Background
Many people with schizophrenia or similar severe mental disorders do not achieve a satisfactory treatment response with ordinary antipsychotic drug treatment. In these cases, various add-on medications are used, among them beta-adrenergic receptor antagonists (beta-blockers).
Objectives
To evaluate the clinical effects of beta-blockers as an adjunct to antipsychotic medication in schizophrenia or similar severe mental disorders.
Search Methods
We searched the Cochrane Schizophrenia Group Trials Register (October 2009) and references of all identified studies for further citations. Where necessary, we also contacted authors of trials for further information.
Selection Criteria
All randomized controlled trials (RCT) comparing beta-blockers with placebo as an adjunct to conventional antipsychotic medication for those with schizophrenia.
Data Collection and Analysis
Data were extracted independently by at least 2 reviewers. For dichotomous data, we calculated relative risks (RR) and their 95% CI on an intention-to-treat basis based on a fixed-effect model. If continuous data were included, we analyzed these data using weighted mean difference with a 95% CI based on a fixed-effect model.
Results
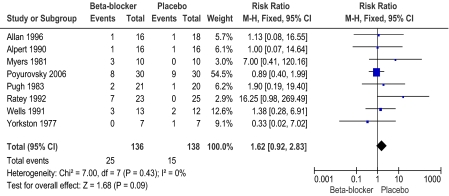
In this 2010 update, 4 additional trials were identified, bringing the total number of included studies to 9 (total n = 282, 8 trials were short term, under/or 12 weeks duration). Overall reporting of data was poor, resulting in much information being lost to this review. No data on mental state were possible to include. Data were reported in graphs with no variances. Adding beta-blockers to antipsychotic treatment seems generally acceptable (n = 274, 8 RCTs, RR leaving study early at 12 weeks = 1.62, CI = 0.92–2.83, figure 1). We found no difference in relapse rate between the 2 treatment groups (n = 68, 2 RCTs, RR at 12 weeks = 3.12, CI = 0.34–28.36). There were few reported general adverse events (n = 48, 1 RCT, RR at 12 weeks = 5.42, CI = 0.27–107.20). The most frequent specific adverse effect was hypotension or symptoms likely to be related to hypotension (n = 274, 8 RCTs, RR at 12 weeks = 1.63 CI = 0.70–3.84).
Fig. 1.
Global state: Leaving the study early—short term.
Authors' Conclusions
Existing evidence is limited and dated. Any possible benefit of adjunctive beta-blocker therapy is obscured by poor reporting within the studies. Important data on quality of life, satisfaction, healthy days, and cost are not available. Considering the number of people whose symptoms are only partially responsive to antipsychotic medication, well conducted and reported trials in this area could be justified. Full details are found in the complete review.1
References
- 1.Shek E, Bardhan S, Cheine MV, Ahonen J, Wahlbeck K. Beta-blocker supplementation of standard drug treatment for schizophrenia. Cochrane Database Syst Rev. 2010 doi: 10.1093/schbul/sbq089. doi: 10.1002/14651858.CD000234. [DOI] [PMC free article] [PubMed] [Google Scholar]