Abstract
Background: Psychosis has been repeatedly suggested to be affected by increases in stress and arousal. However, there is a dearth of evidence supporting the temporal link between stress, arousal, and psychosis during “real-world” functioning. This paucity of evidence may stem from limitations of current research methodologies. Our aim is to the test the feasibility and validity of a novel methodology designed to measure concurrent stress and arousal in individuals with psychosis during “real-world” daily functioning. Method: Twenty patients with psychosis completed a 36-hour ambulatory assessment of stress and arousal. We used experience sampling method with palm computers to assess stress (10 times per day, 10 AM → 10 PM) along with concurrent ambulatory measurement of cardiac autonomic regulation using a Holter monitor. The clocks of the palm computer and Holter monitor were synchronized, allowing the temporal linking of the stress and arousal data. We used power spectral analysis to determine the parasympathetic contributions to autonomic regulation and sympathovagal balance during 5 minutes before and after each experience sample. Results: Patients completed 79% of the experience samples (75% with a valid concurrent arousal data). Momentary increases in stress had inverse correlation with concurrent parasympathetic activity (ρ = −.27, P < .0001) and positive correlation with sympathovagal balance (ρ = .19, P = .0008). Stress and heart rate were not significantly related (ρ = −.05, P = .3875). Conclusion: The findings support the feasibility and validity of our methodology in individuals with psychosis. The methodology offers a novel way to study in high time resolution the concurrent, “real-world” interactions between stress, arousal, and psychosis. The authors discuss the methodology's potential applications and future research directions.
Keywords: psychosis, schizophrenia, stress, arousal, heart rate variability, vagal, cardiac autonomic regulation, experience sampling method, palm computers
Introduction
Following the introduction of the diathesis-stress model of schizophrenia,1 the link between psychosis and stress has been broadly accepted as a constructive model for understanding the course of psychosis in schizophrenia.2,3 The model posits that interactions between a preexisting vulnerability (diathesis) and stressors increase the likelihood that an individual will develop psychosis once a specific individual threshold is surpassed. This model is supported by the world of clinical experience in which patients with schizophrenia often experience their onset of illness or exacerbations in symptoms in relation to stressful events. However, despite its popularity and its ostensible clinical plausibility, there is a dearth of evidence pointing to temporal links between subjective stress, physiological arousal, and psychosis.4 This lack of data may hinder the ability to develop new and more refined models of psychosis, resulting in an impediment in the developments of new treatments.
A number of authors suggested that this paucity of evidence may stem from problems with current research methodologies.3–5 One factor limiting the identification of temporal links between stress and psychosis is the way assessments of stress, as well as psychosis, are conducted. These assessments are typically retrospective and thus vulnerable to the influence of memory difficulties, affective states at assessment time, as well as cognitive biases and reframing.3,4 Even when prospective designs are used (eg, conducting assessments prospectively every 3 mo over a year), the assessments are still based on the participants’ retrospective recollection of stress and psychosis from the past week, past month, or since the previous assessment. These difficulties are particularly critical given the substantial memory deficits experienced by many individuals with schizophrenia,6,7 making the use of retrospective assessments problematic in this population. This view is echoed in the preliminary assessment guidelines for the pharmaceutical industry published by the US Food and Drug Administration.8 Accordingly, patient-reported outcome (PRO) instruments “that require patients to rely on memory, especially if they must recall over a period of time, or to average their response over a period of time may threaten the accuracy of the PRO data. It is usually better to construct items that ask patients to describe their current state than to ask them to compare their current state with an earlier period or to attempt to average their experiences over a period of time.”8(p11)
Another limiting factor is rooted in the fact that retrospective assessments do not provide information about patterns of change in stress over time. Most instruments of stress (and psychosis) typically offer only a single index reflecting the average or the highest level of the stress for a given time period.3 Thus, they do not include information about how stress fluctuates in relation to activities and social contexts, which may vary considerably within the course of a day. Finally, retrospective assessments of stress may not capture minor stressors that may have significant impact on patient's symptoms at the time of occurrence9 but may not be recalled later. Such daily hassles are particularly important given the evidence that such stressors are better predictors of overall stress in individuals with schizophrenia compared with major life events.10 Thus, retrospective assessments of stress may have limited ecological validity, hindering the ability to establish clear temporal relationship between stress and psychosis.
To overcome some of these difficulties, researchers have employed experience sampling method (ESM) to study stress in individuals with psychosis during daily functioning in the “real world.” ESM is an ecologically-valid, time sampling of self-reports developed to study the dynamic process of person-environment interactions.11 Subjects in ESM studies are typically supplied with a digital wristwatch and booklets containing questionnaires about current mood, symptoms, activities, and social context (paper-based ESM). The subjects are instructed to complete a questionnaire upon hearing beeps from the wristwatches, which are typically preprogrammed to beep randomly a number of times a day to elicit experience samples. ESM offers a number of advantages over retrospective assessments, including the assessment of current experiences with limited need of episodic memory input, the potential for inclusion of minor/transient experiences, as well as the possibility to analyze daily fluctuations and patterns of change across activities, social contexts, and time of day. Spearheaded by researchers from the Maastricht group, P.D., Myin-Germeys, and colleagues9,11,12 have found in a series of elegant studies a link between increases in negative mood during daily functioning and momentary elevations in psychotic symptoms in individuals with psychosis. However, the use of paper-based ESM has been criticized as restricted primarily to subjective experiences (ie, subjective stress) due to the challenge of determining the subjects’ precise response time to the ESM questionnaires and the difficulty linking it to concurrent physiological measures of arousal.13
The literature on physiological correlates of stress and psychosis has also suffered from a number of methodological difficulties. Attempts to link psychosis with physiological correlates of stress (arousal) have centered on a number of indices including heart rate variability (HRV), salivary cortisol (SC), and electrodermal activity (EDA). Technological advances over the past decade have made assessment of ambulatory cardiac autonomic functioning a promising index of stress regulation. Cardiac functioning is mediated by the autonomic nervous system (ANS), with both the sympathetic and parasympathetic ANS’ branches innervate the myocardium. The continuous interplay between the branches reflects the ANS’ ability to respond to stressors and return to homeostasis,14 contributing to an individuals’ ability to function effectively within changing environments. The balance between the ANS’ branches can be indexed by the heart's beat-to-beat variability (HRV), with greater HRV reflecting enhanced ANS flexibility and an improved ability to respond to psychological and physiological demands. Use of power spectral analysis of HRV data allows for determination of the relative contribution of the parasympathetic branch to autonomic regulation, as well as the sympathovagal balance. Studies in healthy individuals using spectral analyses of HRV data have demonstrated that negative mood is correlated with sympathetic dominance, whereas positive mood is associated with a shift toward parasympathetic activity.15–18 Similarly, among individuals with schizophrenia, studies of HRV have pointed to a link between parasympathetic (vagal) dysfunction and hyperresponsivity to stressful experiences. Valkonen-Korhonen et al19 found that schizophrenia patients during acute psychosis displayed significantly reduced HRV, suggesting a compromised ability to downregulate stress. Okada et al20 reported that cardiac vagal function decreased when psychotic symptoms were more pronounced. Findings consistent with these results have also been reported by a number of other authors.21–27 These associations appear to be independent of the impact of antipsychotic medications on cardiac autonomic regulation.28,29
HRV offers a number of advantages over SC and EDA. First, it allows for tracking of momentary changes in physiological arousal with a high time resolution (ie, minutes.) In contrast, peak elevations in SC in response to psychological stressors are typically reached 20–40 minutes after the presentation of stressors.30,31 Although attempts have been made to integrate SC with ESM in studies of healthy controls32–34 and depressed patients,35 given the dynamic nature of stress, SC remains a relatively crude index of stress during “real-world” functioning. Second, ambulatory measurement of HRV has been established as valid methodology that has being used extensively as part of standard practice in cardiology. In contrast, there are currently no published studies of ambulatory assessments of EDA in individuals with schizophrenia. This may be in part due to the technical challenges associated with ambulatory “real-world” EDA measurement (ie, the impact of vigorous movements and/or pressure applied on the electrodes.) Thus, the use of HRV as an index of physiological correlates of stress is advantageous as it offers a feasible, high time resolution method to determine autonomic arousal. At the same time, the HRV schizophrenia literature has a number of shortcomings. First, the HRV assessments were “stationary”—subjects were assessed in laboratories, typically during resting state and over relatively brief periods (<1 h.) Therefore, it is unknown whether the findings also apply to fluctuations in psychosis during “real-world” functioning. Similarly, the assessments of psychosis in many of the studies were retrospective.
In summary, previous studies have demonstrated ESM to be a potent methodology that allows the assessment of stress as part of daily functioning in “real-world” environments. However, the use of paper-based ESM makes it difficult to link findings to concurrent physiological correlates of stress. Similarly, HRV has been found to be a promising measure of ambulatory arousal with a high degree of time resolution. However, to date, HRV studies in individuals with psychosis have focused exclusively on “stationary” laboratory assessments with potentially poor ecological validity. As a result, the “real-time, real-world” (ie, in vivo, in situ) temporal relationships between psychosis, stress, and arousal remain uncharted. This lack of high time resolution data may inhibit the testing of more refined models of psychosis and impede the development of novel pharmacological and cognitive-behavioral treatments. Given that psychosis, stress, and arousal are variable phenomena that can fluctuate considerably over brief periods of time, the elucidation of their relationships is contingent on the availability of a methodology that allows for the ambulatory, high time resolution simultaneous assessment of the psychological and physiological indices of stress and psychosis during daily functioning.
Our group and others have previously demonstrated the feasibility and validity of using palm computer to collect ESM data on stress and psychosis from hospitalized schizophrenia patients,13 as well as from outpatients with schizophrenia.36 In this manuscript, we focused on the assessment of stress and arousal—we present data on the feasibility and validity of a novel methodology that takes advantage of the many strengths of paper-based ESM to assess stress during daily functioning, while allowing concurrent ambulatory, high time resolution measurement of arousal. Our specific aims are to assess the feasibility of measuring ambulatory stress and arousal concurrently as part of daily functioning in “real-world” environments; to confirm the validity of using momentary negative mood, measured with ESM with palm computers, as a high time resolution index of subjective stress (this index has been previously assessed using paper-based ESM but not with ESM with palm computers); and to confirm the association between stress and arousal during daily functioning using high time resolution measurement. We hypothesize that patients’ reports of momentary stress will be inversely correlated with parasympathetic activity and positively correlated with sympathovagal balance.
Methods
Participants.
Twenty-eight individuals with psychosis were recruited from patients hospitalized at the New York State Psychiatric Institute (NYSPI). All participants provided written informed consent, and the study was approved by the NYSPI's Institutional Review Board. One subject withdrew from participation prior to the start of the assessment. Data of 7 other participants were excluded from the final data analyses due to equipment malfunction (n = 3), discomfort wearing the Holter monitor (n = 2), a desire to take a shower (n = 1), and a clinical exacerbation (n = 1). Table 1 presents data on the participants’ demographic and clinical information. The participants whose data were excluded from data analyses were not significantly different from those included with regard to their age, education, global assessment of functioning (GAF) scores, or age of onset of psychosis. However, they received significantly higher doses of antipsychotic medication as assessed by chlorpromazine equivalence (t = 2.19, P = .04.).37
Table 1.
Demographics, Clinical Data, and Means and SDs of Ratings Using the Holter During the 36-h Ambulatory Assessment
| Demographics and Clinical Data | n/Average | %/SD |
| Sex (female) | 10 | 50% |
| Racial/ethnic background | ||
| White | 9 | 45% |
| African American | 4 | 20% |
| Hispanic | 4 | 20% |
| Asian | 2 | 10% |
| Multiracial | 1 | 5% |
| Diagnosis | ||
| Schizophrenia | 14 | 70% |
| Schizoaffective disorder | 3 | 15% |
| Delusional disorder | 1 | 5% |
| Depression with psychotic symptoms | 1 | 5% |
| Bipolar I disorder | 1 | 5% |
| Primary language (English) | 16 | 80% |
| Age, y (average) | 30.6 | 8.4 |
| Age of onset of psychotic symptoms, y (average) | 22.1 | 7.7 |
| Education (y) | 14.0 | 1.7 |
| GAF scores (average) | 37.5 | 9.5 |
| Chlorpromazine equivalence (average) | 226.4 | 180.5 |
| Ratings of 36-h ambulatory assessment using of Holter | Mean | SD |
| The LifeShirt was too heavy | 1.06 | 0.24 |
| The LifeShirt was too hot to wear | 1.50 | 0.79 |
| The LifeShirt was comfortable to wear during the day | 4.06 | 0.87 |
| Wearing the LifeShirt interfered with my daily activities | 1.72 | 0.89 |
| The LifeShirt was comfortable to wear during sleep hours | 3.65 | 1.06 |
| The LifeShirt interfered with my sleep during the night | 1.76 | 1.09 |
| I would be interested to participate in a similar study in the future | 3.67 | 0.97 |
| I would recommend to others to participate in a similar study | 4.44 | 0.78 |
Note: n = 20, ratings of using the Holter based on a 5-point Likert scale (from 1 “not at all” to 5 “very much”).
Assessments.
Diagnosis was determined using the Diagnostic Interview for Genetic Studies (DIGS),38 a semistructured diagnostic interview and medical records review that is used to gather diagnostic and course of illness information for the mood, psychotic, and substance use Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) Axis I disorders, as well as functioning (GAF). The DIGS was administered by clinical research interviewers at the masters level or above. A team of clinical research psychologists and psychiatrists made consensus diagnoses based on information collected via the DIGS.
Procedure.
Data were collected during 2005–2007. Assessments were conducted over 2 consecutive days. Arousal was assessed continuously over 36 hours from 10 AM day 1 to 10 PM day 2 (including during sleep time) using the LifeShirt System (LS; VivoMetrics, Inc., Ventura, CA, USA). The LS is a vest-like undergarment embedded with sensors designed to record ambulatory cardiopulmonary data. The electroencephalogram (ECG) electrodes were placed on the left and right upper chest and on the left anterior axillary line at the 10th intercostal space. During the 36-hour period, participants were not able to take a shower. The selection of a 36-hour assessment period reflected a balance between the need to maximize statistical power while also being sensitive to participants’ comfort level during the assessment. We used ESM with palm computers to assess momentary stress. The use of palm computers enabled us to ascertain the precise times the ESM questionnaires were completed. The clocks of the palm computers and LS were synchronized, allowing the temporal linking of ratings of patients’ subjective stress to their concurrent measures of cardiac autonomic regulation. On the morning of the first day of the 36-hour assessment, after being fitted with the LS, participants completed a brief introduction session (∼30 min) to basic operations of palm computers, as well as a practice set of questions on the palm computer. Participants were then provided with palm tungsten T3 computers (Palm OS version 5.2.1). We used the iESP software (version 3.3; Intel Research Center, Seattle, WA) to present questions and collect responses on the palm computers.
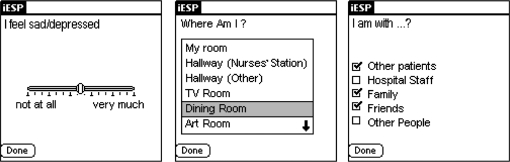
The palm computers were preprogrammed to beep randomly 10 times a day each day (between 10 AM and 10 PM) to elicit 20 experience samples over the 36-hour assessment period. Upon hearing the beep, participants were instructed to respond to brief questionnaires presented on the screen of the palm computer (ie, “I feel sad/depressed”; see figure 1) For each question, participants were asked to indicate on the palm computer's screen the quality of their current experience on a graphical slider. Responses were represented in the output as a value between 1 (“not at all”; leftmost extreme) and 100 (“very much”; rightmost extreme). A stratified time sampling scheme was used to minimize the probability that activations will be concentrated over brief periods. The software was set up to divide each day's assessment period by the number of activations to create 10 equal time windows of 72 min (12 h × 60 min/10 beeps). It then scheduled one questionnaire randomly within each time window. Thus, the potential period between questionnaires ranged from 1 to 143 minutes. Momentary stress (negative mood) was defined as the highest rating (range = 0–100) among the palm computer questions assessing anxiety, loneliness, irritation, sadness, and happiness/relaxation (reversed). All experience samples were then categorized into high vs low stress (negative mood) using the median. The definition of stress along the positive-negative mood dimension is consistent with the ESM psychosis literature.9,11–13
Fig. 1.
Sample Screen Shots of Questions Presented on the Palm Computer Screen.
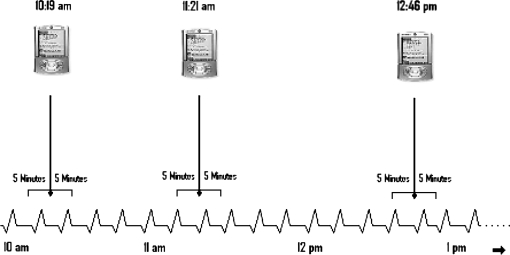
After the completion of the 36-hour assessment, the data from the palm computer and LS were uploaded to a PC computer. The precise time of each questionnaire from the palm computer was identified, and using the VivoLogic software (VivoMetrics, Inc), the corresponding time point on the continuous stream of cardiac autonomic data was marked. For each questionnaire time point (beep), we demarcated a 10-minute epoch (5 min before and after the beep). We then used power spectral analysis to determine the ANS relative parasympathetic contribution and sympathovagal balance during each epoch (see figure 2) For each 10-minute epoch, we calculated spectral power in the low-frequency (LF, 0.04–0.15 Hz) and high-frequency (HF, 0.15–0.40 Hz) bands, the LF/HF ratio, as well as mean heart rate (HR). The HF band reflects parasympathetic contribution to cardiac autonomic regulation, while the LF/HF ratio represents sympathovagal balance.39 The frequency bands were selected based on the guidelines set by task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology.39 The ECG signals were digitized at 200 Hz using the VivoLogic software. Prior to computing fast Fourier transforms, the software subtracted the linear trend and used multiplication of a Hanning window for each segment. The software then averaged and scaled the magnitude power spectrum for each subsegment to form a one-sided periodogram and calculated the area under the curve for each frequency range.
Fig. 2.
Sampling of Momentary Stress (Negative Mood) and Epochs of Physiological Arousal as Part of Daily Functioning in Natural Environments.
Data Analyses.
Data analysis was conducted using SAS (version 9). ESM data have a hierarchical structure in which repeated observations are nested within subjects and therefore are not independent. Because observations from the same subject are more similar than observations from different subjects, the residuals are not independent. Thus, to assess the relationships among symptoms and moods, we analyzed the data using multilevel linear modeling. Multilevel modeling techniques are a variant of unilevel regression analyses, and they are standard for the analysis of ESM data.40
The correlations among stress and measures of autonomic arousal (parasympathetic activity, sympathovagal balance, and heart rate) were estimated using the regression model for each pair of symptoms. Because the measures on each subject are repeated at different time points, and the data include unequal cluster sizes due to the missing values for certain time points, we used generalized estimation equation (GEE)41 adjustment within the regression model to estimate the correlations. Significance level was set to α = .05.
For comparisons between the high- vs low-stress moments, all valid experience samples were divided into high vs low using median scores. We then compared each subject's associated cognitions using z tests with P values based on 2-sided tests. Similar to the assessment of correlations, the GEE adjustment with an autoregressive correlation structure is imposed in order to take into account the dependence of the repeated measures within subject. This method is more effective and powerful than the t test on the subjects’ means across the time points because it allows use of the information from all the repeated measurements rather than an aggregate of them.
Results
Our first aim was to determine the feasibility of using the LS concurrently with ESM with palm computers. Previously, our group has demonstrated the feasibility and validity of using ESM with palm computers alone to collect information from hospitalized individuals with schizophrenia.13 In the present study, the concurrent use of the LS was added and was generally agreeable to the participants––only 3 patients (out of 27) discontinued their participation due to the use of the LS. Table 1 presents the participants’ ratings of comfort level wearing the LS during the 36-hour assessment. The 20 participants who completed the study reported that the LS was overall comfortable to wear during the day, interfered minimally with their daily activities, and was not too heavy or too hot to wear. Similarly, participants reported that the LS was moderately comfortable to wear during sleep hours, and it had limited impact on their sleep. Finally, the participants expressed moderate interest in participating in a similar study in the future, but they highly recommended it to others. Overall, the use of the LS to assess cardiac autonomic regulation over 36 hours in hospitalized individuals with psychosis appears to be feasible and reasonably agreeable to most patients and results in relatively little discomfort.
The 20 patients in the study completed 315 valid experience samples on the palm computers out of a potential of 400 (79%). The distribution of invalid responses was 42 skipped responses (10%), 23 incomplete responses (5%), and 20 palm computer software malfunctions (5%). There was no significant association between GAF scores and number of responses completed. Data on 15 corresponding epochs of cardiac functioning (out of the 315 valid responses) were unusable. Reasons for loss were corrupt data 12 (3%) and LS battery problems 3 (1%). The remaining 300 experience samples with valid palm computer and cardiac functioning data had 186 corresponding epochs with completely intact cardiac functioning data (62%). In the remaining epochs, periods of “noise” were interpolated using the VivoLogic software (VivoMetrics, Inc). The average interpolation time per 10-minute epoch was minimal—7.89 seconds (SD = 18.24), about 1.3% of each epoch assessment time (600 s). Overall, out of the potential 400 experience samples, 300 had valid ESM responses on subjective stress along with valid corresponding cardiac autonomic regulation data (75%). These data were used in the data analyses.
Our second aim was to assess the validity of momentary negative mood, measured using ESM with palm computers as a high time resolution index of subjective stress. For each experience sample, momentary stress (negative mood) was defined as the highest rating (range = 0–100) among the palm computer questions assessing anxiety, loneliness, irritation, sadness, and happiness/relaxation (reversed). We then categorized all experience samples into high vs low stress (negative mood) using the median (=58.50). We compared the high- vs low-stress experience samples on a number of items assessing concurrent thoughts and mental states. Table 2 presents data comparing high vs low moments of stress. As would be expected, moments of high stress were characterized by thoughts that were significantly less pleasant, more confused, described as too fast, and experienced as more difficult to express or get rid of, as well as a statistical trend toward experiencing less control over thoughts.
Table 2.
Differences in Quality of Thoughts During High Vs Low Subjective Stress (Negative Mood) Moments
| Description of Thoughts | Low Stress | High Stress | F | P |
| My thoughts are pleasant | 76.46 | 50.98 | 37.84 | <.0001 |
| My thoughts are confused | 13.86 | 30.78 | 27.67 | <.0001 |
| My thoughts are going too fast | 18.29 | 35.27 | 25.90 | <.0001 |
| I cannot get rid of my thoughts | 18.82 | 41.56 | 40.66 | <.0001 |
| My thoughts are difficult to express | 28.55 | 51.99 | 30.75 | <.0001 |
| I am in control of my thoughts | 69.14 | 60.73 | 3.80 | .0523 |
Note: Number of subjects = 20; number of valid experience samples = 300; all ratings are on a visual slider with a range from 1 “not at all” to 100 “very much”; high- vs low-stress moments are divided based on the median (=58.50); stress––the highest rating among questions assessing momentary negative mood including feelings of anxiety, loneliness, irritation, sadness, and happiness/relaxation (reversed); P values are based on 2-sided tests.
Our final aim was to assess the association between stress and arousal using high time resolution measurement. Table 3 presents information about the relationship between momentary ratings of stress (negative mood) and ambulatory measures of cardiac autonomic regulation. As would be expected, momentary increases in stress were inversely correlated with concurrent epochs of parasympathetic activity as indexed by the HF band measurements, controlling for chlorpromazine equivalence (partial ρ = −.27, P < .0001). Similarly, momentary stress had positive correlation with sympathovagal balance (partial ρ = .19, P = .0008). In contrast, there was no significant relationship between momentary increases in stress and elevations of heart rate (partial ρ = −.05, P = .3875.)
Table 3.
Partial Correlations of Ambulatory Ratings of Subjective Stress and Cardiac Autonomic Regulation (Controlling for Chlorpromazine Equivalence)
| Subjective Stress | Heart Rate | HF | LF | LF/HF | |
| Subjective stress | — | ||||
| Heart rate | −0.05 P = .3875 | — | |||
| HF | −0.27 P <.0001 | 0.02 P = .7498 | — | ||
| LF | −0.07 P = .2525 | 0.22 P = .0001 | 0.54 P <.0001 | — | |
| LF/HF | 0.19 P = .0008 | 0.23 P <.0001 | −0.55 P <.0001 | 0.24 P <.0001 | — |
Note: Number of subjects = 20; number of valid experience samples = 300; stress––the highest rating among palm computer questions assessing momentary negative mood including feelings of anxiety, loneliness, irritation, sadness, and happiness/relaxation (reversed); high frequency (HF)––index of parasympathetic activity; low frequency (LF/HF)––index of sympathovagal balance.
Discussion
The present study assesses the feasibility and validity of a novel methodology for measuring concurrently stress and arousal in individuals with psychosis during daily functioning in natural environments (“in vivo, in situ”). Our findings indicate that the concurrent use of palm computers to assess momentary stress along with the LS to measure ambulatory cardiac autonomic regulation is feasible and valid in this population. The participants’ response rate (79%) to ESM items is comparable with rates reported in the 2 other ESM studies in individuals with psychosis using palm computers (69%–81%),13,36 as well as with studies using paper-based ESM with this population.9,11,12 Similarly, the participants’ ratings of their comfort level during the assessments were overall favorable, with many willing to recommend such a study to others. Our findings are also comparable to comfort level reported in other ESM studies with only palm computers by our group and others.13,36 These results build on our previous findings demonstrating the feasibility and validity of using palm computer to collect ESM data on stress and psychosis in hospitalized schizophrenia patients.13
Our second aim was to confirm the validity of using momentary negative mood, measured using ESM with palm computers, as a high time resolution index of subjective stress. While paper-based ESM has been used extensively to study stress in individuals with psychosis, this index has not been used in studies utilizing ESM with palm computers.13,36 The subjective nature of ESM data (eg, thoughts, moods, mental states) makes the use of customary reliability and validity techniques problematic. The assessment of reliability is complicated by the fact that experiences such as ”I feel anxious” may not necessarily have behavioral expressions, thus making them difficult to verify. As a result, the ascertainment of reliability can be obtained only by evaluation of the validity11––once validity is demonstrated, reliability can be assumed. Delespaul11 suggested a number of methods to validate raw ESM data including the use of face validity, comparison of aggregated data between distinct groups, correlations between similar and dissimilar items, as well as determining associations with available behavioral/external referents. In accord with these recommendations, we found ESM to be valid. The items used in our assessment were designed to maximize face validity by using everyday simple vocabulary. A comparison of quality of thoughts during high- vs low-stress moments indicates differences in the expected direction. Moments of high stress were characterized by thoughts that were significantly less pleasant, more confused, described as too fast, and experienced as more difficult to express or get rid of, as well as a statistical trend toward experiencing less control over thoughts.
Our final aim was to assess the association between stress and arousal during daily functioning using high time resolution measurements. As hypothesized, momentary increases in stress during the flow of daily functioning were associated with parasympathetic withdrawal and increase in sympathovagal balance. Overall, our findings support the use of ESM with palm computers and LS as valid measures of concurrent stress and arousal in individuals with psychosis during the flow of daily functioning in natural environments. The findings are consistent with previous studies in healthy individuals that demonstrated that negative mood was associated with sympathetically dominated power spectrum, whereas positive mood is associated with a shift toward parasympathetic activity.15–18 While the association between stress and parasympathetic activity was highly significant, our findings may in fact underestimate the magnitude of the link between these variables given the influence of the participants’ posture and physical activities during daily functioning.15 Casual daily activities such as standing or walking may have minimal impact on subjective stress but will have significant influence on momentary parasympathetic output, thus potentially minimizing the association between stress and arousal. While it is possible that high-stress moments may be associated with elevated body posture and/or increased physical activity, our pilot data using patient's report of their posture/activities during experience samples (unpublished) do not support this link. However, future studies with more rigorous posture/activity measures (ie, accelerometer) will be needed to clarify this potential confound.
Our group has previously demonstrated the feasibility of using ESM with palm computers to assess stress and psychosis. The addition of the LS offers an exciting new way to study the interactions between stress, arousal, and psychotic symptoms in the “real world.” The methodology allows simultaneous assessment of the stress, arousal, and psychosis; the ability to do it during ambulatory, daily functioning in natural environments; and the possibility to track the dynamic fluctuations in stress, arousal, and psychosis using precise, high time resolution measurement.
Our methodology may be used to further elucidate the mechanisms involved in psychopathology including the temporal links between stress, arousal, and psychosis. For example, the methodology may be used to investigate the impact of increased momentary arousal on severity of psychotic symptoms, shedding light on the physiological and psychological underpinnings of the diathesis-stress model. Similarly, the methodology may allow investigating the “real-world” psychological processes that influence stress and arousal regulation in psychosis. These issues are currently being investigated by D.K. and colleagues. Furthermore, future technological advances may lead to Holter devices with a smaller footprint that will allow increased user convenience and facilitation of longer durations of assessment. Such advances may potentially also allow for the development of more interactive devices that combine cardiac autonomic monitoring technology with palm computer–like devices to be integrated as part of treatment. For example, such ambulatory monitoring devices may be used as part of cognitive-behavioral therapy (CBT) to detect high autonomic arousal during daily functioning and prompt patients to use practiced cognitive strategies to downregulate stress. D.K. and Corcoran42 have recently reported on a case study in which a palm computer was successfully integrated into CBT with an individual at ultra high risk of psychosis.
The present study has a number of limitations. The sample size in the present study was modest; thus, the results should be interpreted with caution. Similarly, the 36-hour assessment period was shorter than what is typical in paper-based ESM schizophrenia studies (6–7 days). The selection of a shorter assessment period was influenced by the study's protocol that required patients not to take showers during the 36-hour assessment period. Thirdly, the present study was carried out in an inpatient unit. The feasibility of using our methodology in outpatients with schizophrenia has not been tested to date. However, Granholm et al36 recently completed a study of outpatient individuals with schizophrenia using palm computers and reported minimal loss/damage to the palm computers. Our group is currently assessing the feasibility and validity of using our methodology in outpatients with schizophrenia residing in the community. Another potential limitation is our selection of 10-minute epochs (5 min before and after the “beeps”). As there are no precedents in schizophrenia studies, this period was selected based on standards used in cardiology studies. It is possible that, given the dynamic nature of stress and psychotic symptoms, shorter epochs (−3/+3 or even −1/+1 min) may be more appropriate. Finally, the assessment of cardiac functioning in this study was completed in medicated patients. While psychiatric medications may impact cardiac functioning, a precise index quantifying this potential relationship is not available. We used chlorpromazine equivalence as crude index to control for this potential link.37 However, Hempel et al43 reported no difference in parasympathetic cardiovascular effects between haloperidol, olanzapine, and risperidone.
In summary, our findings support the concurrent use of ESM with palm computers along with the LS to assess momentary stress and ambulatory cardiac autonomic regulation in individuals with psychosis during daily functioning in “real-world” environments. This methodology may be used to further elucidate the temporal links between stress, arousal, and psychotic symptoms.
Funding
National Institutes of Mental Health (K23MH077653 to D.K., K24MH01699 to D.M.); VivoMetrics, Inc (instrument grant to D.K.).
Acknowledgments
We would like to thank Judy Thompson, PhD, and Samantha Breen, BA, for their help in the preparation of this manuscript.
References
- 1.Zubin J, Spring B. Vulnerability–a new view of schizophrenia. J Abnorm Psychol. 1977;86:103–126. doi: 10.1037//0021-843x.86.2.103. [DOI] [PubMed] [Google Scholar]
- 2.Norman RM, Malla AK. Stressful life events and schizophrenia. I: a review of the research. Br J Psychiatry. 1993;162:161–166. doi: 10.1192/bjp.162.2.161. [DOI] [PubMed] [Google Scholar]
- 3.Norman RM, Malla AK. Stressful life events and schizophrenia. II: conceptual and methodological issues. Br J Psychiatry. 1993;162:166–174. doi: 10.1192/bjp.162.2.166. [DOI] [PubMed] [Google Scholar]
- 4.Phillips LJ, Francey SM, Edwards J, McMurray N. Stress and psychosis: towards the development of new models of investigation. Clin Psychol Rev. 2007;27:307–317. doi: 10.1016/j.cpr.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Gipsen-de Wied. Stress in schizophrenia: an integrative view. Eur J Pharmacol. 2000;405:1–3. doi: 10.1016/s0014-2999(00)00567-7. [DOI] [PubMed] [Google Scholar]
- 6.Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- 7.Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev. 2003;13:43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- 8.Food and Drug Administration (FDA) Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Rockville, MD, USA: Food and Drug Administration. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myin-Germeys I, van Os J, Schwartz JE, Stone AA, Delespaul PA. Emotional reactivity to daily life stress in psychosis. Arch Gen Psychiatry. 2001;58:1137–1144. doi: 10.1001/archpsyc.58.12.1137. [DOI] [PubMed] [Google Scholar]
- 10.Norman RM, Malla AK. A prospective study of daily stressors and symptomatology in schizophrenic patients. Soc Psychiatry Psychiatr Epidemiol. 1994;29:244–249. doi: 10.1007/BF00802047. [DOI] [PubMed] [Google Scholar]
- 11.Delespaul P. Assessing Schizophrenia in Daily Life. Maastricht, The Netherlands: ISPER; 1995. [Google Scholar]
- 12.Delespaul P, deVries M, van Os J. Determinants of occurrence and recovery from hallucinations in daily life. Soc Psychiatry Psychiatr Epidemiol. 2002;37:97–104. doi: 10.1007/s001270200000. [DOI] [PubMed] [Google Scholar]
- 13.Kimhy D, Delespaul P, Corcoran C, Ahn H, Yale S, Malaspina D. Computerized experience sampling method (ESMc): assessing feasibility and validity among individuals with schizophrenia. J Psychiatr Res. 2006;40:221–230. doi: 10.1016/j.jpsychires.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 15.Sloan RP, Shapiro PA, Bagiella E, et al. Effect of mental stress throughout the day on cardiac autonomic control. Biol Psychol. 1994;37:89–99. doi: 10.1016/0301-0511(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 16.McCraty R, Atkinson M, Tiller WA, Rein G, Watkins AD. The effects of emotions on short-term power spectrum analysis of heart rate variability. Am J Cardiol. 1995;76:1089–1093. doi: 10.1016/s0002-9149(99)80309-9. [DOI] [PubMed] [Google Scholar]
- 17.Dishman RK, Nakamura Y, Garcia ME, Thompson RW, Dunn AL, Blair SN. Heart rate variability, trait anxiety, and perceived stress among physically fit men and women. Int J Psychophysiol. 2000;37:121–133. doi: 10.1016/s0167-8760(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 18.Mezzacappa ES, Kelsey RM, Katkin ES, Sloan RP. Vagal rebound and recovery from psychological stress. Psychosom Med. 2001;63:650–657. doi: 10.1097/00006842-200107000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Valkonen-Korhonen M, Tarvainen MP, Ranta-Aho P, et al. Heart rate variability in acute psychosis. Psychophysiology. 2003;40:716–726. doi: 10.1111/1469-8986.00072. [DOI] [PubMed] [Google Scholar]
- 20.Okada T, Toichi M, Sakihama M. Influences of an anticholinergic antiparkinsonian drug, parkinsonism, and psychotic symptoms on cardiac autonomic function in schizophrenia. J Clin Psychopharmacol. 2003;23:441–447. doi: 10.1097/01.jcp.0000088901.24613.b8. [DOI] [PubMed] [Google Scholar]
- 21.Bar KJ, Boettger MK, Koschke M, et al. Non-linear complexity measures of heart rate variability in acute schizophrenia. Clin Neurophysiol. 2007;118:2009–2015. doi: 10.1016/j.clinph.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Bar KJ, Letzsch A, Jochum T, Wagner G, Greiner W, Sauer H. Loss of efferent vagal activity in acute schizophrenia. J Psychiatr Res. 2005;39:519–527. doi: 10.1016/j.jpsychires.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Bar KJ, Wernich K, Boettger S, et al. Relationship between cardiovagal modulation and psychotic state in patients with paranoid schizophrenia. Psychiatry Res. 2008;157:255–257. doi: 10.1016/j.psychres.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Boettger S, Hoyer D, Falkenhahn K, Kaatz M, Yeragani VK, Bar KJ. Altered diurnal autonomic variation and reduced vagal information flow in acute schizophrenia. Clin Neurophysiol. 2006;117:2715–2722. doi: 10.1016/j.clinph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Yi SH, Yoo CS, et al. Heart rate dynamics and their relationship to psychotic symptom severity in clozapine-treated schizophrenic subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:371–378. doi: 10.1016/j.pnpbp.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Olbrich R, Kirsch P, Pfeiffer H, Mussgay L. Patterns of recovery of autonomic dysfunctions and neurocognitive deficits in schizophrenics after acute psychotic episodes. J Abnorm Psychol. 2001;110:142–150. doi: 10.1037//0021-843x.110.1.142. [DOI] [PubMed] [Google Scholar]
- 27.Toichi M, Kubota Y, Murai T, et al. The influence of psychotic states on the autonomic nervous system in schizophrenia. Int J Psychophysiol. 1999;31:147–154. doi: 10.1016/s0167-8760(98)00047-6. [DOI] [PubMed] [Google Scholar]
- 28.Malaspina D, Dalack G, Leitman D, et al. Low heart rate variability is not caused by typical neuroleptics in schizophrenia patients. CNS Spectr. 2002;7:53–57. doi: 10.1017/s1092852900022264. [DOI] [PubMed] [Google Scholar]
- 29.Mujica-Parodi LR, Yeragani V, Malaspina D. Nonlinear complexity and spectral analyses of heart rate variability in medicated and unmedicated patients with schizophrenia. Neuropsychobiology. 2005;51:10–15. doi: 10.1159/000082850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 31.Kirschbaum C, Hellhammer D. Response variability of salivary cortisol under psychological stimulation. J Clin Chem Clin Biochem. 1989;27:237. [PubMed] [Google Scholar]
- 32.Jacobs N, Myin-Germeys I, Derom C, Delespaul P, van Os J, Nicolson NA. A momentary assessment study of the relationship between affective and adrenocortical stress responses in daily life. Biol Psychol. 2007;74:60–66. doi: 10.1016/j.biopsycho.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 33.van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosom Med. 1996;58:447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Ockenfels MC, Porter L, Smyth J, Kirschbaum C, Hellhammer DH, Stone AA. Effect of chronic stress associated with unemployment on salivary cortisol: overall cortisol levels, diurnal rhythm, and acute stress reactivity. Psychosom Med. 1995;57:460–467. doi: 10.1097/00006842-199509000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Peeters F, Nicholson NA, Berkhof J. Cortisol responses to daily events in major depressive disorder. Psychosom Med. 2003;65:836–841. doi: 10.1097/01.psy.0000088594.17747.2e. [DOI] [PubMed] [Google Scholar]
- 36.Granholm E, Loh C, Swendsen J. Feasibility and validity of computerized ecological momentary assessment in Schizophrenia. Schizophr Bull. 2008;34:507–514. doi: 10.1093/schbul/sbm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 38.Nurnberger JI, Jr., Blehar MC, Kaufmann CA, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 39.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 40.Gable SL, Reis HT, Elliot AJ. Behavioral activation and inhibition in everyday life. Pers Soc Psychol. 2000;78:1135–1149. doi: 10.1037//0022-3514.78.6.1135. [DOI] [PubMed] [Google Scholar]
- 41.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 42.Kimhy D, Corcoran CM. Use of palm computer as an adjunct to cognitive behavior therapy with an ultra high risk patient—a case report. Early Interv Psychiatry. 2008;2:234–241. doi: 10.1111/j.1751-7893.2008.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hempel RJ, Tulen JH, van Beveren NJ, Röder CH, Hengeveld MW. Cardiovascular variability during treatment with haloperidol, olanzapine or risperidone in recent-onset schizophrenia [published online ahead of print June 18, 2008] J Psychopharmacol. 2008 doi: 10.1177/0269881108091254. doi:10.1177/0269881108091254. [DOI] [PubMed] [Google Scholar]