Dear Sirs,
Although cerebellar atrophy is a key sign in cerebellar ataxia, interpretation of magnetic resonance imaging (MRI) may be hindered by region-specific age-related degeneration and poor visualization of posterior fossa structures. Direct quantification of true cerebellar cortical volume on MRI is a time-consuming process that may be confounded by complex foliation, which introduces partial volume effects.
It may be simpler to calculate an index for disease progression for the deep cerebellar nuclei (DCN), which mediate virtually all cerebellar outflow along with the vestibular nuclei. Quantitative assessment of the DCN could be invaluable to diagnosis, staging, and prognosis in neurodegenerative diseases with cerebellar involvement. Progress in structural assessment has enabled DCN identification [1–3]; however, there may be variable signal and contrast on T1- and T2-weighted scans such that DCN are sometimes not detectable at all [2, 4].
Recently, our team developed techniques for diffusion tensor imaging (DTI)-based delineation of the dentate and interposed nuclei—the largest, most reliably distinguishable DCN components [4, 5]. Although this indirect method would be expected to systematically overestimate true DCN volumes, it could still produce a clinically relevant DCN index measurement that is indirectly based on volume. As proof of concept, we investigated the sensitivity of these observations to differences in DCN index between ataxia patients and controls. DTI evaluation of the DCN could serve as a simple imaging biomarker of cerebellar disease.
A multi-slice, single-shot EPI sequence achieved whole brain coverage (2.2 mm isotropic resolution) in 19 control participants (6 M/13 F) and 28 patients—18 (11 M/7 F) with idiopathic isolated cerebellar disease (ICD), six (2 M/4 F) with spinocerebellar ataxia type 6 (SCA6), and four (2 M/2F) with olivopontocerebellar atrophy (OPCA). Each sequence utilized 32 diffusion encoding directions and five averaged, minimally-weighted (b0) volumes with a 3T MR scanner (Intera, Philips Medical Systems, Netherlands). Neurological function was assessed using the International Cooperative Ataxia Rating Scale [6] and subsections of the Unified Ataxic Disorders Rating Scale.
Bilateral DCN delineations were performed on DTI colormaps with Medical Image Processing and Visualization software [7] by one rater (A.X.D.), who was naïve to participant status. Measurement accuracy was assessed by an expert neurologist with reference to histological and structural MRI sections [8]. Volumes and ataxia scores were evaluated with Student’s t test. Eight scans were chosen for repeat rating by the original rater and a second rater (J.L.C.).
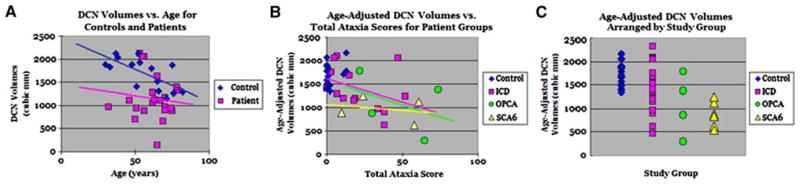
Controls had an average DCN index of 1,722 ± 341 mm3, while patients had an average DCN index of 1,142 ± 494 mm3 (P < 0.001). Analysis of patient groups showed correlations between lower DCN index and higher total ataxia scores, indicating greater disability (Fig. 1) (P < 0.05).
Fig. 1.
Deep cerebellar nuclei (DCN) index in ataxia patients is smaller than expected when compared with control participant data. a Mean DCN index decreases with age, and is smaller in patients compared with controls (P < 0.001). b Age-adjusted DCN index is negatively correlated with ataxia scores in each patient group (P < 0.001). c Age-adjusted DCN index for each group. Although there is considerable overlap between the values in most of the groups, all patient subgroups showed significant differences in DCN index compared to controls (ICD vs. control = P < 0.03, OPCA vs. control = P < 0.002, SCA6 vs. control = P < 0.001). In particular, the SCA6 DCN indices are distinctly lower than the control group values
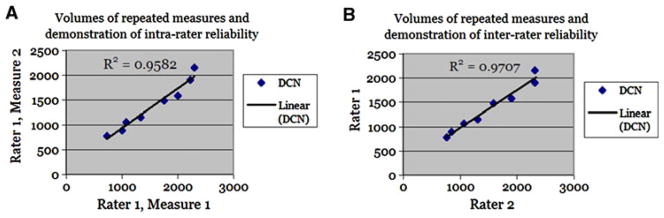
Intra- and inter-rater reliability testing showed high correlation between repeated measurements of DCN structures (R2 = 0.95 and 0.97, Fig. 2).
Fig. 2.
Intra- and inter-rater reliability. The graph on the left shows the intra-rater repeated volumes and reliability correlation score for rater 1 (A.X.D.), whose delineations were used to evaluate our data. The graph on the right shows the inter-rater repeated volumes and reliability correlation score between rater 1 and rater 2 (J.L.C.)
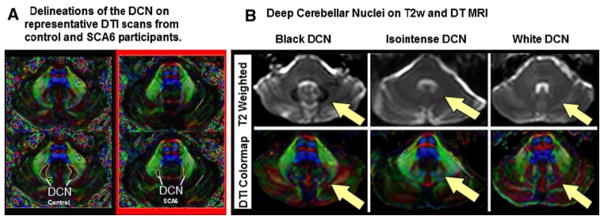
DTI colormaps enabled successful delineation of the DCN for all participants, despite varying degrees of functional deficit, volumetric atrophy, and T2 signal (Fig. 3). Ataxia patients had smaller DCN indices, which were associated with higher ataxia scores. It is important to note that our data show unexpectedly low mean DCN index in all patient groups, including our largest subpopulation (SCA6, Fig. 2b). This supports the possibility that the DCN are affected in SCA6, which is a point of controversy in the literature [9–11]. Of note, our index would be expected to correlate only with macroscopic volumetric loss, whereas “degeneration” classically refers to histopathologic evidence. Presumably, several distinct patterns of DCN involvement exist within the heterogeneous population affected with cerebellar degeneration. It would be of interest to test our method in SCA3 and Friedreich’s ataxia, which show significant dentate involvement.
Fig. 3.
DCN is clearly visualized on DTI images. a Patients with SCA6 (hereditary disease primarily affecting the cerebellum) exhibited reduced DCN index when compared to the control group. b In the T2w images on the left, the DCN appears as a well-defined black area of reduced T2 intensity. In the T2w images in the center, the DCN is isointense to the surrounding white matter and difficult to discern. In the T2w images on the right, the DCN is less well-defined and brighter in intensity than the surrounding white matter. In all of the above images, DCN remains more consistently defined on DTI colormaps than on T2w images
In summary, reliable identification of the DCN is possible with DTI, which exposes clinically relevant differences in DCN characteristics in ataxia.
Acknowledgments
This work was supported by the Arnold-Chiari Foundation, the Robin Zee Fund, the Dana Foundation Program for Brain and Immuno-Imaging, the National Organization for Rare Disorders, the National Alliance for Research on Schizophrenia and Depression, the Office of Naval Research NDSEGF (Landman), and the following NIH grants: K23EY015802, M01-RR00052, R21NS059830, R01NS054255, and R01NS056307. Special acknowledgments to Wade Mayes, Alex H. Sinofsky, and Katherine K. Loya for their invaluable contributions to this work.
Footnotes
Conflict of interest statement None.
Contributor Information
Annie X. Du, Department of Pathology, The Johns Hopkins University School of Medicine, 2-210, 600 N. Wolfe St., Baltimore, MD 21287, USA
Jennifer L. Cuzzocreo, Department of Pathology, The Johns Hopkins University School of Medicine, 2-210, 600 N. Wolfe St., Baltimore, MD 21287, USA
Bennett A. Landman, 324B Clark Hall, 3400 North Charles Street, Baltimore, MD 21218, USA
David S. Zee, Department of Pathology, The Johns Hopkins University School of Medicine, 2-210, 600 N. Wolfe St., Baltimore, MD 21287, USA
Jerry L. Prince, 201B Clark Hall, 3400 North Charles Street, Baltimore, MD 21218, USA
Sarah H. Ying, Email: sying@dizzy.med.jhu.edu, Department of Pathology, The Johns Hopkins University School of Medicine, 2-210, 600 N. Wolfe St., Baltimore, MD 21287, USA
References
- 1.Dimitrova A, Weber J, Redies C, Kindsvater K, Maschke M, Kolb FP, Forsting M, Diener HC, Timmann D. MRI atlas of the human cerebellar nuclei. Neuroimage. 2002;17(1):240–255. doi: 10.1006/nimg.2002.1124. [DOI] [PubMed] [Google Scholar]
- 2.Dimitrova A, Zeljko D, Schwarze F, Maschke M, Gerwig M, Frings M, Beck A, Aurich V, Forsting M, Timmann D. Probabilistic 3D MRI atlas of the human cerebellar dentate/interposed nuclei. Neuroimage. 2006;30(1):12–25. doi: 10.1016/j.neuroimage.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Maschke M, Weber J, Dimitrova A, Bonnet U, Bohrenkämper J, Sturm S, Kindsvater K, Müller BW, Gastpar M, Diener HC, Forsting M, Timmann D. Age-related changes of the dentate nuclei in normal adults as revealed by 3D fast low angle shot (FLASH) echo sequence magnetic resonance imaging. J Neurol. 2004;251(6):740–746. doi: 10.1007/s00415-004-0420-5. [DOI] [PubMed] [Google Scholar]
- 4.Landman BA, Du AX, Mayes WD, Prince JL, Ying SH. Diffusion tensor imaging enables robust mapping of the deep cerebellar nuclei. Organization for Human Brain Mapping; Chicago, Illinois: 2007. [Google Scholar]
- 5.Du AX, Landman BA, Zee DS, Prince JL, Ying SH. Diffusion tensor imaging reveals disease-specific dentate nucleus changes in cerebellar degeneration. Organization for Human Brain Mapping; Chicago: 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B. International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the world federation of neurology. J Neurol Sci. 1997;145(2):205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- 7.McAuliffe MJ, Lalonde FM, McGarry D, Gandler W, Csaky K, Trus BL. Medical image processing, analysis, and visualization in clinical research. Proceedings of the 14th IEEE symposium on computer-based medical systems; 2001. pp. 381–386. [Google Scholar]
- 8.Duvernoy HM. The human brain stem and cerebellum. Springer; Berlin: 1995. [Google Scholar]
- 9.Murata Y, Kawakami H, Yamaguchi S, Nishimura M, Kohriyama T, Ishizaki F, Matsuyama Z, Mimori Y, Nakamura S. Characteristic magnetic resonance imaging findings in spinocerebellar ataxia 6. Arch Neurol. 1998;55(10):1348–1352. doi: 10.1001/archneur.55.10.1348. [DOI] [PubMed] [Google Scholar]
- 10.Tashiro H, Suzuki SO, Hitotsumatsu T, Iwaki T. An autopsy case of spinocerebellar ataxia type 6 with mental symptoms of schizophrenia and dementia. Clin Neuropathol. 1999;18(4):198–204. [PubMed] [Google Scholar]
- 11.Schulz JB, Borkert J, Wolf S, Schmitz-Hübsch T, Rakowicz M, Mariotti C, Schoels L, Timmann D, van de Warrenburg B, Dürr A, Pandolfo M, Kang JS, Mandly AG, Nägele T, Grisoli M, Boguslawska R, Bauer P, Klockgether T, Hauser TK. Visualization, quantification and correlation of brain atrophy with clinical symptoms in spinocerebellar ataxia types 1, 3 and 6. Neuroimage. 2010;49:158–168. doi: 10.1016/j.neuroimage.2009.07.027. [DOI] [PubMed] [Google Scholar]