducted by gas chromatography by the Vanderbilt Mouse Metabolic Phenotyping Center.
Statistics
Statistical significance between 2 groups was determined using a Student’s t test, and significance between multiple groups was determined using 1-way ANOVA with Newman–Keuls multiple comparison test for post hoc analysis. A value of P<0.05 was considered statistically significant, and all statistical analyses were performed using GraphPad Prism software.
Results
ApoE−/− Mice Have Decreased Percentages and Absolute Numbers of Peripheral iNKT Cells
Previously, we demonstrated that apoE−/− mice have an age-associated decrease in iNKT cell numbers.9 However, it was not determined whether this apparent decrease was due to systemic changes in iNKT cells or was the result of impaired iNKT cell development in the thymus. To address this question, we compared iNKT cell numbers in the spleen, liver, and thymus of older (≥16 week old) age-matched B6 and apoE−/− mice. As previously reported,9 we observed a significant decrease in the percentages of iNKT cells (TCRβint tetramer+) in livers and spleens of apoE−/− mice compared with B6 mice (Figure 1A). Given that there was no difference in the numbers of total lymphocytes in liver and spleen, the decrease in iNKT cell percentages translates into a decrease in absolute numbers of iNKT cells (Figure 1B). Thymic iNKT cell percentages and absolute numbers did not differ between apoE−/− and B6 mice (Figure 1A and 1B). In addition, surface markers of thymic iNKT cell differentiation, CD44 and NK1.1, were not different between the 2 strains (Figure 1C). The data suggest that defects in iNKT cell numbers occur in the periphery and are not due to decreased thymic differentiation.
Figure 1.
Decreased iNKT cell numbers and functions in apoE−/− mice. A, iNKT cell percentages in the thymus, spleen, and liver of B6 and apoE−/− mice. Shown are representative dot plots of 3 experiments with 3 mice per group. B, Absolute iNKT cell numbers in the spleen and thymus. Bars represent the mean and SE. Data are representative of 3 experiments. C, Flow cytometry analyses of NK1.1 and CD44 on iNKT cells from the thymus of B6 and apoE−/− mice. D, Splenocytes from B6 and apoE−/− mice were cultured for 72 hours in the presence of the indicated concentrations of α-GalCer. IFN-γ and IL-4 were determined by ELISA. Data points represent the mean and SE of 3 mice in each group. Shown is 1 representative experiment of 3. E, Mice were injected IP with 4 µg α-GalCer, and cytokine production was determined by ELISA. Serum IFN-γ was measured 24 hours after injection, and IL-4 was measured 2 hour after injection. Shown are the mean and SD of 3 mice per group. F, Mice were injected with 4 µg α-GalCer, and 24 hours after injection, activation of splenic B cells, NK cells, T cells, and DCs was determined by flow cytometry. Shown are representative histograms of 5 mice in each group. *P<0.05 as determined by Student’s t test.
Because of this, we hypothesized that spontaneous hyperlipidemia could affect iNKT cell responsiveness. Not surprisingly, when we stimulated whole splenocytes with α-GalCer in vitro, we observed blunted IL-4 and IFN-γ production by apoE−/− splenocytes compared with age-matched controls (Figure 1D). Notably, the decrease in cytokine production in apoE−/− cultures was greater than what would be expected if it was due only to decreased absolute iNKT cell numbers. To determine whether this degree of iNKT cell hyporesponsiveness also occurred in vivo, we injected apoE−/− and B6 mice with 4 µg/mouse of α-GalCer or vehicle IP. At 2 and 24 hours following injection (times associated with peak iNKT cell-mediated IL-4 and IFN-γ production, respectively), we observed that similar to in vitro analyses, the in vivo response to α-GalCer was also blunted in apoE−/− mice, as indicated by serum IFN-γ and IL-4 (Figure 1E). This was associated with a decreased ability to trans-activate other immune cells downstream of iNKT cell activation, such as B cells, NK cells, T cells, and DCs (Figure 1F).
Decreased Cytokine Production in ApoE−/− Mice Following α-GalCer Stimulation Is iNKT Cell Specific
Although the apoE−/− mice had a 2-fold decrease in the absolute numbers of iNKT cells in the spleen and liver, the cytokine response to α-GalCer stimulation in vitro and in vivo was disproportionately suppressed and could not be completely attributed to decreased iNKT cell numbers. Because in vivo activation or in vitro stimulation of splenocytes with α-GalCer allows for the potential of multiple cellular cytokine sources, we conducted intracellular cytokine staining on iNKT cells isolated from apoE−/− and B6 mice. Following a 4-hour in vitro stimulation with α-GalCer, we observed a significant decrease in IFN-γ positive splenic iNKT cells in apoE−/− mice compared with controls (Figure 2A). The percentage of IL-4 positive iNKT cells was reduced in apoE−/− mice compared with B6 mice but did not reach statistical significance (Figure 2B).
Figure 2.
Decreased cytokine production is iNKT cell specific. Splenocytes from B6 and apoE−/− mice were stimulated for 4 hours in vitro with 25 ng/mL of α-GalCer, and intracellular IFN-γ and IL-4 were determined by flow cytometry. A, Representative dot plots from 3 experiments. B, Percentages of IFNγ+ (left panel) and IL-4+ (right panel) iNKT cells. *P<0.05 as determined by Student’s t test.
iNKT From ApoE−/− Mice Cells Resemble Exhausted, Chronically Activated Cells
Because we observed a decrease in the ability of apoE−/− splenic iNKT cells to produce cytokines on stimulation with α-GalCer, we examined the ability of iNKT cells to expand in vivo following specific activation. Previous studies have demonstrated that on in vivo activation with α-GalCer, iNKT cells respond by initially contracting at 24 hours and then expanding 3 days following α-GalCer injection.15 iNKT cells that have been rendered anergic due to a previous antigen exposure, however, fail to respond in this manner.15 Therefore, to test the hypothesis that iNKT cells in hyperlipidemic apoE−/− mice display an anergic phenotype, we injected B6 or apoE−/− mice with 4 µg/mouse of α-GalCer (or vehicle) and tracked changes in the splenic iNKT cell population over time. As shown in Figure 3A, the B6 iNKT cells responded as expected following α-GalCer injection. Although iNKT cells from apoE−/− mice initially contracted 24 hours following injection, these cells showed blunted expansion at 3 days. Quantitatively, this resulted in a significant decrease in the ability of iNKT cells from apoE−/− mice to proliferate in response to α-GalCer stimulation (Figure 3B, top panel). This difference was also significant following normalization to the baseline absolute numbers of iNKT cells (Figure 3B, bottom panel). To determine whether these changes in iNKT cell responses had physiological consequences, we compared apoE−/− and B6 mice in iNKT cell-dependent ConA-induced hepatitis. As expected, livers from apoE−/− mice showed decreased inflammatory infiltration compared with B6 mice, indicating decreased iNKT cell function (Figure 3C). Therefore, iNKT cells from apoE−/− mice respond to antigen stimulation in vivo similar to those from wild-type mice rendered anergic by repeated activation, and this abrogation of iNKT cell function is relevant to modulation of disease pathology.
Figure 3.
iNKT cells from apoE−/− mice display an anergic phenotype. A, B6 and apoE−/− mice were injected with 4 µg α-GalCer, and splenic iNKT cells were analyzed by flow cytometry at 0 hours, 24 hours, 3 days, 7 days, and 1 month following injection. Shown are representative dot plots of 3 mice per group gating on B220− cells. B, Flow cytometry data as shown by percent tetramer+ cells (gated on B220−TCRβint; top panel). Fold change over baseline in percent tetramer+ cells (bottom panel). C, B6 and apoE−/− mice were injected IV with 350 µg ConA in 200 µL PBS and killed 24 hours later. ConA-induced hepatitis (black arrowheads show areas of lymphocyte infiltration) was assessed by hematoxylin and eosin staining of paraffin-embedded liver sections. Shown are representative sections from 4 mice per group.
Changes in iNKT Cell Activation Are Cell Autonomous and Not Due to Defective Antigen Presentation
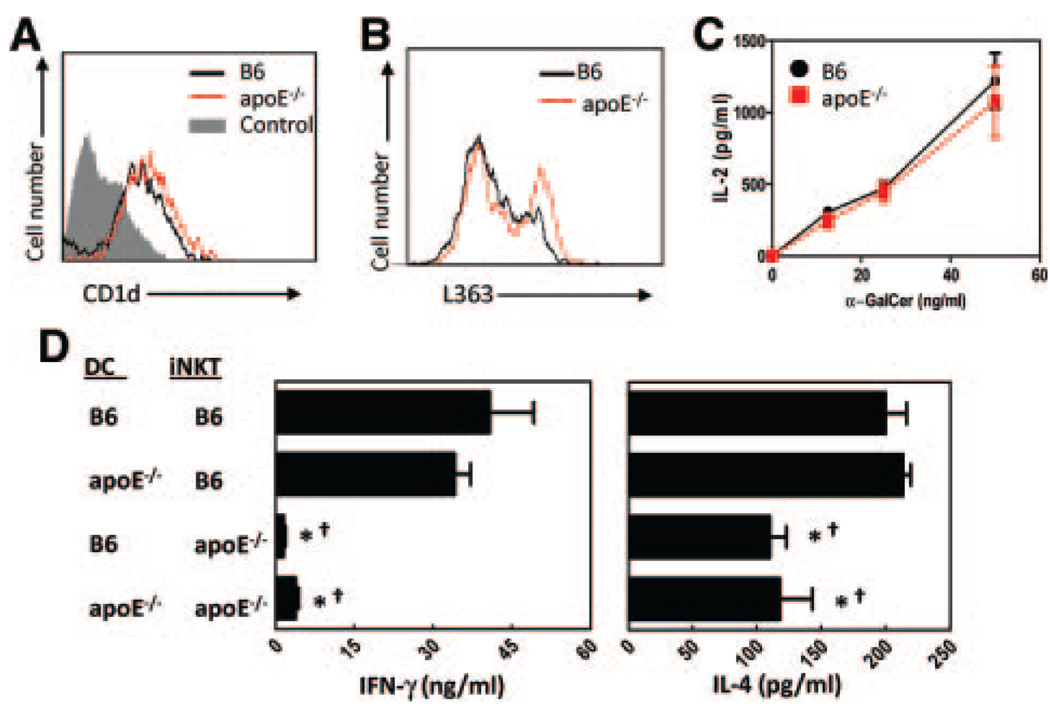
Given that CD1d on APCs is required for presentation of glycolipid antigen to iNKT cells, and previous reports suggested that apoE increases uptake and presentation of glycolipid antigens by DCs,19 we isolated splenic DCs from B6 and apoE−/− mice and compared CD1d expression. Flow cytometry analyses demonstrated that CD1d expression was not decreased on apoE−/− DCs compared with B6 DCs (Figure 4A). To examine differences in glycolipid antigen presentation, splenocytes from B6 and apoE−/− mice were loaded with the α-GalCer precursor galactosyl(α1 to 2)galactosylce-ramide (GGC) in vitro and then incubated with a monoclonal antibody specific to CD1d:α-GalCer complexes (L363).20 GGC must undergo lysosomal processing to be loaded on CD1d molecules for surface presentation. Flow cytometry analyses showed that apoE−/− DCs exhibited increased CD1d loading and presentation of processed GGC at the cell surface (Figure 4B). In addition, to demonstrate that the in vivo changes that we observed in iNKT cells from apoE−/− mice were not due to defective ability of apoE−/− DCs to process and present antigen, we grew bone marrow-derived DCs from B6 and apoE−/− mice. DCs were pulsed in vitro with α-GalCer and then incubated with the Vα14+ iNKT cell hybridoma DN32.D3. We observed that DCs from both strains of mice were able to activate the iNKT cell hybridoma with equal efficiency (Figure 4C). Finally, when lethally irradiated low-density lipoprotein receptor−/− mice were reconstituted with B6 bone marrow (to restore the apoE-low-density lipoprotein receptor axis on hematopoietic cells, yet retain dyslipidemia), similar decreases in iNKT cell functions were observed when mice were fed chow diet (supplemental Figure I, available online at http://atvb.ahajournals.org).
Figure 4.
DCs from apoE−/− mice retain the ability to activate iNKT cells. A, CD11c+ splenic DC expression of CD1d as determined by flow cytometry. B, Splenocytes from B6 and apoE−/− mice were incubated 18 hours with 50 ng/mL GGC, then stained with 1 µg/mL L363 monoclonal antibody to detect surface CD1d:α-GalCer complexes. Flow cytometry analyses were performed by gating on CD11c+ cells. Shown are representative histograms of 2 experiments using 3 mice per group. C, CD11c+ DCs were purified from spleens of B6 and apoE−/− mice, loaded with 50 ng/mL α-GalCer for 30 minutes, then incubated with the iNKT cell hybridoma DN32.D3 for 48 hours. Activation of the hybridoma, as measured by IL-2 production in culture supernatants, was determined by ELISA. α-GalCer alone (with no DC) did not result in IL-2 production. D, Purified splenic DCs were incubated with 50 ng/mL α-GalCer for 30 minutes, then added to purified iNKT cells and cocultured for 72 hours. IFN-γ (left panel) and IL-4 (right panel) were determined by ELISA. *P<0.05 when compared with B6 DCs cultured with B6 iNKT cells, and †P<0.05 when compared with apoE−/− DCs cultured with B6 iNKT cells, as determined by 1-way ANOVA.
To determine whether iNKT cells from apoE−/− mice can respond to wild-type APCs, splenic DCs were isolated from B6 and apoE−/− mice, pulsed with α-GalCer, and mixed with iNKT cells from B6 or apoE−/− mice. Analysis of cultured supernatants demonstrated that although the apoE−/− DCs were able to stimulate cytokine production by B6 iNKT cells, the apoE−/− iNKT cells had a reduced ability to respond to B6 DCs (Figure 4D). Collectively, these data demonstrate that in our system, absence of apoE does not directly affect antigen presentation or iNKT cell responsiveness but that changes are more likely due to a dyslipidemic environment.
iNKT Cells From ApoE−/− Mice Appear To Be Spontaneously Activated
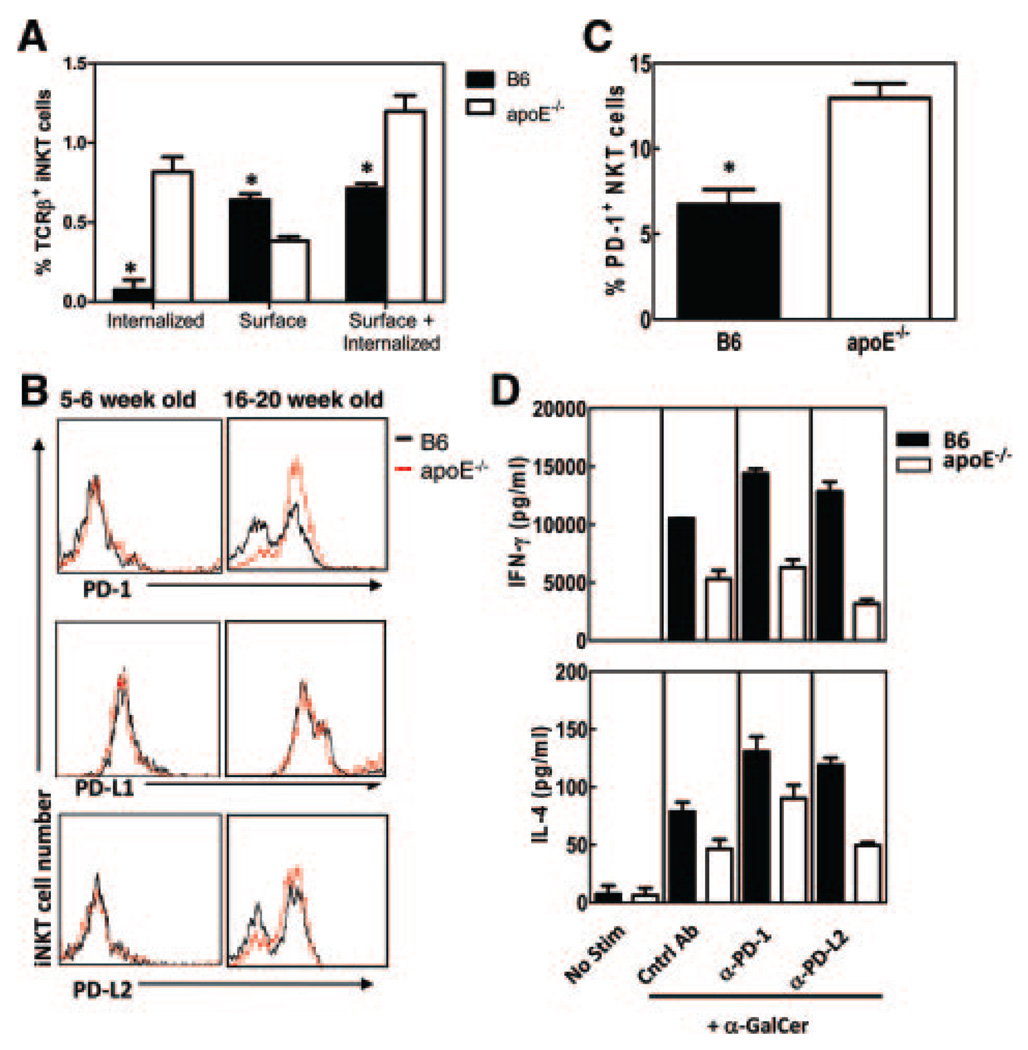
It has been previously demonstrated that on activation, the surface TCR expression on iNKT cells is downregulated via internalization.21 To determine whether this phenomenon was responsible for the apparent decrease in absolute numbers of iNKT cells in apoE−/− mice, we performed intracellular and extracellular staining on splenocytes using CD1d/α-GalCer tetramers and anti-TCRβ antibodies. We observed that spleens of B6 mice had similar percentages of iNKT cells following both intracellular and extracellular staining (Figure 5A, comparing surface with surface+internalized). Because surface receptors may be stained during the intracellular staining process, these data suggest that the majority of the control iNKT cells have not internalized their TCR and are not activated. In contrast, intracellular staining of apoE−/− splenocytes demonstrated an increase in the percentage of iNKT cells that had internalized their TCRβ, supporting the hypothesis that iNKT cells from hyperlipidemic animals are spontaneously activated. Interestingly, when the numbers of iNKT cells that had internalized their TCR were accounted for, the apoE−/− mice had increased numbers of iNKT cells compared with B6 control mice. These data suggest that iNKT cells from apoE−/− mice are spontaneously activated and have internalized their TCR, accounting for the decreased detection by conventional surface staining.
Figure 5.
iNKT cells from apoE−/− mice have increased PD-1 expression. A, iNKT cells from 16- to 20-week-old B6 and apoE−/− mice were stained for internalized and surface TCRβ expression and analyzed by flow cytometry. Internalized TCRβ was determined by subtracting surface TCRβ from surface+internalized TCRβ. Shown are representative histograms from 3 mice per group. B, Ex vivo PD-1, PD-L1, and PD-L2 expression on iNKT cells from 5- to 6-week-old (left panels) and 16- to 20-week-old (right panels) B6 and apoE−/− mice. Shown are representative histograms from 3 experiments. C, Percent PD-1+ iNKT cells ex vivo in 16- to 20-week-old B6 and apoE−/− mice. D, Splenocytes were cultured in vitro in the presence or absence of α-GalCer, with 50 µg/mL of control immunoglobulin, anti-PD-1, or anti-PD-L2 antibodies. After 72 hours, IFN-γ (top panel) and IL-4 (bottom panel) were determined by ELISA in culture supernatants. *P<0.05 as determined by Student’s t test. No stim indicates no stimulation.
Recent studies have demonstrated that T cells from mice with chronic viral infection express increased levels of the inhibitory markers programmed death-1 (PD-1) receptor and PD-1 ligand-1 (PD-L1).22 Increased expression of PD-1 and PD-L1 has also been demonstrated on iNKT cells rendered anergic by α-GalCer stimulation.23,24 Given that iNKT cells from hyperlipidemic apoE−/− mice appear to be spontaneously anergic both ex vivo and in vivo, we examined the expression of the inhibitory markers PD-1, PD-L1, PD-L2, and Ly49 on iNKT cells of naive B6 and apoE−/− mice. Flow cytometry analyses demonstrated that consistent with previous reports, iNKT cells from B6 mice have some constitutive expression of PD-1, PD-L1, and PD-L2 (Figure 5B, right panels). However, the percentage of PD-1 positive iNKT cells was significantly increased in apoE−/− mice (Figure 5C). These increases were not observed in iNKT cells from young 5- to 6-week-old apoE−/− mice (Figure 5B, left panels), demonstrating that changes in iNKT cells were acquired and not directly the result of developmental effects of apoE−/− deficiency. In addition, apoE−/− iNKT cells had increased expression of the inhibitory receptor Ly49 (data not shown). These data indicate that apoE−/− iNKT cells exhibit an exhausted phenotype perhaps due to increased activation by accumulating endogenous lipid antigens.
PD-1 Blockade Can Neither Rescue Nor Prevent Downregulation of iNKT Cell Responses in ApoE−/− Mice
To determine whether blockade of PD-1 could restore apoE−/− iNKT cell responses as previously described,23 we incubated whole splenocytes from naive B6 and apoE−/− mice with 50 ng/mL α-GalCer in the presence of a PD-1 blocking antibody or isotype control antibody. Analysis of 72-hour supernatants demonstrated a partial increase in cytokine secretion in apoE−/− mice (Figure 5D). However, PD-1 blockade did not result in normalization of cytokine levels in supernatants of apoE−/− mice. These data suggest that blocking PD-1 cannot rescue the anergic phenotype of apoE−/− iNKT cells.
Previous work by Parekh et al15 demonstrated that although PD-1 blockade could not rescue anergic iNKT cells, it could prevent the development of anergy in this cell population if conducted before activation with α-GalCer. To determine whether this also applied to our apoEv model, we injected apoE−/− mice with anti-PD-1 or control rat immunoglobulin beginning at weaning (≈4 weeks of age). Mice received antibody for 6 weeks, after which responses to α-GalCer were analyzed. We observed that PD-1 blockade could not prevent induction of iNKT cell anergy in apoE−/− mice (data not shown). These data suggest that upregulation of PD-1 is not the primary mechanism by which iNKT cells become nonresponsive in apoE−/− mice.
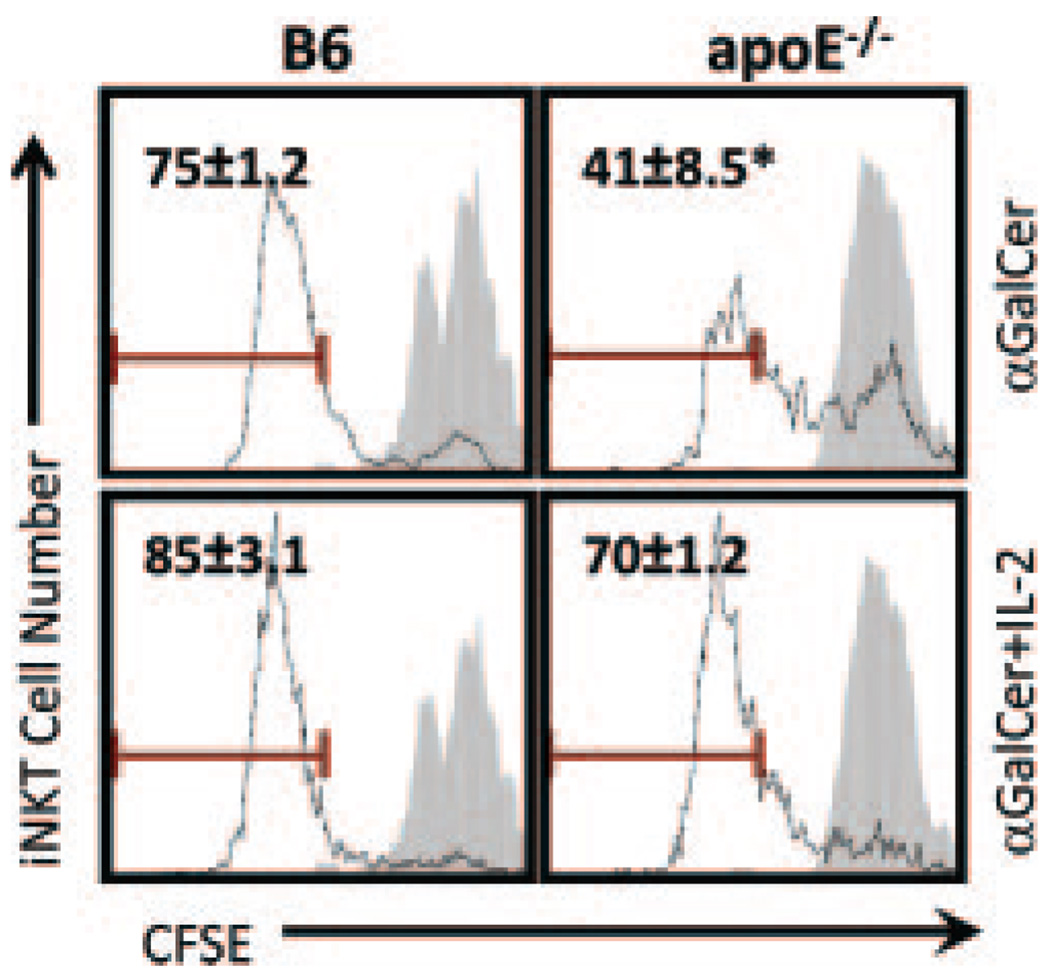
Exogenous IL-2 Restores ApoE−/− iNKT Cell Proliferation In Vitro
It is known that administration of exogenous cytokines, such as IL-2, can overcome conventional T cell exhaustion, as well as iNKT cell anergy.15 Given that iNKT cells from apoE−/− mice display an anergic-like phenotype, we tested the ability of exogenous IL-2 to restore iNKT cell responses in vitro. Splenocytes from B6 and apoE−/− mice were labeled with CFSE and stimulated with α-GalCer in the presence or absence of IL-2. As shown by the CFSE dilution profile of tetramer+ cells (Figure 6), iNKT cells from apoE−/− mice displayed decreased proliferation in response to α-GalCer as compared with B6 iNKT cells. Consistent with previous studies,15 addition of IL-2 resulted in restoration of the ability of apoE−/− iNKT cells to proliferate. These data indicate that iNKT cells from hyperlipidemic apoE−/− mice are anergic and that IL-2 is sufficient to largely overcome this anergy in vitro.
Figure 6.
IL-2 overcomes iNKT cell anergy in vitro. Splenocytes from B6 and apoE−/− mice were labeled with CFSE and cultured for 24 hours with α-GalCer (100 ng/mL) with or without IL-2 (50 U/mL). The cells were then washed, and cultures were continued for an additional 72 hours without α-GalCer, in the presence or absence of IL-2. After culture, CFSE dilution was analyzed on iNKT cells. Shown are representative histograms from 3 mice per group. *P<0.05 as determined by Student’s t test.
Discussion
iNKT cells are a unique subset of T cells that recognize glycolipid antigens and play a role in the regulation of inflammation and immunity. Furthermore, our laboratory9 and others10–12 have shown that activation of iNKT cells is proatherogenic. During conditions of spontaneous hyperlipidemia in apoE−/− mice9 and diet-induced dyslipidema,11 iNKT cell numbers and functions are decreased. However, the etiology behind these changes is not yet known. Modulation of iNKT cell responses is being clinically tested as a possible therapeutic for cancer,25 diabetes,26 and HIV27 in humans. In addition, it has been hypothesized that activating iNKT cells during immunization is a superior vaccination strategy.28 Thus, it is imperative to understand how the lipid environment might dampen or skew the iNKT cell response.
In the current study, we sought to examine the mechanisms by which chronic hyperlipidemia might decrease iNKT cell numbers and functions. We observed that apoE−/− mice have decreased numbers of iNKT cells in spleens and livers compared with B6 controls and that iNKT cells present in the spleens of apoE−/− mice have an impaired ability to respond to exogenous stimulation by the glycolipid α-GalCer. This decreased cytokine production seemed to affect IFN-γ responses more than IL-4, suggesting that T helper 1 responses may be more affected than T helper 2.
It is well established that glycolipids play an important role in the development of iNKT cells within the thymus and that normal thymic development of these iNKT cells requires the recognition of natural lipid ligands presented by CD1d. Previous studies have suggested that the endogenous glycosphingolipid isoglobotrihexosylceramide is important for iNKT cell development and self-recognition.29 Because a spontaneous increase in circulating lipids, as seen in apoE−/− mice, could affect the development of iNKT cells and subsequently lead to decreased peripheral numbers, we analyzed thymic iNKT cells from apoE−/− and B6 mice by flow cytometry. Our data suggest that decreased iNKT cell numbers in apoE−/− mice are not the result of defects in thymic development, because numbers and maturation markers of iNKT cells in the thymus remained largely unchanged. Instead, our data support the hypothesis that the iNKT cell defect results from changes in the periphery. This is in stark contrast to studies in mice with deficiencies in the Niemann–Pick type C1 and C2 proteins, where reductions in iNKT cell numbers were observed in the thymus and peripheral lymphoid tissues.30–32 This was also found to be the case in mouse models of other lysosomal lipid storage diseases, such as mice deficient for α- or β-galactosidase or hexosaminidases A and S.33 Therefore, decreased numbers of iNKT cells in the peripheral lymphoid organs of apoE−/− mice suggest that the endogenous glycolipids necessary to elicit normal selection of iNKT cells in the thymus are not affected by lipid accumulation.
Recent evidence has shown that B cell-mediated activation of iNKT cells is enhanced by apoE and is dependent on the low-density lipoprotein receptor.34 In addition, findings from Van den Elzen et al19 have shown that circulating lipoproteins, such as very low-density lipoproteins, can enhance iNKT cell responses to glycolipid antigen presented by DCs. These experiments elegantly demonstrated that apoE may enhance glycolipid uptake. However, there was no evidence that glycolipid and apoE interact in vivo or that apoE was necessary for normal iNKT cell responses to exogenous lipid antigen. Although the absence of apoE and the effect this might have on antigen uptake is still an important consideration in our system, we have several pieces of evidence suggesting that the absence of apoE alone cannot explain the difference in iNKT cell activation observed in hyperlipidemic mice. First, in our system, DCs from apoE−/− mice did not appear to be compromised in their ability to present antigen to B6 iNKT cells. Additionally, CD1d loading of GGC and presentation on the cell surface, as measured by L363 antibody staining, was not compromised in apoE−/− DCs. Therefore, we conclude that although apoE may increase iNKT activation under normolipidemic conditions, the functional defects that we observe in apoE−/− mice cannot be completely attributed to the lack of apoE.
It has been shown previously in atherosclerosis studies that lipids, such as the glycosphingolipid β-glucosylceramide35 and the disialoganglioside GD3,36 accumulate in the serum of apoE−/− mice as well as in humans. In addition, natural activation of iNKT cells during microbial infection is often dependent on both IL-12 and presentation of endogenous glycolipid antigen by DCs.37 Thus, a possible explanation for the iNKT cell hyporesponsiveness that we observe in apoE−/− mice is that these cells are chronically activated by increased levels of endogenous glycolipid. Although recent data from VanderLaan et al12 suggest that endogenous iNKT antigen was not present in the serum of apoE−/− mice fed a high-fat diet, it is possible that presence of an endogenous ligand in tissue, such as in the liver, spleen, or lymph node, and not serum is responsible for the changes in iNKT cells that we observed. Alternatively, given that glycolipid antigens are processed and loaded onto CD1d within the APC after uptake, lysosomal accumulation of lipids during hyperlipidemia may play a role in the differences that we observe. Supporting this possibility, a recent study from Bai et al38 shows that lipid exchange within the lysosome is regulated by lipid structure as well as acidic lysosomal pH. However, our results indicate that DCs from apoE−/− mice retain their ability to activate iNKT cells, suggesting that the iNKT cell hyporesponsiveness in apoE−/− mice is due to chronic activation rather than defective antigen presentation.
Previous studies have shown that repeated activation of conventional T cells, such as in chronic viral infection, results in T cell exhaustion.39 Similarly, repeated activation of iNKT cells by α-GalCer also results in a functionally unresponsive anergic phenotype.15 Our data show that apoE−/− iNKT cells display a phenotype similar to those rendered anergic due to repeated activation. This evidence supports our hypothesis that spontaneous dyslipidemia, as observed in apoE−/− mice, leads to chronic activation of iNKT cells. Several recent reports have shown that the inhibitory receptor PD-1 is upregulated on exhausted T cells due to chronic activation.40,41 In addition, PD-1 has been implicated in the induction and maintenance of iNKT cell anergy.23 In a recent study by Parekh et al,24 it was shown that blocking PD-1/PD-L interactions could prevent iNKT cell anergy but could not overcome anergy once established. Our study illustrates that hyperlipidemic apoE−/− mice have spontaneously increased PD-1 expression on iNKT cells, although blocking PD-1 in vitro was not able to restore responsiveness to α-GalCer stimulation. Consistent with our data, recent clinical findings in HIV-infected humans indicated that these patients have increased PD-1 expression on iNKT cells, and PD-1 blockade did not restore iNKT cell function.36 Taken together, these data suggest that in our system, decreased functionality of chronically activated iNKT cells is not dependent on PD-1 expression and may be irreversible once established.
It is known that iNKT cells become undetectable with tetramer soon after in vivo stimulation with α-GalCer due to surface TCR downregulation.18 Our flow cytometry analyses demonstrate that there is a significant increase in the intracellular TCRβ in the apoE−/− mice as compared with B6 mice. Importantly, these studies were performed ex vivo without exogenous stimulation. In addition, we have shown that hyperlipidemic apoE−/− mice have modest but significant increases in myristic acid (14:0) and palmitic acid (16:0) (supplemental Figure II). Although the physiological relevance of this increase is not tested in the current study, the serum concentrations that we observe are in excess of fatty acid concentrations that have been shown to activate APCs through toll-like receptor activation.42 Therefore, it is possible that saturated fatty acid signaling in DCs via toll-like receptor 4 leads to the production of IL-12, thus activating iNKT cells by the alternative or indirect pathway.43 Collectively, these data support the hypothesis that the hyperlipidemic environment of the apoE−/− mice spontaneously activates the iNKT cells, rendering them unresponsive to further stimulation. Further studies to test this hypothesis are warranted.
In summary, our results indicate that an increase in circulating lipids leads to decreased iNKT cell functionality and that this dyslipidemia-associated decrease is iNKT cell intrinsic. The functional characteristics of iNKT cells are important to understand not only in chronic conditions of dyslipidemia, such as atherosclerosis, but also in diseases for which therapeutic approaches involving manipulation of iNKT cells are being considered. In addition, given that similar decreases in iNKT cell numbers and functions are observed in wild-type mice fed a high-fat diet,11 caution may be warranted in immunologic studies involving the use of lipid-laden diets and therapies that give rise to dyslipidemia.
Supplementary Material
Acknowledgments
We thank Curtis Gabriel and Nekeithia Wade for critical reading of the manuscript.
Sources of Funding
This work was supported by National Institutes of Health (NIH) National Research Service Award Training Grant in Mechanisms of Vascular Disease 5T32HL07751 (to N.A.B.), NIH Grant R01HL089310 (to A.S.M.), NIH Grant R01HL089667 (to L.V.K.), and NIH Grant R01AI45889 (to S.A.P). Y.V.M.-F. received support from the Irvington Institute Fellowship Program of the Cancer Research Institute.
Footnotes
Disclosures
S.A.P. is a paid consultant for Vaccinex, Inc., (Rochester, NY). No other authors have any financial conflicts to disclose.
References
- 1.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 2.Bezbradica JS, Stanic AK, Matsuki N, Bour-Jordan H, Bluestone JA, Thomas JW, Unutmaz D, Van Kaer L, Joyce S. Distinct roles of dendritic cells and B cells in Va14Ja18 natural T cell activation in vivo. J Immunol. 2005;174:4696–4705. doi: 10.4049/jimmunol.174.8.4696. [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 4.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roelofs-Haarhuis K, Wu X, Nowak M, Fang M, Artik S, Gleichmann E. Infectious nickel tolerance: a reciprocal interplay of tolerogenic APCs and T suppressor cells that is driven by immunization. J Immunol. 2003;171:2863–2872. doi: 10.4049/jimmunol.171.6.2863. [DOI] [PubMed] [Google Scholar]
- 6.Wilson MT, Singh AK, Van Kaer L. Immunotherapy with ligands of natural killer T cells. Trends Mol Med. 2002;8:225–231. doi: 10.1016/s1471-4914(02)02325-0. [DOI] [PubMed] [Google Scholar]
- 7.Wang B, Geng YB, Wang CR. CD1-restricted NK T cells protect nonobese diabetic mice from developing diabetes. J Exp Med. 2001;194:313–320. doi: 10.1084/jem.194.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh AK, Wilson MT, Hong S, Olivares-Villagomez D, Du C, Stanic AK, Joyce S, Sriram S, Koezuka Y, Van Kaer L. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801–1811. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Major AS, Wilson MT, McCaleb JL, Ru Su Y, Stanic AK, Joyce S, Van Kaer L, Fazio S, Linton MF. Quantitative and qualitative differences in proatherogenic NKT cells in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:2351–2357. doi: 10.1161/01.ATV.0000147112.84168.87. [DOI] [PubMed] [Google Scholar]
- 10.Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren HG, Hansson GK, Berne GP. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J Exp Med. 2004;199:417–422. doi: 10.1084/jem.20030997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakai Y, Iwabuchi K, Fujii S, Ishimori N, Dashtsoodol N, Watano K, Mishima T, Iwabuchi C, Tanaka S, Bezbradica JS, Nakayama T, Taniguchi M, Miyake S, Yamamura T, Kitabatake A, Joyce S, Van Kaer L, Onoe K. Natural killer T cells accelerate atherogenesis in mice. Blood. 2004;104:2051–2059. doi: 10.1182/blood-2003-10-3485. [DOI] [PubMed] [Google Scholar]
- 12.VanderLaan PA, Reardon CA, Sagiv Y, Blachowicz L, Lukens J, Nissenbaum M, Wang CR, Getz GS. Characterization of the natural killer T-cell response in an adoptive transfer model of atherosclerosis. Am J Pathol. 2007;170:1100–1107. doi: 10.2353/ajpath.2007.060188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu L, Gabriel CL, Parekh VV, Van Kaer L. Invariant natural killer T cells: innate-like T cells with potent immunomodulatory activities. Tissue Antigens. 2009;73:535–545. doi: 10.1111/j.1399-0039.2009.01256.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Lalani S, Parekh VV, Vincent TL, Wu L, Van Kaer L. Impact of bacteria on the phenotype, functions, and therapeutic activities of invariant NKT cells in mice. J Clin Invest. 2008;118:2301–2315. doi: 10.1172/JCI33071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun N, Wade NS, Wakeland EK, Major AS. Accelerated atherosclerosis is independent of feeding high fat diet in systemic lupus erythematosus-susceptible LDLr(−/−) mice. Lupus. 2008;17:1070–1078. doi: 10.1177/0961203308093551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van den Elzen P, Garg S, Leon L, Brigl M, Leadbetter EA, Gumperz JE, Dascher CC, Cheng TY, Sacks FM, Illarionov PA, Besra GS, Kent SC, Moody DB, Brenner MB. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005;437:906–910. doi: 10.1038/nature04001. [DOI] [PubMed] [Google Scholar]
- 20.Yu KO, Im JS, Illarionov PA, Ndonye RM, Howell AR, Besra GS, Porcelli SA. Production and characterization of monoclonal antibodies against complexes of the NKT cell ligand α-galactosylceramide bound to mouse CD1d. J Immunol Methods. 2007;323:11–23. doi: 10.1016/j.jim.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson MT, Johansson C, Olivares-Villagomez D, Singh AK, Stanic AK, Wang C-R, Joyce S, Van Kaer L. The response of natural killer T cells to glycolipid antigens in characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci U S A. 2003;19:10913–10918. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963–970. doi: 10.1016/j.molimm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 23.Chang WS, Kim JY, Kim YJ, Kim YS, Lee JM, Azuma M, Yagita H, Kang CY. Cutting edge: programmed death-1/programmed death ligand 1 interaction regulates the induction and maintenance of invariant NKT cell anergy. J Immunol. 2008;181:6707–6710. doi: 10.4049/jimmunol.181.10.6707. [DOI] [PubMed] [Google Scholar]
- 24.Parekh VV, Lalani S, Kim S, Halder R, Azuma M, Yagita H, Kumar V, Wu L, Kaer LV. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J Immunol. 2009;182:2816–2826. doi: 10.4049/jimmunol.0803648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motohashi S, Nakayama T. Clinical applications of natural killer T cell-based immunotherapy for cancer. Cancer Sci. 2008;99:638–645. doi: 10.1111/j.1349-7006.2008.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fletcher MT, Baxter AG. Clinical application of NKT cell biology in type I (autoimmune) diabetes mellitus. Immunol Cell Biol. 2009;87:315–323. doi: 10.1038/icb.2009.5. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Xu XN. NKT cells in HIV-1 infection. Cell Res. 2008;18:817–822. doi: 10.1038/cr.2008.85. [DOI] [PubMed] [Google Scholar]
- 28.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 29.Zhou D, Mattner J, Cantu C, III, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 30.Sagiv Y, Hudspeth K, Mattner J, Schrantz N, Stern RK, Zhou D, Savage PB, Teyton L, Bendelac A. Cutting edge: impaired glycosphingolipid trafficking and NKT cell development in mice lacking Niemann-Pick type C1 protein. J Immunol. 2006;177:26–30. doi: 10.4049/jimmunol.177.1.26. [DOI] [PubMed] [Google Scholar]
- 31.Schrantz N, Sagiv Y, Liu Y, Savage PB, Bendelac A, Teyton L. The Niemann-Pick type C2 protein loads isoglobotrihexosylceramide onto CD1d molecules and contributes to the thymic selection of NKT cells. J Exp Med. 2007;204:841–852. doi: 10.1084/jem.20061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schumann J, Facciotti F, Panza L, Michieletti M, Compostella F, Collmann A, Mori L, De Libero G. Differential alteration of lipid antigen presentation to NKT cells due to imbalances in lipid metabolism. Eur J Immunol. 2007;37:1431–1441. doi: 10.1002/eji.200737160. [DOI] [PubMed] [Google Scholar]
- 33.Gadola SD, Silk JD, Jeans A, Illarionov PA, Salio M, Besra GS, Dwek R, Butters TD, Platt FM, Cerundolo V. Impaired selection of invariant natural killer T cells in diverse mouse models of glycosphingolipid lysosomal storage diseases. J Exp Med. 2006;203:2293–2303. doi: 10.1084/jem.20060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allan LL, Hoefl K, Zheng DJ, Chung BK, Kozak FK, Tan R, Van den Elzen P. Apolipoprotein mediated lipid antigen presentation in B cells provides a pathway for innate help by NKT cells. Blood. 2009 doi: 10.1182/blood-2009-04-211417. [DOI] [PubMed] [Google Scholar]
- 35.Garner B, Priestman DA, Stocker R, Harvey DJ, Butters TD, Platt FM. Increased glycosphingolipid levels in serum and aortae of apolipoprotein E gene knockout mice. J Lipid Res. 2002;43:205–214. [PubMed] [Google Scholar]
- 36.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003;198:173–181. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 38.Bai L, Sagiv Y, Liu Y, Freigang S, Yu KO, Teyton L, Porcelli SA, Savage PB, Bendelac A. Lysosomal recycling terminates CD1d-mediated presentation of short and polyunsaturated variants of the NKT cell lipid antigen αGalCer. Proc Natl Acad Sci U S A. 2009;106:10254–10259. doi: 10.1073/pnas.0901228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 41.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8 + T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 42.Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol. 2005;174:5390–5397. doi: 10.4049/jimmunol.174.9.5390. [DOI] [PubMed] [Google Scholar]
- 43.Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol. 2007;19:354–364. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.