There have been many reports that the administration of free radical scavenger compounds can interdict oxygen free radicals and limit this cause of cell damage. Four such drugs, superoxide dismutase (1, 2), allopurinol (3), coenzyme Q10 (4), and glutathione (5) have been evaluated in different organs using a variety of experimental models, but there is little information about their comparative value. In this study, we have attempted to provide such information in male Lewis rats weighing 220–260 g. The animals were given water ad libitum for 12 h, anesthetized by intraperitoneal injection of 40 mg/kg sodium pentobarbital (Nembutal), and submitted to cross-clamping of the hepatic arterial and portal venous branches to the lateral and median lobes of the liver. This procedure (6) imposes a standard ischemic insult on about two-thirds of the liver, without causing splanchnic venous stasis since portal blood flow is well accommodated by the right and right caudate liver lobes that are left perfused. The perfused hepatic fraction is large enough to prevent hepatic failure and consequent deterioration of the animal during the ensuing 24 hr, after which the abdominal incision was reopened. Blood was taken from the abdominal aorta for liver function tests, and the animals were killed. The extent of ischemic necrosis in the damaged liver fragment was measured by making 3 thin (less than 2-mm) slices from each devascularized lobe and by staining these with tetranitro blue tetrazolium (TNBT, Sigma Corporation, Sigma Chemical Company, St. Louis, MO) in a phosphate buffer solution at pH 7.4 (6). The stained necrotic margin was measured by tracing it with a computer digitizer and Summa Sketch MM1201.
In control experiments, it was shown that crossclamping for 150 min was necessary before a high degree of necrosis was produced in the devascularized lobes. This was determined with biochemical and morphologic criteria (Fig. 1) in 36 experiments using crossclamp times of 30, 50, 90, 120, 150, and 180 min (n=6 at each time). SGOT and necrosis data from the 150-min crossclamping were the controls used for comparison with the test groups (Table 1).
Figure 1.
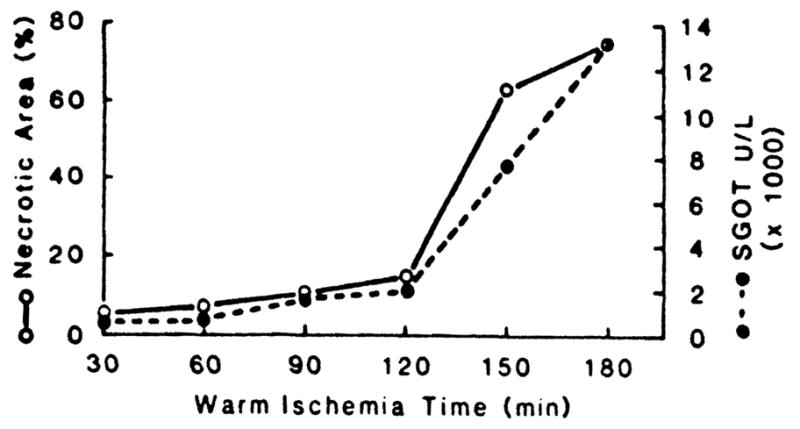
Relation of crossclamp time to the area of necrosis in the liver slices and to SGOT rise. Mean ± SE for the 150-min ischemia data are given in Table 1. (n=6 at each time.)
Table 1.
Dose-effective test of various scavengers with 150 min ischemia
| Scavenger | Dosage (mg/kg) | n | SGOT (U/L) (mean ± SE) | Necrotic area (%) (mean ±SE) |
|---|---|---|---|---|
| No treatment | 0 | 6 | 7193.1±968.2 | 62.6±7.0 |
| SOD | 10 | 6 | 4676.0±938.9 | 24.9±3.5 |
| 20a | 6 | 2724.3±724.4 | 12.2±1.4 | |
| 30 | 5 | 2737.8±804.1 | 20.9±4.2 | |
| Allopurinol | 50 | 5 | 8731.6±2107.5 | 64.7±8.1 |
| 100 | 6 | 5182.4±1078.1 | 38.1±7.8 | |
| 200a | 4 | 6302.7±1908.1 | 38.0±6.3 | |
| Coenzyme Q10 | 5 | 6 | 11,549.3±1852.4 | 73.7±7.0 |
| 10a | 6 | 7806.7±1696.9 | 56.2±4.8 | |
| 20 | 5 | 12,600.0±1506.7 | 52.6±8.8 | |
| Glutathione | 250 | 4 | 13,273.3±2811.6 | 72.5±6.1 |
| 500a | 6 | 9941.3±1404.5 | 75.0±3.8 | |
| 1000 | 5 | 14,518.4±1862.7 | 96.7±0.7 |
Most effective dose used for experiments shown in Figure 2.
Drugs tested were: (1) superoxide dismutase (human Du/Z SOD, Pharmacia AB Uppsala, Sweden), (2) allopurinol (Aldrich Chemical Company, Inc., Milwaukee, WI), (3) coenzyme Q10 (Eisai Company, Tokyo, Japan), and (4) a reduced form of glutathione (Aldrich Chemical Company, Inc., Milwaukee, WI). For each of these agents, 3 doses were tried (Table 1). The drugs were administered intravenously into the internal jugular vein 5 min (SOD) or 60 min (allopurinol, co-enzyme, and glutathione) before beginning 150 min of ischemia. The most effective of these doses (Table 1) were used for the more complete experiments summarized in Figure 2. Data were expressed as mean ±SE. Comparison of group means was with the Student’s t test (unpaired), in which a probability of error less than 0.025 was considered as significant.
Figure 2.
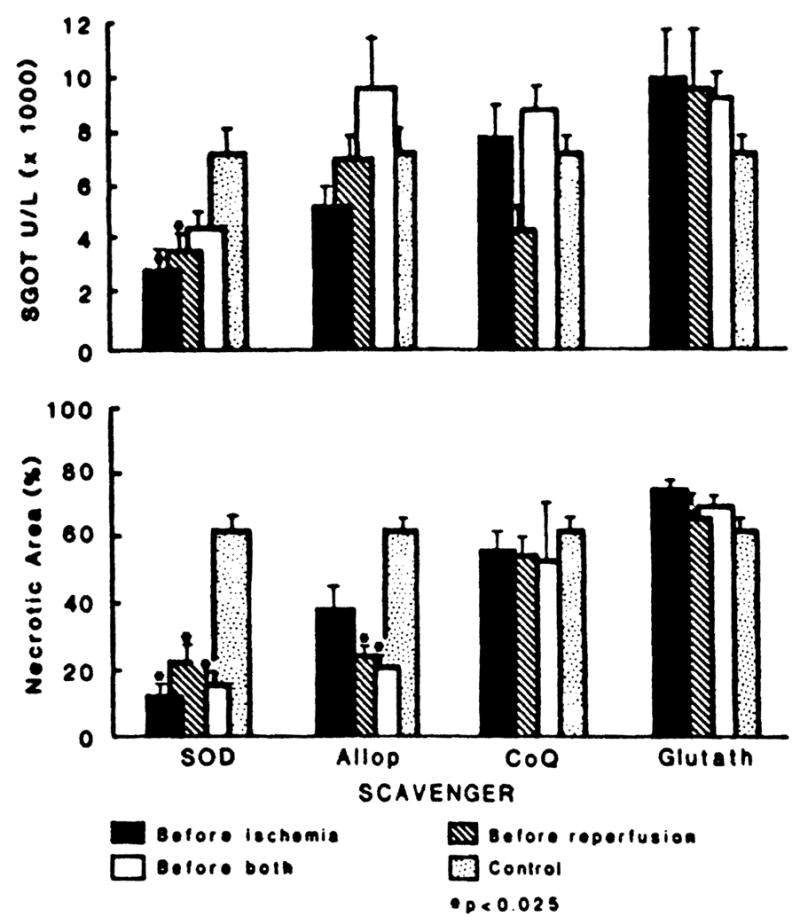
Effect of scavengers on SGOT levels and on necrotic area, 24 hr after 150-min of ischemia. Agents were administered intravenously 5 min (SOD) or 60 min (allop., CoQ, glutath.) before ischemia, 5 min before reperfusion (all 4 agents), or both. (SOD) superoxide dismutase; (Allop) allopurinol; (CoQ) coenzyme Q10; (glutath) glutathione; (U/L) units per liter; (*)P<0.025 compared with controls.)
Superoxide dismutase was the most effective agent. The rises in SGOT were reduced with SOD treatment before ischemia, or before reperfusion; treatment at both times was less effective than at either time alone (Fig. 2). The extent of necrotic tissue was significantly reduced with all 3 variations of timing, but the effect was most pronounced with the preischemia dose alone (Fig. 2). Allopurinol reduced the SGOT rise only if given before ischemia (Fig. 2). However, the area of necrosis was reduced with all 3 variations of treatment timing. Coenzyme Q10 may have reduced the SGOT rise when given before reperfusion but there was no significant effect on the extent of necrosis with any of the treatment variations (Fig. 2). With glutathione treatment, the SGOT rises were higher than in untreated animals but the extent of measured ischemic necrosis was not influenced (Fig. 2).
The superoxide anion, hydrogen peroxide, and the hydroxyl radical are the most important free radicals produced at the time of reperfusion. At this time, molecular oxygen is used to convert ischemic accumulations of hypoxanthine to xanthine (7). Because the oxygen free radicals may act on the endothelial cells, causing their swelling and increased permeability (7, 8), the scavenger drugs theoretically could protect the graft micro-vasculature. The most effective of the four scavenger drugs in our study was superoxide dismutase, which to our surprise was most potent when given before the ischemic injury. Most previous reports have suggested that SOD will not be effective unless it is given just before reperfusion (9). An effect of treatment at this time also was seen in our studies, but it was no stronger than treatment 5 min before the ischemia. SOD given before reperfusion did not add to the benefit of the preischemic treatment. A benefit with allopurinol was also noted, confirming the work of others (3, 10) but this was far less striking. Since coenzyme Q10 is known to have antioxidant as well as membrane-stabilizing properties, and has been said to protect from reperfusion injury (4), it was particularly disappointing to see no ameliorating effect of this agent on the extent of tissue necrosis. The same was true of glutathione, which is said to have a direct antioxidant effect (5).
Caution is necessary in reaching conclusions in the rat either about the efficacy or lack thereof of any agent as it may apply to the human liver. Southards et al. (11) have pointed out that the rat may not be a suitable animal for research on free radical scavengers. They noted that the ratio of superoxide dismutase to xanthine oxidase in the rat liver was much lower than in the dog or human and suggested that the rat liver could be more sensitive to oxygen-derived free radical damage than in other species. If so, positive results from SOD therapy could be too easily obtained in the rat and could overestimate what might be expected in humans. Species differences also could be responsible for underrating other potentially useful substances, such as coenzyme Q10, glutathione, and other drugs classified as scavengers but with a biochemical mechanism different from SOD.
Footnotes
This work was supported by Research Grants from the Veterans Administration and by Project Grant DK 29961 from the National Institutes of Health, Bethesda, MD.
References
- 1.Atalla SL, Toledo-Pereyra LH, MacKenzie GH, et al. Influence of oxygen-derived free radical scavengers on ischemic livers. Transplantation. 1985;40:584. doi: 10.1097/00007890-198512000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Koyama I, Bulkley GB, Williams GM, et al. The role of oxygen free radicals in mediating the reperfusion injury of cold preserved ischemic kidney. Transplantation. 1985;40:590. doi: 10.1097/00007890-198512000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Castillo M, Toledo-Pereyra LH, Prough D, et al. Effective timing of allopurinol administration in the ischemic liver. Transplantation. 1989;47:727. doi: 10.1097/00007890-198904000-00033. [DOI] [PubMed] [Google Scholar]
- 4.Marubayashi S, Dohi K, Ezaki H, et al. Preservation of ischemic rat liver mitochondrial functions and liver viability with coenzyme Q10. Surgery. 1982;91:631. [PubMed] [Google Scholar]
- 5.McCay PB, Gibson DD, Fong KL, et al. Effect of glutathione peroxidase activity on lipid peroxidation in biological membranes. Biochem Biophys Acta. 1976;431:459. [PubMed] [Google Scholar]
- 6.Frederiks WM, Fronik GM, Hesseling JMG. A method for quantitative analysis of the extent of necrosis in ischemic rat liver. Exp Mol Pathol. 1984;41:119. doi: 10.1016/0014-4800(84)90012-1. [DOI] [PubMed] [Google Scholar]
- 7.McCord JM. Oxygen derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 8.Bulkley GB. The role of oxygen free radicals in human disease processes. Surgery. 1983;94:407. [PubMed] [Google Scholar]
- 9.Hoshino T, Maley WR, Bulkley GB, et al. Ablation of free radical-mediated reperfusion injury for the salvage of kidneys taken from non-heartbeating donors. Transplantation. 1988;45:284. doi: 10.1097/00007890-198802000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Adkinson D, Hollwarth ME, Benoit JN, et al. Role of free radicals in ischemia-reperfusion injury to the liver. Physiol Scand. 1986;548:101. [PubMed] [Google Scholar]
- 11.Southard JH, Marsh DL, McAnulty JF, et al. The importance of O2 derived free radical injury to organ preservation and transplantation. Transplant Proc. 1987;19:1380. [PubMed] [Google Scholar]


