Abstract
Full-term pregnancies are associated with long-term reductions in maternal risk of breast cancer, but the biological determinants of the protection are unknown. Experimental observations suggest that human Chorionic Gonadotropin (hCG), a major hormone of pregnancy, could play a role in this association. A case-control study (242 cases, 450 controls) nested within the Northern Sweden Maternity Cohort included women who had donated a blood sample during the first trimester of a first full-term pregnancy. Total hCG was determined on Immulite 2000 analyzer. Odds ratios (OR) and 95% confidence intervals (CI) were estimated through conditional logistic regression. Maternal breast cancer risk decreased with increasing hCG (upper tertile OR, 0.67; CI, 0.46-0.99) especially for pregnancies before age 25 (upper tertile OR, 0.41; CI, 0.21-0.80). The association diverged according to age at diagnosis: risk was reduced after age 40 (upper tertile OR: 0.60; CI, 0.39-0.91) and appeared to increase before age 40 (upper tertile OR: 1.78; CI, 0.72-4.38). Risk was reduced among those diagnosed 10 years or longer after blood draw (upper tertile OR, 0.60; CI, 0.40-0.90), but not so among those diagnosed within 10 years (upper tertile OR, 4.33; CI, 0.86-21.7). These observations suggest that the association between pregnancy hCG and subsequent maternal risk of breast cancer is modified by age at diagnosis. While the hormone appears to be a determinant of the reduced risk around or after age 50, it might not confer protection against, or it could even increase the risk of, cancers diagnosed in the years immediately following pregnancy.
Keywords: Breast cancer; Risk assessment; Hormonal carcinogenesis; Reproductive, hormonal, and related risk factors: Serum biomarkers of endogenous exposure; Human Chorionic Gonadotropin; Cohort studies
Introduction
Women who have carried to term at least one pregnancy experience approximately a one-fourth reduction in their lifetime risk of breast cancer, as compared to nulliparous, which is stronger the younger the age at first pregnancy (1). Multiparity (2) and extended breastfeeding (3) further enhance this protection. Ample epidemiological evidence supports the association consistently across populations in all continents (1), but not all women benefit from the protection. Some, especially those who delay first pregnancy beyond age 30, may experience a substantial increase in the risk of breast cancer that peaks after about 5-7 years and slowly declines for up to 15 years following pregnancy (4).
In spite of the weight of epidemiological evidence, our understanding of the biological determinants of the effect of pregnancy on breast cancer, both the lifetime protection and the transient post-pregnancy increase, remain sketchy. An extensive literature on mammary carcinoma in rodents shows unequivocally not only that pregnancy, especially when occurring early in life, inhibits carcinogen-induced tumors, but also that the same effect could be achieved through the administration of specific hormones of pregnancy, including estradiol plus progesterone and human chorionic Gonadotropin (hCG), to virgin animals (5-7). In line with these observations, a large population-based case-control study in California (8) reported that young women who had been treated with daily injections of hCG for weight reduction were at considerably reduced risk of breast cancer, an association particularly evident among the nulliparous. Thus, the combined human and experimental evidence suggests that the hormones that are of relevance to the normal progression of pregnancy are also relevant to the profound physiological changes in the breast that occur during pregnancy. Such hormonally induced changes might be important in protecting the gland from carcinogenic transformation.
To our knowledge, only a preliminary study that served to set the stage for the present research has addressed the association of maternal breast cancer risk and circulating hCG during pregnancy (9). The preliminary study, a case-control of 173 cases and 254 controls nested in the same prospective maternity cohort in North Sweden as the present study, suggested that women in the upper tertile of first trimester hCG were at reduced risk of breast cancer, a reduction especially evident among those who were past the median lag-time of 14 years after pregnancy (OR, 0.53: CI, 0.27-1.03). However, because the study included a mix of primiparous and non-primiparous subjects at baseline blood draw, separating the effect of age at first birth from that of parity on the hCG-breast cancer risk association was not possible.
The current study addresses the hypothesis that lifelong maternal breast cancer risk is reduced in women who experience elevations in circulating hCG during the first trimester of a first full-time pregnancy, a protective effect expected to be stronger the younger the age of the mother at pregnancy. The hypothesis is rooted in seminal experimental observations showing that the strong differentiating potential of hCG on mammary cells would drive the formation of a complex, well differentiated glandular structure refractory to carcinogenic insults provided that exposure to the hormone (through a healthy pregnancy or chemo-intervention) precedes exposure to carcinogens capable of initiating still undifferentiated breast cells (10).
Materials and Methods
Study design and population
Study subjects were part of the North Sweden Maternity Cohort at the University of Umeå, Sweden, established in 1975 and described previously (9, 11). The cohort includes residents of one of the four northernmost counties of Sweden recruited while attending a Maternity Care Unit, run by the Swedish National Health Care System, providing pre- and post-natal care free of charge to all pregnant women. Blood samples are drawn from all patients during the latter part of the first trimester, or the early weeks of the second. Blood sera are shipped frozen to a central repository at the University Hospital in Umeå where laboratory analyses are performed for clinical purposes. Leftover specimens are stored at −20°C and preserved for research. The cohort’s biological bank contains approximately 110,000 serum samples from approximately 83,000 women.
In a case-control study nested within the cohort, of interest were cohort members with blood specimens drawn during a primiparous pregnancy leading to the delivery at full term (pregnancy lasting > 259 days) of a singleton live or stillborn child. In the maternity cohort database, available information was limited to personal identity number, name, sample serial number and location of sampling. The identification and selection of eligible case and control study subjects required a multi-step approach involving record linkages with nationwide databases made possible by the availability for all cohort subjects of the unique 10-digit personal identification number assigned to every person born in, or legally resident of, Sweden.
The first record linkage was performed with the Swedish Birth Register, a nationwide information system gathering data from all standardized medical records used in every maternal care and delivery units since 1973 (12). Over 99% of all births are registered and the quality and completeness of information is excellent. Through this linkage, the information available was expanded to include parity, date of each delivery and gestational age at birth. All primiparous subjects 40 or younger at blood draw who had given birth to a full-term live or stillborn singleton child were included in the study file.
The next record linkage was performed with the Swedish Cancer Registry to identify potentially eligible breast cancer cases and exclude women ineligible to be controls from the study file. The Cancer Registry, maintained by the Board of Health and Welfare, was founded in 1958 and covers the whole Swedish population with completeness of registration estimated to be 96.3% (13). Of interest were women first diagnosed with invasive epithelial breast cancer during the interval spanning from one year after delivery until June 8, 2007. A total of 315 potentially eligible case subjects were identified. Suitable serum samples were available for 289 (92%) of the cases, including 57 (18%) who were part of our previous report (8), and those were considered for the current study. As reported previously (8), excluded were also all samples collected after January 1, 1988, because the stability and quality of hormonal measurements on such samples was frequently compromised as a consequence of changes in specimen preparation procedures that the clinical laboratory had adopted on that date. There was no evidence of degradation for samples collected prior to this time, during which the vast majority of specimens from breast cancer cases and controls included in the study had been drawn. Samples judged to be of poor quality at inspection (severe hemolysis, discoloration) and those containing less than 700 μl of serum were excluded from study.
For each case (index case), potential controls were selected at random among cohort members in the study file who were alive and free of cancer at the time of the case’s diagnosis. Two controls were sought for each case, individually matched on age at sampling (± 6 months) and date of sampling (± 3 months). Lists of up to 14 potential controls were drawn for each case. New controls were selected for the 57 cases included in our preliminary report.
For each case and four of her potential controls, a full copy of the relevant maternity and delivery records was requested from the pertinent hospital in order to estimate gestational day at blood draw from the dates of blood draw and last menstrual period and to retrieve additional information for the study. Of the 1,504 requested (289 cases, 1,210 controls), 1,359 were obtained (88% of the cases, 91% of the controls). If information on date of blood draw was missing, gestational age at blood draw was greater than 120 days, or there was no biological specimen available for assay, the subject was excluded (6 cases, 120 controls). If the 4 initial controls selected for a given case were found to be ineligible, the records of 4 new potential controls were requested until at least one eligible control was identified, or all potential controls selected initially were found to be ineligible. If a matched set included a case and at least one individually matched control, the remainder of the information from the medical records was abstracted, which included: menstrual cycle characteristics and contraceptive methods used before index pregnancy, smoking, maternal weight and height at enrollment, treatments for infertility or other hormonal treatments during pregnancy, pregnancy conditions, including pre-eclampsia and hypertension, child’s gender, weight, length and placental weight. Sixteen subjects (4 cases, 12 controls) who had been treated with hormones during pregnancy, 3 cases without eligible controls and all controls for ineligible cases were excluded. In total, 242 cases and 450 controls were included in the study.
This study was approved annually by the Regional Ethics Committee of Umeå, Sweden. All information allowing the identification of human subjects was removed from the study database used for data preparation, editing and statistical analyses. Serum specimens used in laboratory analyses were labeled using anonymous codes.
Laboratory analyses
Total hCG and cotinine were measured in the laboratory of Clinical Chemistry, University Hospital in Umeå, Sweden, where technicians and their supervisors were unaware of any information concerning the specimens, including exposure and blind quality control status. Cotinine, a reliable indicator of recent exposure to tobacco, was used to complement medical record information, as smoking during pregnancy is known to lower hCG (8,13). Serum specimens of individually matched case and control subjects were always included in the same laboratory run. In addition to routine laboratory quality controls, a pool of serum from the cohort was created at the beginning of the study and 2 aliquots, undistinguishable from the test samples were inserted in each laboratory run. Both total hCG and cotinine were quantified by solid-phase competitive chemiluminescence assays on an Immulite 2000 Siemens analyzer. For hCG, the intra- and inter-run coefficients of variation estimated from analyses of laboratory quality controls spiked with 44 mIU/mL hCG were 1.8% and 13.7%, whereas those spiked with 427 mIU/mL hCG were 7.4% and 7.2%, respectively. The total (intra- + inter-batch) coefficient of variation based on the quality controls from the blind pool was 5.0 percent. The intra- and inter-batch coefficients of variation for cotinine were 5.5% and 9.9% at 18 ng/mL concentration and 5.6% and 8.5% at 60 ng/mL, respectively.
Statistical Analysis
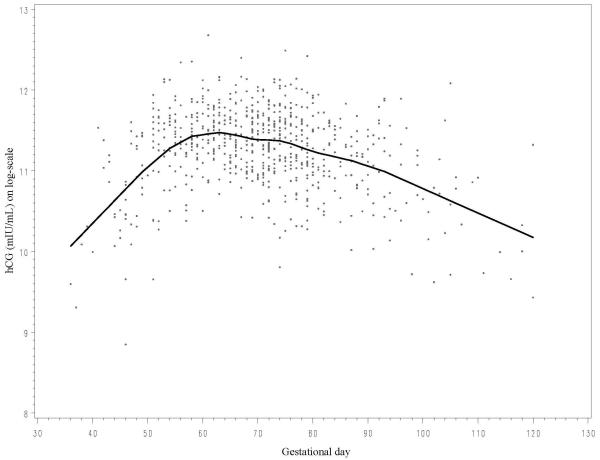
Inter-individual hCG concentrations varied considerably during the gestational age of interest showing a sharp tendency to increase until around day 60 and decreasing progressively thereafter (Figure 1). Individual matching for gestational day would have been nearly impossible to achieve since it would have required obtaining birth delivery records from a much larger pool of potential controls in order to identify two for each set matching precisely. Therefore, in all statistical analyses the variability in hCG was taken into account following the approach of Richardson and Coll. (9,14). In summary, prior to analyses, original hCG values were naturally log-transformed to limit heteroscedasticity. The mean curve of hCG variation was estimated based on all data points using local linear regression (15), a nonparametric smoothing technique that employs weighted regression and uses specific subsets of the data to estimate the curve at each point. hCG values for each study subject were computed as the difference (residual) between the assay value and the estimated mean determined for the day of gestation when the sample was drawn. The term “adjusted” hCG refers to hCG residuals and not to the original measured values. There were no outliers exceeding 3 times the inter-quartile range of concentrations.
figure 1.
Pearson correlation coefficients were used to relate adjusted hCG concentrations to specific characteristics of interest (e.g., age, height, weight). Subgroup differences in adjusted hCG (e.g., child’s gender, maternal smoking) were examined among controls using Generalized Linear Models. Kappa statistic was used to compare categorical values of reported maternal smoking at baseline and measured cotinine concentrations.
Mixed effect models with fixed effect for case-control status and maternal age and random effect for matched set were used to compare adjusted hormone levels in cases and controls. The conditional logistic regression model was used to calculate odds ratios (OR) and corresponding 95% confidence intervals (CI) associated with increasing adjusted hCG. In analyses utilizing the entire study population, subjects were classified in tertiles using the frequency distribution of the controls. Tests for trend were computed by treating adjusted hCG concentrations as ordered categorical variables quantitatively scored as ‘0’, ‘1’ and ‘2’ for tertiles 1 to 3, respectively. Likelihood ratio tests were used to assess statistical significance. Additionally, risk estimates were calculated on continuous scale of adjusted hCG for sub-group analyses with small number of cases. The ORs correspond to a change of 1 natural-log-transformed unit of hCG, or a ~ 2.7 change in hCG. Associations of breast cancer with adjusted hCG were explored within strata of age at blood sampling (< 25, 25-30, ≥ 30), age at cancer diagnosis (<40, 40–50, ≥50) and lag time between blood donation and date of diagnosis of the index case (<10, 10-15, 15-20 and ≥20; and <10 vs. ≥10). Formal tests of heterogeneity between the odds ratios in different subgroups were based on chi-square statistics, calculated as the deviations of logistic regression coefficients observed in each of the subgroups, relative to the overall regression coefficient (16). Potential confounders considered were maternal height, weight, self-reported smoking at blood donation, cotinine concentrations, baby’s height, weight, gender, placental weight, and parity by index date (cancer diagnosis). For inclusion in final models, a confounder had to alter point estimates by 10% or more. All tests of statistical significance were two-sided and considered significant if P-values were < 0.05.
Results
Table 1 reports relevant characteristics of the study population. Mean age at sampling was 27 for both cases and controls (range, 16.7-39.2). Blood had been drawn between the 8th and 14th gestational week for 81 percent of both cases and controls, before the 8th week for 15 percent and after the 14th week for 4 percent. Mean gestational day at sampling was 70.8 for cases and 70.3 for controls. Mean age at diagnosis was 45.5 years (range, 25.5- 62.7). Most of the cases (76 percent) had been diagnosed before age 50. Mean lag time between blood draw and cancer diagnosis was 18.5 years (range, 2.7- 30.5). The two groups were highly comparable on all characteristics.
Table 1.
Umeå, Sweden, 1975-2008. Selected characteristics of study subjects: means (standard deviation) or frequencies (percentage)
| Characteristics | Cases (242) |
Controls (450) |
|---|---|---|
| Maternal age at sampling | 27 (4.2) | 27 (4.1) |
| Gestational day | 71 (14.5) | 70 (14.1) |
| Age at diagnosis | 46 (6.4) | |
| Time from blood draw to diagnosis (years) |
19 (6.0) | |
| Primi-gravida | 200 (83%) | 354 (79%) |
| Maternal weight at enrolment (kg) | 61 (8.3) | 62 (9.5) |
| Maternal height at enrolment (cm) | 165 (5.6) | 165 (5.7) |
| Mothers reporting smoking at enrolment* | 74 (31%) | 119 (27%) |
| Cotinine ≥ 25 ng/mL | 87 (36%) | 142 (32%) |
| Child male gender | 133 (55%) | 232 (52%) |
| Child weight at birth (g) | 3,475 (439.8) |
3,444 (458) |
| Child length at birth (cm) | 50 (2.2) | 50 (2.0) |
| Placental weight (g) ** | 577 (119.5) | 579 (116.7) |
| Parity *** | ||
| 1 | 59 (24%) | 84 (19%) |
| 2 | 127 (52%) | 232 (52%) |
| >2 | 56 (23%) | 134 (30%) |
235 cases, 435 controls
206 cases, 385 controls
Number of children born before index date of cancer diagnosis
Among controls, adjusted hCG concentrations were inversely correlated with maternal weight (r= −0.14, p=0.003), were lower in mothers smoking while pregnant than in non-smokers (−0.119 vs. 0.0998 mIU/mL; p<0.0001) and in those carrying a male (−0.056 vs. 0.127, p<0.0001). There was an excellent correspondence between reported smoking and cotinine (cutoff, 25 ng/mL) (Kappa=0.84). Adjusted hCG was lower in cases than in controls (−0.03 vs 0.03; p < 0.098) corresponding to original concentrations at gestational day 70 of 94,080 and 97,416 mIU/mL, respectively. The results of conditional logistic regression analyses are reported in Table 2. In analyses including all case-control sets, there was a 33 percent decrease in risk of breast cancer with increasing adjusted hCG (upper tertile OR, 0.67; CI, 0.46-0.99; ptrend, 0.04). In sub-group analyses according to age at hCG measurement, the inverse association appeared to be limited to those whose blood was drawn at young age (upper tertile OR, 0.41; CI 0.21-0.80; ptrend, 0.01), but the null hypothesis of homogeneity between groups was not rejected (p =0.09).
Table 2.
Umeå, Sweden, 1975-2008. ORs and CI within tertiles* of adjusted hCG concentrations
| hCG Concentration |
pTrend | pHomo | |||
|---|---|---|---|---|---|
| Low | Intermediate | High | |||
| All subjects | |||||
| Ref. | 0.69 (0.47-1.01) | 0.67 (0.46-0.99) | 0.04 | ||
| Cases/Controls | 103/150 | 71/150 | 68/150 | . | |
| By age at sampling | |||||
| Maternal age < 25.0 | Ref. | 0.56 (0.29-1.07) | 0.41 (0.21-0.80) | 0.01 | 0.09 |
| Cases/Controls | 42/49 | 22/50 | 16/50 | . | |
| Maternal age ≥ 25.0 | Ref. | 0.77 (0.48-1.24) | 0.84 (0.52-1.35) | 0.45 | . |
| Cases/Controls | 62/100 | 49/100 | 51/101 | . | |
| By age at diagnosis | |||||
| Maternal age < 40 | Ref. | 1.17 (0.44-3.09) | 1.78 (0.72-4.38) | 0.20 | 0.03 |
| Cases/Controls | 11/26 | 12/26 | 20/26 | . | |
| Maternal age ≥ 40 | Ref. | 0.59 (0.39-0.91) | 0.60 (0.39-0.91) | 0.01 | . |
| Cases/Controls | 91/124 | 55/124 | 53/124 | . | |
| By lag time (years) between sampling and diagnosis | |||||
| < 10.0 | Ref. | 2.69 (0.53-13.6) | 4.33 (0.86-21.7) | 0.07 | 0.02 |
| Cases/Controls | 4/15 | 8/15 | 13/15 | . | |
| ≥ 10.0 | Ref. | 0.60 (0.40-0.91) | 0.60 (0.40-0.90) | 0.01 | . |
| Cases/Controls | 99/135 | 60/135 | 58/135 | . | |
Based on the frequency distribution of controls
In sub-group analyses with cutoff at age at diagnosis 40 years (Table 2) the risk of maternal breast cancer associated with relative elevations of adjusted hCG appeared to diverge substantially between the two groups (pheterogeneity=0.03), with markedly reduced risk among those in the older group (upper tertile OR, 0.60; CI, 0.39-0.91; ptrend, 0.01) and increased risk in the younger group (upper tertile OR, 1.78; CI, 0.72-4.38; ptrend, 0.20). The association between hCG and breast cancer risk appear to differ according to lag-time between sampling and date of diagnosis of index cases (pheterogeneity =0.02) with high risk among those diagnosed within 10 years (upper tertile OR, 4.33; CI, 0.86-21.7) and marked risk reduction among those with a longer lag-time (upper tertile OR, 0.60; CI, 0.40-0.90). Note that the <10 years group included only 25 cases.
Table 3 reports on analyses performed on a continuous scale within subgroups of age at sampling, age at diagnosis and lag-time to diagnosis. The strong inverse risk association with increasing concentrations of adjusted hCG when sampled at young age (<25) persisted (OR, 0.52; CI, 0.29-0.94) and was also present in analyses limited to those >40 at diagnosis (OR, 0.43; CI, 0.21-0.87) and those with lag-time >10 years between blood draw and diagnosis (OR, 0.45; CI, 0.24-0.84). Within groups of age at diagnosis (< 40, 40-49 and ≥ 50) there was evidence of progressive change in risk from relatively elevated in the youngest group (OR, 1.79; CI, 0.76-4.18) to markedly reduced in the oldest (OR, 0.37; CI, 0.16-0.88). Within groups of increasing lag-time (<10, ≥10 years), the risk was substantially reduced within the longer lag-time group (OR, 0.61; CI, 0.42-0.90), but not within the shorter where, on the contrary, there was evidence that the risk could be somewhat increased (OR, 8.72; CI, 1.50-50.78). Age at cancer diagnosis and lag-time between sampling and diagnosis were highly correlated (r=0.73) and there was no evidence of effect modification between the two time-dependent covariates.
Table 3.
Umeå, Sweden, 1975-2008. Relative risk change in one unit of adjusted hCG concentrations
| Cases / Controls | OR (continuous) | p | pHomo | |
|---|---|---|---|---|
| By age at sampling | ||||
| < 25 | 80 / 149 | 0.52 (0.29 - 0.94) | 0.03 | 0.14 |
| 25-30 | 110 / 209 | 1.10 (0.65 - 1.88) | 0.72 | . |
| ≥ 30 | 52 / 92 | 0.55 (0.21 - 1.46) | 0.23 | . |
| By age at diagnosis | ||||
| < 40 | 43 / 78 | 1.79 (0.76 - 4.18) | 0.18 | 0.04 |
| 40-50 | 142 / 271 | 0.71 (0.44 - 1.13) | 0.15 | . |
| ≥ 50 | 57 / 101 | 0.37 (0.16 - 0.88) | 0.02 | . |
| By lag time between sampling and diagnosis | ||||
| < 10 | 25 / 45 | 8.72 (1.50 - 50.78) | 0.02 | 0.04 |
| 10-15 | 31 / 56 | 0.65 (0.23 - 1.82) | 0.42 | . |
| 15-20 | 82 / 153 | 0.53 (0.27 - 1.02) | 0.06 | . |
| ≥ 20 | 104 / 196 | 0.67 (0.39 - 1.14) | 0.14 | . |
| < 10 | 25 / 45 | 8.72 (1.50 - 50.78) | 0.02 | 0.004 |
| ≥ 10 | 217 / 405 | 0.61 (0.42 - 0.90) | 0.01 | . |
Adjustments for reported smoking, maternal weight and height, placental weight, child’s weight, length and gender, and parity by index date of diagnosis did not influence the above associations. Exclusion of study subjects whose blood had been drawn beyond gestational day 98 (12 cases, 34 controls) and of those who had been included in the preliminary study (57 cases) had no appreciable effect.
Discussion
This study shows that hCG - one of the key hormones of pregnancy – may play a major role in the long established, but biologically poorly understood, lifetime protection that a pregnancy affords the mother. Women who had comparatively elevated concentrations of hCG in serum drawn during the first trimester of a primiparous pregnancy experienced a 30 percent reduction in the risk of breast cancer.
These observations are in line with the study’s hypothesis that hCG plays an important role in conferring protection against breast cancer among women who experience their first delivery early in life. The nulliparous breast is especially susceptible to toxicants and carcinogens (17,18), which could lead to transformations in immature epithelial cells that in turn can become the site of cancer initiation, as shown in animal experiments (19,20). The timely differentiation of immature mammary cells following a pregnancy at early age would prevent the initiation of cancer (21) owing to hCG’s strong growth inhibitory, pro-apoptotic, anti-proliferative and tumor suppressor activity (22) .
The most striking association pertains to the evident inverse association with increasing concentrations of hCG for maternal cancers diagnosed after age 40, which becomes considerably stronger after age 50, whereas elevated hCG appears to have no effect, or perhaps even increase the risk of cancer diagnosed at young age and/or within 10 years after a first full-term pregnancy. Even though a threshold effect rather than a dose-response model could always be argued, in our view such findings are of extraordinary interest in that they suggest that while in most women the powerful hormonal actions of hCG that pertain to the physiological maturation of the breast exert a lasting beneficial effect in protecting the gland from cancer, in some the same events do not confer any protection, or might even be associated with increased risk of cancer at young age. It is possible that among the latter the hormone interacts with other factors, such as individual genetic susceptibility, physiologically altered immunocompetence of pregnancy, exposure to environmental and/or infectious agents, or pre-existing pre-cancerous lesions (e.g., somatic mutations) hastening the early emergence of an invasive cancer.
The study is prospective in design, a characteristic that excludes reverse-causation, or the presence of an as yet undetected cancer being the cause of hCG alterations in pregnancy. A prominent feature is that the study included exclusively women who were primiparous at the time of blood drawing and whose pregnancy was carried out to full-term (37 or more weeks of gestation). Because full-term pregnancies beyond the first are associated with additional reduction in risk and, more importantly, hormone concentrations lower than in the first (23,24), the inclusion of non-primiparous subjects would have rendered extremely difficult, if not impossible, to disentangle the effect of a first pregnancy from that of subsequent ones, an issue which should be addressed in specifically designed projects. Additionally, in statistical analyses a woman’s full pregnancy history prior to cancer diagnosis was taken into account without any evident influence on point estimates. Cases and controls were tightly matched for age and date of blood draw and detailed information on covariates was secured through the retrieval of individual medical records from the hospital of delivery, supplemented through record linkages with high-quality nationwide registries.
Some major limitations should be considered. First, the temperature in the facility where serum specimens had been kept in storage was relatively high (−20°C). Evidence that degradation hadn’t occurred comes from observing that the distribution of original hCG measurements according to gestational age closely reflected what would be expected from clinical experience (Figure 1). Also reassuring was that original hCG concentrations were not correlated with length of time in storage in cases and in controls (r=0.04 and 0.07, respectively). Second, information on gestational age, a critically important piece of information, was estimated with some degree of approximation. It is therefore possible that adjusted hCG levels were subject to error in measurement. However, error would be expected to be non-differential with respect to case/control status and therefore would most likely lead to an attenuation of relative risk estimates. Third, the association of hCG with breast cancer may differ according to intrinsic tumor subtypes (luminal, HER2-positive, basal and normal), an information that was not available for the vast majority of study subjects. Indeed, the association of breast cancer risk with reproductive risk factors has been shown to vary by subtype (25,26), including inverse association with parity and age at first birth for hormone receptor-positive tumors and no association with hormone receptor-negative tumors. Finally, the strong correlation between age at diagnosis of index cases and lag time between pregnancy and date of index diagnosis precluded meaningful analyses of interaction between these two time-dependent covariates. Therefore, it was not possible to determine whether these two important variables were independently associated with risk or whether only one of them mattered and the association with the other was entirely attributable to the high correlation. The study was based on a single measurement at a single point in time in the course of a first pregnancy, a further potential limitation. It should be considered, however, that hCG is detectable virtually exclusively in pregnancy and that the hormone exerts a powerful stimulus inducing a profound differentiation of the breast that transforms the immature, nulliparous breast in a mature organ (22). It has been shown that there is a rather strong correlation between hCG concentrations in consecutive pregnancies (r=0.42). In studies of Down syndrome, a condition characterized by extremely elevated hCG, high hCG concentrations during a pregnancy are 3-times more likely to be associated with similarly high levels in consecutive pregnancies (27). Thus, the early part of a first full-term pregnancy is quite reasonably the most relevant period to relate hCG concentrations to long-term risk.
A final comment concerns the appropriateness of the laboratory assay employed in the study to measure hCG. The assay quantifies total hCG, including but not limited to the regular (or intact) isoform, which predominates in the first trimester of all normal pregnancies. Two of the hCG variants, the hyperglycosylated regular and hyperglycosylated free β-subunit have been shown to possess important physiological functions distinct from that of regular hCG (27). The hyperglycosylated variants have a fundamental autocrine role in regulating invasion of the myometrium at implantation in addition to strong anti-apoptotic and pro-angiogenetic properties. Therefore, it could not be ruled out that the excess risk observed among women who develop early breast cancer might be in part related to exposure to a pattern of elevated circulating hCG isoforms that is different from that associated with reduced risk of cancer arising at older ages.
This is the first epidemiological study reporting evidence that elevations in circulating hCG during the first trimester of a primiparous, full-term pregnancy completed at early age may be an important determinant of the long noted, lasting protection against maternal breast cancer that an early pregnancy affords the mother. In the past century or so, in all populations, age at first pregnancy has progressively been delayed and the average number of children per woman reduced (28) even though large differences remain between populations and social groups. While the societal and economic forces that are at the roots of such profound, rapid changes are unlikely to be modifiable, a better understanding of the factors mediating this protective association could ultimately lead to the development of chemo-preventive interventions seeking to mimic nature’s plans.
Acknowledgments
Grant support: USPHS grants CA114329 (to P. Toniolo), CA120061 (to A. Lukanova) and Cancer Center grant CA16087 from the National Cancer Institute, NIH, Department of Health and Human Services.
This project was the result of productive discussions held during research planning meetings of the International Consortium on Pregnancy and Health (ICPH). We thank the women of Västerbotten County, Sweden, for their willing contribution to the project. The authors are indebted to Yelena Afanasyeva, Anne Marie Ahren, Soren Holmgren, Ritu Andersson and Lena Selbrand for their technical assistance.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
References
- 1.Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2:133–40. doi: 10.1016/S1470-2045(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 2.Hinkula M, Pukkala E, Kyrrönen P, Kauppila A. Grand multiparity and the risk of breast cancer: population-based study in Finland. Cancer Causes Control. 2001;12:491–500. doi: 10.1023/a:1011253527605. [DOI] [PubMed] [Google Scholar]
- 3.Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50 302 women with breast cancer and 96 973 women without the disease. Lancet. 2002;360:187–95. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 4.Liu Q, Wuu J, Lambe M, Hsieh SF, Ekbom A, Hsieh CC. Transient increase in breast cancer risk after giving birth: postpartum period with the highest risk (Sweden) Cancer Causes Control. 2002;13:299–305. doi: 10.1023/a:1015287208222. [DOI] [PubMed] [Google Scholar]
- 5.Russo IH, Russo J. Role of hormones in mammary cancer initiation and progression. J Mammary Gland Biol Neoplasia. 1998;3(1):49–61. doi: 10.1023/a:1018770218022. [DOI] [PubMed] [Google Scholar]
- 6.Medina D. Mammary developmental fate and breast cancer risk. Endocr Relat Cancer. 2005;12:483–495. doi: 10.1677/erc.1.00804. [DOI] [PubMed] [Google Scholar]
- 7.Guzman RC, Yang J, Rajkumar L, Thordarson G, Chen X, Nandi S. Hormonal prevention of breast cancer: mimicking the protective effect of pregnancy. Proc Natl Acad Sci USA. 1999;96:2520–25. doi: 10.1073/pnas.96.5.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernstein L, Hanisch R, Sullivan-Halley J, Ross RK. Treatment with human chorionic gonadotropin and risk of breast cancer. Cancer Epidemiol Biom Prev. 1995;4:437–40. [PubMed] [Google Scholar]
- 9.Lukanova A, Andersson R, Wulff M, et al. Human chorionic gonadotropin and alpha-fetoprotein concentrations in pregnancy and maternal risk of breast cancer: a nested case-control study. Am J Epidemiol. 2008;168:1284–91. doi: 10.1093/aje/kwn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo J, Russo IH. Molecular Basis of Breast Cancer. Springer-Verlag; Heidelberg: 2004. [Google Scholar]
- 11.Lukanova A, Toniolo P, Zeleniuch-Jacquotte A, et al. Insulin-like growth factor I in pregnancy and maternal risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2489–93. doi: 10.1158/1055-9965.EPI-06-0625. [DOI] [PubMed] [Google Scholar]
- 12.Cnattingius S, Ericson A, Gunnarskog J, Kallen B. A quality study of a medical birth registry. Scand J Soc Med. 1990;18:143–148. doi: 10.1177/140349489001800209. [DOI] [PubMed] [Google Scholar]
- 13.Barlow L, Westergren K, Holmberg L, Talbäck M. The completeness of the Swedish Cancer Register – a sample survey for year 1998. Acta Oncol. 2009;48:27–33. doi: 10.1080/02841860802247664. [DOI] [PubMed] [Google Scholar]
- 14.Richardson BE, Hulka BS, Peck JL, et al. Levels of maternal serum alpha-fetoprotein (AFP) in pregnant women and subsequent breast cancer risk. Am J Epidemiol. 1998;148:719–727. doi: 10.1093/oxfordjournals.aje.a009691. [DOI] [PubMed] [Google Scholar]
- 15.Cleveland WS, Loader C. Smoothing by local regression: Priciples and Methods. In: Schimek MG, editor. Statistical Theory and Computational Aspects of Smoothing. 1st ed. Springer; New York: 1996. pp. 113–20. [Google Scholar]
- 16.Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. 1991;10:1665–1677. doi: 10.1002/sim.4780101105. [DOI] [PubMed] [Google Scholar]
- 17.De Bruin ML, Sparidans J, van t Veer MB, et al. Breast cancer risk in female survivors of Hodgkin s lymphoma: lower risk after smaller radiation volumes. J Clin Oncol. 2009;27:4229–31. doi: 10.1200/JCO.2008.19.9174. [DOI] [PubMed] [Google Scholar]
- 18.Key J, Hodgson S, Omar RZ, et al. Meta-analysis of studies of alcohol and breast cancer with consideration on the methodological issues. Cancer Causes Control. 2006;17:759–70. doi: 10.1007/s10552-006-0011-0. [DOI] [PubMed] [Google Scholar]
- 19.Russo J, Tay LK, Russo IH. Differentiation of the mammary gland and susceptibility to carcinogenesis: a review. Breast Cancer Res Treat. 1982;2:5–73. doi: 10.1007/BF01805718. [DOI] [PubMed] [Google Scholar]
- 20.Russo J, Balogh GA, Chen J, et al. The concept of stem cell in the mammary gland and its implication in morphogenesis, cancer and prevention. Front Biosci. 2006;11:151–72. doi: 10.2741/1788. [DOI] [PubMed] [Google Scholar]
- 21.Russo J, Balogh GA, Russo IH. Full-term pregnancy induces a specific genomic signature in the human breast. Cancer Epidemiol Biomarkers Prev. 2008;17:51–66. doi: 10.1158/1055-9965.EPI-07-0678. [DOI] [PubMed] [Google Scholar]
- 22.Rao CV, Li X, Manna SK, Lei ZM, Aggarwal BB. Human chorionic gonadotropin decreases proliferation and invasion of breast cancer MCF-7 cells by inhibiting NF-kappaB and AP-1 activation. J Biol Chem. 2004;11:25503–510. doi: 10.1074/jbc.M400683200. [DOI] [PubMed] [Google Scholar]
- 23.Arslan AA, Zeleniuch-Jacquotte A, Lukanova A, et al. Effects of parity on pregnancy hormonal profiles across ethnic groups with a diverse incidence of breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2123–30. doi: 10.1158/1055-9965.EPI-06-0470. [DOI] [PubMed] [Google Scholar]
- 24.Chen T, Lundin E, Grankvist K, et al. Maternal hormones during early pregnancy: a cross-sectional study. Cancer Causes Control. 2010;21:719–27. doi: 10.1007/s10552-009-9500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–39. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwan ML, Kushi LH, Weltzien E, et al. Epidemiology of breast cancer subtypes in two prospective cohorts of breast cancer survisors. Breast Cancer Res. 2009;11:R31. doi: 10.1186/bcr2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wald NJ, et al. Prenat Diagn. 2004;24(5):389–392. doi: 10.1002/pd.890. [DOI] [PubMed] [Google Scholar]
- 28.Cole LA. New discoveries on the biology and detection of human chorionic gonadotropin. Reprod Biol Endocrinol. 2009;7:8–45. doi: 10.1186/1477-7827-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heck KE, Schoendorf KC, Ventura SJ, Kiely JL. Delayed childbearing by education level in the United States, 1969-1994. Matern Child Health J. 1997;1:81–8. doi: 10.1023/a:1026218322723. [DOI] [PubMed] [Google Scholar]