Abstract
Background: Neurological soft signs (NSS) are hypothesized as candidate endophenotypes for schizophrenia, but their prevalence and relations with clinical and demographic data are unknown. The authors undertook a quantification (meta-analysis) of the published literature on NSS in patients with schizophrenia and healthy controls. A systematic search was conducted for published articles reporting NSS and related data using standard measures in schizophrenia and healthy comparison groups. Method: A systematic search was conducted for published articles reporting data on the prevalence of NSS in schizophrenia using standard clinical rating scales and healthy comparison groups. Meta-analyses were performed using the Comprehensive Meta-analysis software package. Effect sizes (Cohen d) indexing the difference between schizophrenic patients and the healthy controls were calculated on the basis of reported statistics. Potential moderator variables evaluated included age of patient samples, level of education, sample sex proportions, medication doses, and negative and positive symptoms. Results: A total of 33 articles met inclusion criteria for the meta-analysis. A large and reliable group difference (Cohen d) indicated that, on average, a majority of patients (73%) perform outside the range of healthy subjects on aggregate NSS measures. Cognitive performance and positive and negative symptoms share 2%–10% of their variance with NSS. Conclusions: NSS occur in a majority of the schizophrenia patient population and are largely distinct from symptomatic and cognitive features of the illness.
Keywords: neurological soft signs, meta-analysis, schizophrenia
Introduction
Schizophrenia is a loose and complex neuropsychiatric syndrome characterized by a range of cognitive1 and psychophysiological deficits.2 Beginning with the seminal review by Heinrichs and Buchanan,3 the study of neurological soft signs (NSS) has provided an additional and increasingly important perspective on the illness. Conventionally defined as nonlocalizing abnormalities without diagnostic specificity, NSS involve observable defects in sensory integration (SI), motor coordination (MC), and inhibition. Tsuang and colleagues4,5 have argued that these defects reflect genetic and nongenetic processes underpinning the predisposition to psychotic illness. Moreover, the assumption of nonfocal neural representation is being revised in light of evidence that NSS have identifiable cerebral correlates.6–8 Current views consider these signs as covariates of attention,9–13 verbal ability,14,15 and visual-spatial memory.10,16,17 It follows that NSS and cognitive findings have emerged as candidate endophenotypes for schizophrenia-spectrum disorders.18,19
Evaluating the strength of evidence in support of NSS as key indicators of psychotic illness has been complicated by a number of potentially confounding variables including duration of illness, medication doses, and the use of different measurement techniques. Although systematic reviews addressing the issues of NSS in schizophrenia have appeared in the past decades, all were narrative reviews (eg, Bombin et al,18 Heinrichs and Buchanan,3 Bombin et al20). Although valuable, narrative reviews do not quantify the strength or consistency of evidence in a field or test hypotheses about moderators underlying the variability between study findings. Meta-analytic techniques of research synthesis provide the most objective way of assessing the magnitude and consistency of differences between people with and without schizophrenia.21,22 Research synthesis aggregates data on the same or highly similar dependent measures from multiple independent studies, expresses differences between schizophrenia patients and healthy comparison subjects in pooled SD units (ie, the effect size), and yields CIs for mean effect sizes.23
Recent meta-analyses suggest that deficits in cognitive measures consistently distinguish a majority of schizophrenia patients from healthy, nonpsychiatric subjects.21,24,25 However, little is known about the relative prevalence of NSS in schizophrenia patients and healthy samples. In addition, there are a few published estimates of the strength of association between soft signs, symptoms, and neurocognitive functions in the schizophrenia population. Moreover, the relative prevalence of NSS in relatives of schizophrenia patients remains unknown in terms of quantification and consistency across the research literature. Thus, although NSS are regarded as candidate endophenotypes for schizophrenia, it is not clear whether data obtained from relatives parallel the deficits found in patients.
In light of these considerations, we undertook a meta-analysis to address a number of issues concerning the magnitude, consistency, and mediation of NSS in schizophrenia. First, we aimed to determine the average standardized difference in NSS between patients with schizophrenia and healthy research participants. Second, we were interested in estimating the average magnitude of association between NSS, psychiatric symptoms, and neurocognitive functions in the schizophrenia population. Third, we sought to evaluate potential moderator variables that may contribute to differences between patients and healthy participants. These moderators included patients’ ages, level of education, sex, medication, and negative and positive symptom severity.
Methods
Potential articles were identified through a comprehensive search using literature databases of Elsevier Science, EBSCOHost (PsychINFO, PsychARTICLE), and MedLine between 1966 and January 2008. The key words were “neurological soft signs,” “neurological signs,” “soft signs,” “neurological abnormalit*,” “motor coordination,” “sensory integration,” “disinhibition,” “complex motor sequencing,” “Luria task,” “fist-edge-palm,” “schizophrenia,” “schizotypal,” and “schizotypy.” Additional articles were obtained from the reference lists of the initial article base and from a search from January 2007 through January 2008 of journals that frequently published articles on NSS in schizophrenia. This strategy was used to minimize the possibility of overlooking very recent articles not included in computerized databases. These journals included Acta Psychiatrica Scandinavica, American Journal of Psychiatry, Archives of General Psychiatry, British Journal of Psychiatry, Journal of Abnormal Psychology, European Archives of Psychiatry and Clinical Neuroscience, Journal of Nervous and Mental Diseases, Neuropsychology, Neuropsychologia, Psychiatry Research, Psychological Medicine, Schizophrenia Bulletin, and Schizophrenia Research. These search procedures yielded an initial pool of 172 potential articles for inclusion.
The following criteria were used to select studies in the initial pool for quantitative analysis. Each study required at least one of the criteria: (1) patients and controls’ scores means or t value obtained from NSS instruments such as the Neurological Evaluation Scale (NES)26 and the Cambridge Neurological Inventory (CNI)27; (2) the correlation coefficient between NSS and psychiatric symptom ratings from instruments including the Brief Psychiatric Rating Scale (BPRS),28 Positive and Negative Syndrome Scale (PANSS),29 the Scale for Assessment of Positive Symptoms,30 and the Scale for Assessment of Negative Symptoms31; and (3) the correlation coefficient between NSS and scores of neurocognitive performance from standard tests such as the Wechsler Adult Intelligence Scale,32,33 Trail Making Test,34 and Wisconsin Card Sorting Test,35 etc.
This procedure yielded a study base of 57 published reports, with 40 contrasting NSS (included total and subscale) schizophrenic and healthy control samples, 14 reporting correlation coefficients between NSS and neurocognitive scores, and 18 reports of correlation coefficients between NSS and symptom scores.
We recorded potential moderator variables including (1) the name of the first author, year of publication, and the order for sorting; (2) schizophrenia diagnostic criteria used (Diagnostic and Statistical Manual of Mental Disorders [Third Edition], Diagnostic and Statistical Manual of Mental Disorders [Third Edition Revised], or Diagnostic and Statistical Manual of Mental Disorders [Fourth Edition]; International Classification of Diseases, Ninth Revision or Tenth Revision); (3) descriptions of basic information including sample size, gender, age, education, age on set, duration of illness, chronic vs first-episode schizophrenia; (4) NSS scale (ie, NES, CNI, Condensed Neurological Examination,36 etc) and NSS score of patients and controls (mean, SD, t value, P value); (5) correlation coefficient comparing patients’ NSS scores with their symptoms scores; and (6) correlation coefficient comparing patients’ NSS scores with their neurocognitive function test score.
Data Analysis
All analyses were performed using the Comprehensive Meta-analysis (CMA) software package.37 Effect sizes (Cohen d) indexing the difference between schizophrenia patients and the healthy controls were calculated on the basis of reported statistics (mean of schizophrenia sample minus the mean of the healthy control group, divided by the pooled SD). When means and SDs were not available, effect size d was computed from t or F values or estimated from exact P values. Standard meta-analytic methods were adopted to obtain mean effect sizes weighted for study variance and averaged across primary studies.23 Stability of the mean effect was estimated by its 95% CI. In addition, the homogeneity statistic, Q, was calculated to test whether individual study effect sizes for any given variable likely reflect a single common population effect size. A significant Q statistic indicates heterogeneity of the individual study effect sizes, implying multiple underlying effect populations and the need to analyze potential moderators of effect size variability.23
Due to the existence of the hypothetical “file-drawer problem,”38 whereby mean effect sizes based on published findings may be overturned by the existence of discrepant unpublished findings,39 it is recommended that researchers use estimation procedures to assess the likelihood of this possibility. Thus, a fail-safe number estimates the number of unpublished studies with nil or minimal effect sizes required to reduce an overall effect size to some specified negligible value.38,40 We set this negligible level at 0.2 and assumed a value of 0.1 for hypothetically “missing” or unpublished studies. A second method is a 2-step approach that uses both visual inspection of graphic data and estimation procedures. First, individual study effect sizes are plotted against their SEs, giving rise to a “funnel” shape or distribution in the ideal situation and suggesting the absence of bias. On the other hand, skewing and asymmetry in the effect distribution imply a publication bias.41 Duval and Tweedie42 developed a method whereby “missing” study effects that correct the hypothetical bias and graphic asymmetry are estimated and included along with the published studies to yield an adjusted mean effect size. This procedure was carried out with the CMA software. Finally, moderator variables evaluated in relation to both uncorrected and corrected effect sizes included age of patient samples, level of education, sample sex proportions, medication doses, and negative and positive symptoms.
Results
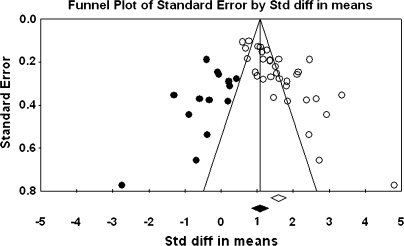
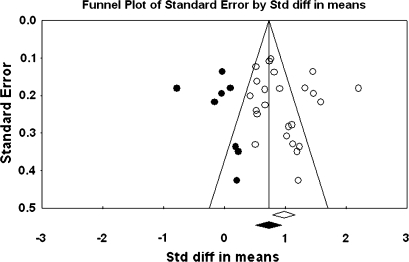
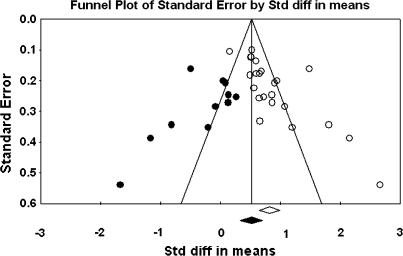
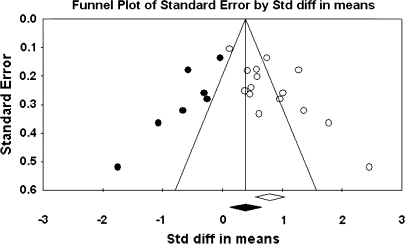
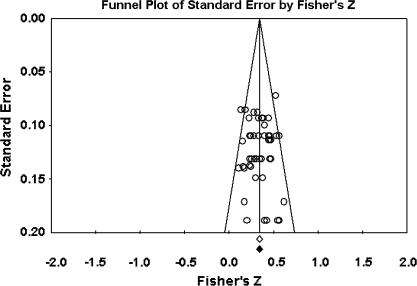
The primary study base yielded 34 schizophrenia-control comparisons of NSS total score data drawn from 33 independent studies. One study43 was excluded because it reported the same data as Ismail et al,44 which was included in the study base. All these comparisons were reported as being statistically significant in the original studies. Grand mean effect sizes for total and selective NSS scores along with their 95% CIs and homogeneity statistics are presented in table 1. The aggregated NSS total score mean effect was large, corresponding to 73% separation of joint schizophrenia and control distributions.45 However, the individual effects were not homogeneous as indexed by the Q statistic. Moreover, the funnel plot (figure 1) showed a higher concentration of studies on the right side of the mean, suggesting a bias against publishing small studies with no effect. Therefore, the CMA recomputed the effect size using the method of Duval and Tweedie40 described previously. The number of “missing” studies and adjusted effect size and adjusted Q statistic are shown in table 2. The adjusted standard difference in means is lower than uncorrected results for NSS total and for every subscale comparison. Nevertheless, the fail-safe number of studies required to overturn the mean effect size was 341, which is sufficiently large to make the existence of large numbers of unpublished negligible findings unlikely. Analysis of data derived from first-episode schizophrenia and chronic patient samples also revealed large mean effect sizes that were statistically indistinguishable. Similarly, there was no evidence for greater sensitivity of type of NSS instrument (ie, NES vs CNI). However, mean d‘s and associated CIs show that NSS indexed by a total score, rather than subscales indexing sensory and motor items, provides the most sensitive discrimination of patients and controls. Mean effect sizes for all subscale scores were less than d = 1.0. At the same time, all quantified literatures with the exception of studies reporting MC subscale scores based on the NES revealed significant heterogeneity as indexed by Q tests (figures 1–4).
Table 1.
Results of Meta-analyses of Differences in NSS Total Scores and Subscale Scores Between Schizophrenia Patients and Healthy Controls
| Number of Studies | Number of Schizophrenic Patients | Number of Healthy Controls | Std Diff | SE (95% CI) | Q Value | Fail-Safe N | |
| Contrasting NSS total score | 33 | 2345 | 1984 | 1.591 | 0.109 (1.377, 1.805) | 277.093* | 341 |
| NSS measured by NES or modified NES | 22 | 1382 | 1229 | 1.554 | 0.137 (1.285, 1.822) | 170.285* | 219 |
| NSS measured by CNI | 5 | 688 | 553 | 1.404 | 0.275 (0.866, 1.943) | 65.172* | 47 |
| First-episode schizophrenia only | 9 | 646 | 717 | 1.526 | 0.238 (1.060, 1.992) | 97.806* | 74 |
| Chronic/mixed | 24 | 1699 | 1267 | 1.613 | 0.120 (1.378, 1.848) | 160.826* | 272 |
| Contrasting NSS-MC score | 24 | 1926 | 1510 | 0.977 | 0.094 (0.793, 1.161) | 128.686* | 175 |
| NSS measured by NES or modified NES | 14 | 990 | 742 | 0.878 | 0.088 (0.705, 1.051) | 31.253 | 93 |
| NSS measured by CNI | 8 | 871 | 728 | 1.110 | 0.211 (0.697, 1.523) | 93.596* | 64 |
| First-episode schizophrenia only | 7 | 661 | 610 | 0.968 | 0.140 (0.693, 1.242) | 26.016* | 51 |
| Chronic/mixed | 17 | 1265 | 900 | 0.979 | 0.126 (0.731, 1.226) | 102.661* | 125 |
| Contrasting NSS-SI score | 23 | 1839 | 1456 | 0.823 | 0.087 (0.652, 0.994) | 104.487* | 106 |
| NSS measured by NES or modified NES | 15 | 1022 | 774 | 0.872 | 0.122 (0.633, 1.111) | 68.838* | 65 |
| NSS measured by CNI | 7 | 778 | 660 | 0.739 | 0.140 (0.464, 1.014) | 33.144* | 34 |
| First-episode schizophrenia only | 7 | 600 | 574 | 0.754 | 0.170 (0.421, 1.086) | 35.659* | 22 |
| Chronic/mixed | 16 | 1239 | 882 | 0.854 | 0.100 (0.659, 1.049) | 58.989* | 88 |
| Contrasting NSS-MSeq (NES) score | 15 | 1001 | 721 | 0.795 | 0.127 (0.546, 1.044) | 71.407* | 62 |
| First-episode schizophrenia only | 6 | 482 | 414 | 0.677 | 0.204 (0.276, 1.078) | 31.001* | 16 |
| Chronic/mixed | 9 | 519 | 307 | 0.874 | 0.156 (0.569, 1.179) | 30.220* | 54 |
| Contrasting NSS-disinhib (CNI) score | 8 | 909 | 711 | 0.970 | 0.180 (0.617, 1.322) | 69.388* | 56 |
Note: NSS = neurological soft signs; Std Diff = standard difference in means; NES = Neurological Evaluation Scale; CNI = Cambridge Neurological Inventory; MC = motor coordination; SI = sensory integration; MSeq = complex motor sequencing; disinhib = disinhibition.
Q value heterogeneous, P < .05.
Fig. 1.
Funnel Plot for the Meta-analysis of Differences in Neurological Soft Signs Total Scores Between Schizophrenia Patients and Healthy Controls.
Table 2.
Adjusted Results of Meta-analyses of Differences in NSS Total Scores and Subscale Scores Between Schizophrenia Patients and Healthy Controls
| Number of Studies Missing | Adjusted Std Diff (Adjusted 95% CI) | Adjusted Q Value | |
| Contrasting NSS total score | 14 | 1.077 (0.844, 1.310) | 561.558* |
| NSS measured by NES or modified NES | 9 | 1.041 (0.751, 1.331) | 342.637* |
| NSS measured by CNI | 2 | 0.950 (0.339, 1.561) | 144.857* |
| First-episode schizophrenia only | 4 | 0.848 (0.340, 1.357) | 205.437* |
| Chronic/mixed | 10 | 1.176 (0.918, 1.433) | 332.353* |
| Contrasting NSS-MC score | 8 | 0.726 (0.512, 0.941) | 308.755* |
| NSS measured by NES or modified NES | 3 | 0.749 (0.550, 0.948) | 62.317* |
| NSS measured by CNI | 2 | 0.838 (0.381, 1.295) | 175.308* |
| First-episode schizophrenia only | 3 | 0.757 (0.447, 1.066) | 66.126* |
| Chronic/mixed | 0 | — | — |
| Contrasting NSS-SI score | 11 | 0.516 (0.332, 0.700) | 232.101* |
| NSS measured by NES or modified NES | 7 | 0.517 (0.267, 0.768) | 139.946* |
| NSS measured by CNI | 0 | — | — |
| First-episode schizophrenia only | 3 | 0.432 (0.085, 0.780) | 67.380* |
| Chronic/mixed | 2 | 0.755 (0.539, 0.971) | 82.997* |
| Contrasting NSS-Mseq (NES) score | 7 | 0.389 (0.116, 0.663) | 183.344* |
| First-episode schizophrenia only | 3 | 0.234 (−0.196, 0.664) | 86.628* |
| Chronic/mixed | 1 | 0.777 (0.440, 1.113) | 41.204* |
| Contrasting NSS-disinhib (CNI) score | 2 | 0.710 (0.287, 1.132) | 169.308* |
Note: NSS = neurological soft signs; Std Diff = standard difference in means; NES = Neurological Evaluation Scale; CNI = Cambridge Neurological Inventory; MC = motor coordination; SI = sensory integration; MSeq = complex motor sequencing; disinhib = disinhibition.
Q value heterogeneous, P < .05.
Fig. 2.
Funnel Plot for the Meta-analysis of Differences in Neurological Soft Signs Motor Coordination Scores Between Schizophrenia Patients and Healthy Controls.
Fig. 3.
Funnel Plot for the Meta-analysis of Differences in Neurological Soft Signs Sensory Integration Scores Between Schizophrenia Patients and Healthy Controls.
Fig. 4.
Funnel Plot for the Meta-analysis of Differences in Neurological Soft Signs Complex Motor Sequencing Scores Between Schizophrenia Patients and Healthy Controls.
Fig. 5.
Funnel Plot for the Meta-analysis of Correlation Between NSS Total Scores and Neurocognitive Test Scores in Schizophrenia Patients.
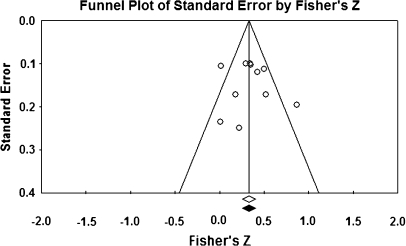
Pearson correlation coefficients between NSS total and subscale scores and aggregated and selective neurocognitive test scores are presented in tables 3 and 4. Several literatures were represented by small numbers of primary studies.11,13,15,17,46–50 However, it is noteworthy that NSS total, MC, and SI associate significantly with cognitive performance collapsed across tasks. In addition, with 10 primary studies reporting data from executive function tasks, the relationship between cognition and NSS total scores is similar in magnitude to the collapsed findings. Correlations between NSS scales and symptom severity (PANSS, BPRS) are presented in tables 5 and 6. Significant relationships were found between NSS total and both total and negative symptoms, with shared variances of 10%–12%. In contrast, while CIs for the mean value for NSS total and positive symptoms excluded 0, the effect size was relatively modest with less than 4% shared variance (figures 5 and 6).
Table 3.
Results of Meta-analyses of Correlations of NSS and Neurocognitive Functions
| Number of Studies | Number of Subjects | Correlation (95% CI) | Q Value | |
| Correlation between NSS total score and cognitive abilities | 48 | 3789 | −0.331 (−0.362, −0.299) | 54.972 |
| NSS-MC subscale | 24 | 1816 | −0.332 (−0.378, −0.283) | 28.199 |
| NSS-SI subscale | 31 | 2435 | −0.374 (−0.409, −0.339) | 30.589 |
| NSS-Mseq subscale | 6 | 489 | −0.313 (−0.392, −0.23) | 3.253 |
| NSS-disinhib subscale | 17 | 1653 | −0.177 (−0.251, −0.101) | 37.535* |
| Correlation between NSS total score and verbal memory | 4 | 383 | −0.305 (−0.394, −0.21) | 0.955 |
| (WMS-R verbal pairs; paired associate learning; logical memory—immediate, delayed; CVLT) | ||||
| NSS-MC subscale | 5 | 423 | −0.296 (−0.397, −0.182) | 5.71 |
| NSS-SI subscale | 6 | 508 | −0.354 (−0.429, −0.274) | 2.687 |
| NSS-disinhib subscale | 4 | 338 | −0.058 (−0.166, 0.05) | 2.037 |
| Correlation between NSS total score and nonverbal memory | 6 | 608 | −0.374 (−0.485, −0.252) | 13.851* |
| (WMS-R visual reproduction—immediate, delayed; visual pairs; ROCFT) | ||||
| NSS-MC subscale | 4 | 338 | −0.396 (−0.483, −0.3) | 1.942 |
| NSS-SI subscale | 7 | 554 | −0.417 (−0.485, −0.345) | 1.748 |
| NSS-Mseq subscale | 1 | 85 | −0.3 (−0.482, −0.093) | NA |
| NSS-disinhib subscale | 4 | 338 | −0.117 (−0.241, 0.009) | 3.95 |
| Correlation between NSS total score and motor | 4 | 360 | −0.299 (−0.392, −0.291) | 0.363 |
| (Finger tapping test, Purdue pegboard task) | ||||
| Correlation between NSS total score and attention | 8 | 579 | −0.292 (−0.382, −0.197) | 9.892 |
| (TMT-A, B time; WAIS-R—digit span; reading span test; Stroop; Continuous Performance Test; SART) | ||||
| NSS-MC subscale | 2 | 82 | −0.274 (−0.6, 0.13) | 3.147 |
| NSS-SI subscale | 4 | 252 | −0.282 (−0.393, −0.161) | 2.058 |
| NSS-Mseq subscale | 1 | 85 | −0.26 (−0.448, −0.05) | NA |
| NSS-disinhib subscale | 1 | 51 | −0.015 (−0.289, 0.262) | NA |
| Correlation between NSS total score and IQ | 6 | 580 | −0.336 (−0.443, −0.218) | 10.952 |
| (WAIS-R—full scale, verbal, performance; WAIT-III; MWT-B, Ammons Quick Test) | ||||
| NSS-MC subscale | 4 | 353 | −0.26 (−0.474, −0.017) | 12.788* |
| NSS-SI subscale | 5 | 456 | −0.468 (−0.541, −0.388) | 4.306 |
| NSS-Mseq subscale | 1 | 79 | −0.18 (−0.386, 0.043) | NA |
| NSS-disinhib subscale | 2 | 246 | −0.35 (−0.456, −0.234) | 0 |
| Correlation between NSS total score and spatial ability | 5 | 233 | −0.268 (−0.386, −0.141) | 1.958 |
| (WAIS-R block design, picture arrangement, picture completion) | ||||
| NSS-MC subscale | 2 | 62 | −0.355 (−0.109, −0.56) | 0.017 |
| NSS-SI subscale | 1 | 85 | −0.36 (−0.532, −0.159) | 0 |
| Correlation between NSS total score and executive function | 10 | 641 | −0.361 (−0.428, −0.29) | 8.974 |
| (WCST categories achieved, preservative errors, total errors) | ||||
| NSS-MC subscale | 6 | 440 | −0.335 (−0.417, −0.248) | 1.427 |
| NSS-SI subscale | 4 | 246 | −0.209 (−0.328, −0.084) | 0.486 |
| NSS-Mseq subscale | 3 | 240 | −0.377 (−0.482, −0.261) | 0.213 |
| NSS-disinhib subscale | 4 | 431 | −0.289 (−0.141, 0.424) | 7.445 |
| Correlation between NSS total score and language function | 4 | 344 | −0.354 (−0.257, 0.445) | 2.597 |
| (WAIS-R vocabulary, WRAT-vocabulary, VF) | ||||
| NSS-MC subscale | 1 | 118 | −0.374 (−0.52, −0.207) | NA |
| NSS-SI subscale | 4 | 334 | −0.384 (−0.473, −0.288) | 1.748 |
| NSS-disinhib subscale | 1 | 118 | −0.147 (−0.204, −0.089) | NA |
Note: NSS = neurological soft signs; MC = motor coordination; SI = sensory integration; MSeq = complex motor sequencing; disinhib = disinhibition; WMS-R = Wechsler Memory Scale-Revised; CVLT = California Verbal Learning Test; ROCFT = Rey Auditory Verbal Learning Test; NA, not available; TMT-A, B = Trail-Making Test—A, B; WAIS-R = Wechsler Adult Intelligence Scale-Revised; WAIT-III = Wechsler Adult Intelligence Test-III; SART = Sustained Attention to Response Test; MWT-B = Mehrfachwahl-Wortschatz-Intelligenz Test; WCST = Wisconsin Card Sorting Test; WRAT = Wide Range Achievement Test; VF = verbal fluency from Controlled Word Association Test; NA = Not Available.
Q value heterogeneous, P < .05.
Table 4.
Adjusted Results of Meta-analyses of Correlations of NSS and Neurocognitive Functions
| Number of Studies | Number of Studies Missing | Adjusted Correlation (Adjusted 95% CI) | Adjusted Q Value | |
| Correlation between NSS total score and cognitive abilities | 47 | 0 | — | — |
| NSS-MC subscale | 24 | 0 | — | — |
| NSS-SI subscale | 31 | 0 | — | — |
| NSS-Mseq subscale | 6 | 0 | — | — |
| NSS-disinhib subscale | 17 | 0 | — | — |
| Correlation between NSS total score and verbal memory | 4 | 0 (right side 2) | — | — |
| (WMS-R verbal pairs; paired associate learning; logical memory—immediate, delayed; CVLT) | ||||
| NSS-MC subscale | 5 | 0 | — | — |
| NSS-SI subscale | 6 | 2 | −0.325 (−0.394, −0.253) | 5.248 |
| NSS-disinhib subscale | 4 | 0 (right side 1) | ||
| Correlation between NSS total score and nonverbal memory | 6 | 1 | −0.344 (−0.455, −0.221) | 18.183 |
| (WMS-R visual reproduction—immediate, delayed; visual pairs; ROCFT) | ||||
| NSS-MC subscale | 4 | 0 | — | — |
| NSS-SI subscale | 7 | 0 | — | — |
| NSS-Mseq subscale | 1 | NA | — | — |
| NSS-disinhib subscale | 4 | 2 | −0.056 (−0.187, 0.078) | 9.192 |
| Correlation between NSS total score and motor | 4 | 1 | −0.29 (−0.378, −0.197) | 0.673 |
| (Finger tapping test, Purdue pegboard task) | ||||
| Correlation between NSS total score and attention | 8 | 0 | — | — |
| (TMT-A, B time; WAIS-R—digit span; reading span test; Stroop; Continuous Performance Test; SART) | ||||
| NSS-MC subscale | 2 | NA | — | — |
| NSS-SI subscale | 4 | 0 | — | — |
| NSS-Mseq subscale | 1 | NA | — | — |
| NSS-disinhib subscale | 1 | NA | — | — |
| Correlation between NSS total score and IQ | 6 | 0 (right side 2) | — | — |
| (WAIS-R—full scale, verbal, performance; WAIT-III; MWT-B; Ammons Quick Test) | ||||
| NSS-MC subscale | 4 | 0 (right side 2) | — | — |
| NSS-SI subscale | 5 | 1 | −0.451 (−0.529, −0.367) | 6.142 |
| NSS-Mseq subscale | 1 | NA | — | — |
| NSS-disinhib subscale | 2 | NA | — | — |
| Correlation between NSS total score and spatial ability | 5 | 0 | — | — |
| (WAIS-R block design, picture arrangement, picture completion) | ||||
| NSS-MC subscale | 2 | NA | — | — |
| NSS-SI subscale | 1 | NA | — | — |
| Correlation between NSS total score and executive function | 10 | 2 | −0.341 (−0.412, −0.266) | 12.746 |
| (WCST categories achieved; preservative errors; total errors; SET) | ||||
| NSS-MC subscale | 6 | 2 | −0.316 (−0.391, −0.237) | 2.937 |
| NSS-SI subscale | 4 | 0 (right side 2) | — | — |
| NSS-Mseq subscale | 3 | NA | — | — |
| NSS-disinhib subscale | 4 | NA | — | — |
| Correlation between NSS total score and language function | 4 | 1 | −0.329 (−0.412, −0.240) | 3.946 |
| (WAIS-R vocabulary; WRAT-vocabulary; VF) | ||||
| NSS-MC subscale | 1 | NA | — | — |
| NSS-SI subscale | 4 | 1 | −0.371 (−0.456, −0.279) | 2.461 |
| NSS-disinhib subscale | 1 | NA | — | — |
Note: NSS = neurological soft signs; MC = motor coordination; SI = sensory integration; MSeq = complex motor sequencing; disinhib = disinhibition; WMS-R = Wechsler Memory Scale-Revised; CVLT = California Verbal Learning Test; ROCFT = Rey Auditory Verbal Learning Test; NA, not available; TMT-A, B = Trail-Making Test—A, B; WAIS-R = Wechsler Adult Intelligence Scale-Revised; SART = Sustained Attention to Response Test; WAIT-III = Wechsler Adult Intelligence Test-III; MWT-B = Mehrfachwahl-Wortschatz-Intelligenz Test; WCST = Wisconsin Card Sorting Test; SET = Six-Elements Test; WRAT = Wide Range Achievement Test; VF = verbal fluency from Controlled Word Association Test; NA = Not Available.
Table 5.
Results of Meta-analyses of Correlations of NSS and Clinical Symptoms in Schizophrenia Patients
| Number of Studies | Number of Schizophrenic Patients | Corr (95%CI) | Q Value | |
| Correlation between NSS total score and symptom total score | 11 | 696 | 0.327 (0.213, 0.432) | 23.341* |
| NSS-MC subscale | 7 | 516 | 0.293 (0.142, 0.430) | 18.262* |
| NSS-SI subscale | 7 | 537 | 0.237 (0.154, 0.316) | 5.323* |
| NSS-Mseq subscale | 7 | 515 | 0.216 (0.107, 0.319) | 9.171 |
| Correlation between NSS total score and symptom-positive score | 10 | 529 | 0.192 (0.067, 0.312) | 16.578 |
| NSS-MC subscale | 6 | 434 | 0.153 (0.003, 0.296) | 11.493* |
| NSS-SI subscale | 6 | 434 | 0.143 (−0.028, 0.306) | 14.854* |
| NSS-Mseq subscale | 6 | 394 | 0.095 (−0.017, 0.205) | 5.989 |
| Correlation between NSS total score and symptom-negative score | 15 | 758 | 0.346 (0.260, 0.426) | 20.728 |
| NSS-MC subscale | 7 | 484 | 0.252 (0.140, 0.357) | 9.183 |
| NSS-SI subscale | 6 | 434 | 0.194 (0.100, 0.284) | 1.627 |
| NSS-Mseq subscale | 8 | 467 | 0.217 (0.099, 0.330) | 11.122 |
Note: NSS = neurological soft signs; MC = motor coordination; SI = sensory integration; MSeq = complex motor sequencing; Corr = Correlation coefficient.
Q value heterogeneous, P < .05.
Table 6.
Adjusted Results of Meta-analyses of Correlations of NSS and Clinical Symptoms in Schizophrenia Patients
| Number of Studies Missing | Adjusted Std Diff (Adjusted 95% CI) | Adjusted Q Value | Fail-Safe N | |
| Correlation between NSS total score and symptom total score | 0 | — | — | 14 |
| NSS-MC subscale | 2 | 0.224 (0.071, 0.367) | 28.755* | 6 |
| NSS-SI subscale | 2 | 0.206 (0.125, 0.285) | 8.762* | 3 |
| NSS-Mseq subscale | 2 | 0.156 (0.030, 0.276) | 18.682* | 1 |
| Correlation between NSS total score and symptom-positive score | 2 | 0.155 (0.032, 0.273) | 20.538 | — |
| NSS-MC subscale | 2 | 0.086 (−0.068, 0.236) | 18.769* | NS |
| NSS-SI subscale | 2 | 0.034 (−0.151, 0.217) | 30.806* | NS |
| NSS-Mseq subscale | 1 | 0.077 (−0.035, 0.187) | 7.52 | NS |
| Correlation between NSS total score and symptom-negative score | 5 | 0.296 (0.205, 0.382) | 33.183 | 22 |
| NSS-MC subscale | 0 | — | — | 4 |
| NSS-SI subscale | 0 | — | — | — |
| NSS-Mseq subscale | 2 | 0.177 (0.062, 0.287) | 15.376 | 2 |
Note: NSS = neurological soft signs; Std Diff = standard difference in means; MC = motor coordination; SI = sensory integration; MSeq = complex motor sequencing; NS, not significant.
Q value heterogeneous, P < .05.
Fig. 6.
Funnel Plot for the Meta-analysis of Correlation Between NSS Total Scores and Symptom Scores in Schizophrenia Patients.
Moderator Variables
Heterogeneity statistics reported in tables 1–6 revealed the presence of significant effect variability in many of the NSS literatures in the study base. Accordingly, it was of interest to assess moderator variables that may vary with effect magnitudes in these literatures. We examined the effect of several potential moderator variables, including mean age of patient samples, duration of illness, and education. Sample sex proportions, medication status, and symptom severity could not be considered as moderators due to inadequate reporting of relevant data in the primary studies (table 7).
Table 7.
Analyses of Potential Moderators of Effect Size of Differences in NSS Total scores Between Patients With Schizophrenia and Healthy Controls
| Number of Studies | Number of Schizophrenic Patients | Number of Healthy Controls | P Value | |
| Contrasting NSS total score of schizophrenia vs controls | ||||
| Age (y) | 30 | 2069 | 1573 | .008* |
| Duration of illness | 15 | 867 | 675 | .045* |
| Education | 22 | 1451 | 1127 | .67 |
| NSS measured by NES or modified NES | ||||
| Age (y) | 20 | 1151 | 939 | .07 |
| Duration of illness | 11 | 437 | 356 | .02* |
| Education | 14 | 690 | 572 | .16 |
| Chronic/mixed | ||||
| Age (y) | 23 | 1687 | 1255 | .05 |
| Duration of illness | 10 | 702 | 528 | .16 |
| Education | 17 | 1286 | 980 | .67 |
| Contrasting NSS-MC score of schizophrenia vs controls | ||||
| Age (y) | 22 | 1662 | 1111 | .50 |
| Duration of illness | 11 | 712 | 543 | .86 |
| Education | 18 | 1257 | 841 | .91 |
| NSS measured by NES or modified NES | ||||
| Age (y) | 13 | 844 | 503 | .65 |
| Duration of illness | 8 | 318 | 236 | .73 |
| Education | 10 | 486 | 300 | .93 |
| Chronic/mixed | ||||
| Age (y) | 17 | 1265 | 900 | .60 |
| Duration of illness | 8 | 543 | 407 | .65 |
| Education | 12 | 930 | 597 | .92 |
| Contrasting NSS-SI score of schizophrenia vs controls | ||||
| Age (y) | 21 | 1575 | 1057 | .16 |
| Duration of illness | 12 | 744 | 575 | .13 |
| Education | 17 | 1170 | 787 | .23 |
| NSS measured by NES or modified NES | ||||
| Age (y) | 14 | 876 | 535 | .041* |
| Duration of illness | 9 | 350 | 268 | .21 |
| Education | 11 | 518 | 332 | .54 |
| Chronic/mixed | ||||
| Age (y) | 16 | 1239 | 882 | .18 |
| Duration of illness | 9 | 647 | 500 | .07 |
| Education | 13 | 1034 | 690 | .56 |
| Contrasting NSS-Mseq (NES) score of schizophrenia vs controls | ||||
| Age (y) | 14 | 855 | 482 | .029* |
| Duration of illness | 8 | 290 | 193 | .57 |
| Education | 12 | 557 | 354 | .15 |
| Chronic/mixed | ||||
| Age (y) | 9 | 519 | 307 | .037* |
| Duration of illness | 5 | 193 | 118 | .10 |
| Education | 8 | 421 | 257 | .52 |
Note: NSS = neurological soft signs; NES = Neurological Evaluation Scale; MC = motor coordination; SI = sensory integration; MSeq = complex motor sequencing.
P < .05.
In our analysis, age was a significant moderator of the NSS total score contrast between schizophrenia and control groups, whereby study effect sizes decreased as mean ages of patient samples increased. However, age was not a significant moderator when only NES data were analyzed. Age also moderated the complex motor sequencing (MSeq) but not MC and SI scores contrasting schizophrenic patients and controls. Moreover, age was not a significant moderator for any correlation coefficient between NSS total and symptoms. In addition, the correlation coefficients between NSS total and IQ increased with the mean age of patient samples.
On the other hand, duration of illness was a significant moderator of the NSS total effect between schizophrenia and control groups. Duration of illness was also a significant moderator for the correlation coefficient between NSS total and IQ. However, duration of illness was not a significant moderator of the correlation between NSS total and symptoms. Finally, education did not moderate any NSS total score contrast between schizophrenia and control samples and was not a significant moderator of correlations between NSS and symptom severity or neurocognitive performance.
In the current study, we would also like to explore the handedness and treatment effect as moderator previously, but many studies reported results without concrete data. Besides, different methods varied across these studies.
There were only 7 studies concerning the association of handedness and soft signs, but 3 of them51–53 reported no significant correlations between handedness and NSS; 3 studies55–57 indicated that there were significant differences between mixed-handed and hand-preference patients. One study57 presented a trend for an association between left-handedness and NSS. Among these studies, 2 studies51,52 did not report data, while others used different methods (correlation coefficient or t test) resulting in insufficient data for us to compute the effect size of handedness on soft signs.
Although there were 30 studies (see table 8) that mentioned the relationship between treatment and NSS, many of them did not mention actual descriptive data or useable data for computation. Among these 30 studies, only 5 studies reported the relevant results. Therefore, we cannot calculate the effect size moderated by treatment. Besides, the majority of studies demonstrated nonsignificant results suggesting that treatment might not be a significant moderator.
Table 8.
Summary of the Relationships Between Treatment and NSS
| Measure | Data | Results | Studies (First Author, Published Year) |
| Correlation between NSS and treatment | Reported data | Significant | King, 199158 |
| Nonsignificant | Bartko, 198859; Lane, 199651; Braun, 199546; Merriam, 199014; Jahn, 200648 | ||
| No data | Significant | Liddle, 198760 (included nonsignificant results) | |
| Nonsignificant | Liddle, 198760; Cox, 197961; Rossi, 199036; Schroder, 199262; Cuesta, 199610; Ismail, 199844; Flyckt, 199963; Arango, 200064; Chen, 200065; Biswas, 200766 | ||
| Contrast medication: low NSS vs high NSS | Reported data | Significant | Flashman, 199615 |
| Nonsignificant | Das, 200467 | ||
| No data | Significant | — | |
| Nonsignificant | Bersani, 200468,69; Dazzan, 200469 | ||
| Contrast NSS low dose vs high doses | Reported | Significant | Boks, 200570 (included nonsignificant results) |
| Nonsignificant | Gureje, 198871; Aydemir, 200572; Boks, 200570 | ||
| No data | Nonsignificant | Chen, 200573 | |
| Contrast NSS baseline vs posttreated | Reported | Nonsignificant | Scheffer, 200474; Sevincok, 200675 |
| No data | Nonsignificant | Emsley, 200576; Mittal, 200777 | |
| No data | Significant | Picchioni, 200678 | |
| Nonsignificant | Griffich, 199852 |
Note: NSS, neurological soft signs.
In addition, only 2 studies79,80 compared the typical and atypical antipsychotics effect on NSS and found nonsignificant difference.
There were very limited studies on the issue of comorbidity on NSS. In the literature, only one study compared the schizophrenia with and without obsessive-compulsive disorder (OCD). The authors indicated that OCD-schizophrenic patients had higher scores on total-NSS than non–OCD-schizophrenic group. Therefore, it is difficult to calculate and combine the results in the current meta-analysis.
Discussion
Our meta-analytic review of NSS in schizophrenia suggests that the illness expresses itself strongly in these basic motor and sensory deficiencies, with mean effects similar or larger in magnitude than those reported in neurobehavioral and neurobiological literatures.19,81 The most robust findings were obtained with a summary index of NSS. In addition, we found evidence of associations between NSS and both cognitive performance and negative symptoms. Moreover, effect sizes were significantly moderated by age and duration of illness. Therefore, the substantial difference between schizophrenia patients and controls suggests that NSS meet one essential criterion (association with illness) of an endophenotype for schizophrenia.
Contrast of NSS Between Schizophrenia Patients and Healthy Controls
Our findings show a large and reliable effect indicating that, on average, a substantial majority of patients (73%) perform outside the range of healthy control subjects on aggregate NSS measures.21 The average effect size obtained compares favorably with those reported for cognition, psychophysiology, frontal-temporal brain volumes, and regional physiology.24,25,82 The evidence is complicated by the presence of considerable effect heterogeneity, which may reflect the use of difference instruments and patient sample characteristics.83 Hence, more detailed moderator analysis and more comprehensive reporting practices will become increasingly important as the NSS literature expands. Nonetheless, our preliminary quantification of the NSS effect confirms the value of this literature in the search for schizophrenia disease markers and endophenotypes.
Previous studies reported that the frequency of NSS increased with age in a sample of schizophrenic patients.84 However, we found that effect sizes based on summary measures of NSS decreased in magnitude with increases in the age of patient samples. This relationship may reflect in part the increased frequency of NSS with age in healthy people.36,84 Thus, higher rates of NSS in older control samples may have attenuated the effect found in younger samples. We found an inverse relationship between NSS and age in healthy control samples, which clearly contradicts this interpretation. On the other hand, data from patient samples showed a relation between illness duration and NSS in our study base. This implies that less chronic, and presumably younger, samples may include patients with higher rates of neurological deficit. The implication is partially supported by reports of an inverse relationship between age and verbal memory effect sizes in the neurocognitive literature on schizophrenia.1 Nonetheless, a similar relationship has not been found in neuroimaging literatures.82
In terms of chronicity-related moderation of the NSS effect, we found no statistically significant difference between first-episode schizophrenia and more chronic patients. However, we did find an association between the overall NSS effect and duration of illness. On the other hand, Chen et al84 previously reported a relationship between NSS and duration of illness that became nonsignificant when age and education were taken into account in the analysis. It seems unlikely that chronicity is a major factor in amplifying or diminishing the NSS-schizophrenia association, but the question should be answerable as the literature expands and provides a more comprehensive study base for moderator analysis.
Several studies85,86 have investigated the association between NSS and gender. While Bjorck et al85 reported no difference between male and female patients, Duggal et al86 illustrated that performance in motor sequencing tasks may be influenced by sex-bound variables. Unfortunately, we were unable to address gender differences in our meta-analysis because of inadequate reporting of gender composition in the primary studies.
Only 2 studies reported data relevant to the issue of comorbidity and NSS findings. Sevincok et al87 compared schizophrenia patients with and without OCD and indicated that OCD-schizophrenia patients had higher scores than non–OCD-schizophrenia patients on total-NSS. In terms of substance abuse co-occurring with the illness, Bersani et al88 compared cannabis-consuming and -nonconsuming schizophrenia patients. The results showed that NSS were more prevalent in nonconsuming patients. Unfortunately, no conclusions can be reached with such limited data. The question of NSS rates in conditions comorbid with schizophrenia is an important issue that requires more research attention from investigators.
NSS and Neurocognitive and Symptom Scores
This analysis compiled correlation coefficients between NSS and neurocognitive and symptom test scores reported in our primary study base. Cognitive and NSS data share approximately 10% of their variance, implying that these data reflect associated, but distinct, aspects of neurobehavioral function in schizophrenia. Specific relationships were found between sensory and motor soft signs and several aspects of cognitive ability including spatial, executive, and language performance. However, only SI signs were significantly related to IQ measures. This suggests that IQ test performance may require input and coordination of sensory data, whereas motor system dysfunction associates with disturbances in systems underlying spatial and executive processing.
The correlations between NSS and clinical symptoms were relatively modest but significant. The correlation coefficients between NSS total and total, positive and negative symptoms scores indicate shared variance from 2% to 10%, with the weakest relation between NSS and positive symptom severity. Hence, our results confirm previous studies demonstrating that NSS are more prominent in patients with negative symptoms than in those with positive symptoms.18
Limitations and Future Research
The current study has several limitations. First, the presence of significant effect variability in some analyses means that average effect sizes may not represent adequately the underlying populations, which may include important subsets of patients.83 This is especially notable in the contrast between patients and controls. Second, potential moderators including age and duration of illness were only coarsely estimated with sample means. Therefore, the relationships between NSS and these factors are not clear and require more refined and detailed analysis. Third, although differences in effect as a function of NSS instrument (NES vs CNI) were examined, a comprehensive analysis of scale differences and score composition was not conducted. Notwithstanding these limitations, this meta-analysis has shown, for the first time, that NSS occur in a majority of the schizophrenia patient population and are similar to or exceed psychophysiological, cognitive, and neuroanatomic findings as indicators or correlates of schizophrenic illness. Reporting limitations in the literature with respect to key moderators including gender and chronicity reduce the inferences that can be made about the generality of these findings. These limitations should be addressed through improved reporting practices. In addition, important questions remain concerning prevalence of specific soft signs in the illness and the sources of heterogeneity and effect variability in this population.
Funding
Research Initiation Fund of the 100-Scholar Programme (O7CX031003); Institute of Psychology, Chinese Academy of Sciences (Research Fund KSCX2-YW-R-131); National Science Foundation of China (30770723); National Basic Research Programme (973 Programme No. 2007CB512302).
Acknowledgments
All authors report no competing interests.
Appendix
Appendix I: NSS Scales.
| Scale | Items |
| NES26 | MC: intention tremor, balance, gait, hopping, finger-thumb opposition, disdiadochokinesia, finger-to-nose test |
| SI: bilateral extinction, audiovisual integration, graphaesthesia, stereognosis, R-L confusion extinction | |
| Mseq: fist-edge-palm, fist-ring, Ozeretsky, go/no-go, rhythm tapping | |
| CNI27 | MC: finger tapping (L/R), finger-thumb opposition (L/R), fist-edge-palm, Ozeretsky |
| SI: extinction, finger agnosia, stereognosis, graphaesthesia, L-R orientation | |
| Disinhib: saccade blink, saccade head, wink, mirror movement of fist-edge-palm, mirror movement of disdiadochokinesia (L/R), go/no-go test | |
| 23 items from Krebs89 | Gait, tandem walk, Romberg (balance), standing heel-to-toe, tongue protrusion, finger-to-nose, RL confusion, RL recognition, hand-face, lateral preference, apraxia, alternative movement of foot speed (R/L), foot dysrhythmia (R/L), alternative movements of hand speed (R/L), hand dysrhythmia (R/L), finger opposition (R/L), fist-edge-palm (R/L), mirror movements (R/L), abnormal movement and posture, RL asymmetry, stereognosia, constructive apraxia, graphaesthesia (R/L) |
| Heidelberg Scale62 | Station and gait, tandem walking, R/L orientation, speech articulation, primitive reflexes, Ozeretsky test, pronation/supination, diadochokinesis, finger-to-thumb opposition, 2-point discrimination, fist-edge-palm, finger-to-nose, face-hand sensory, graphaesthesia, stereognosis, mirror movements, arm-holding test |
Note: R, right; L, left; disinhib, disinhibition.
Appendix II: Studies Included in Meta-analysis
- Arango C, Bartko JJ, Gold JM, Buchannan RW. Prediction of neuropsychological performance by neurological signs in schizophrenia. Am J Psychiatry. 1999;156:1349–1357. doi: 10.1176/ajp.156.9.1349. [DOI] [PubMed] [Google Scholar]
- Aydemir C, Goka E, Kisa C, Kurt A, Yuksel FV. Dyskinesia and soft neurological signs in schizophrenia: a comparative study. Int J Psychiatry Clin Pract. 2005;9:238–243. doi: 10.1080/13651500500329150. [DOI] [PubMed] [Google Scholar]
- Bachmann S, Bottmer C, Schröder J. Neurological soft signs in first-episode schizophrenia: a follow-up study. Am J Psychiatry. 2005;162:2337–2343. doi: 10.1176/appi.ajp.162.12.2337. [DOI] [PubMed] [Google Scholar]
- Bersani G, Orlandi V, Gherardelli S, Pancheri P. Cannabis and neurological soft signs in schizophrenia: absence of relationship and influence on psychopathology. Psychopathology. 2002;35:289–295. doi: 10.1159/000067064. [DOI] [PubMed] [Google Scholar]
- Biswas P, Malhotra S, Malhotra A, Gupta N. Comparative study of neurological soft signs in schizophrenia with onset in childhood, adolescence. Acta Psychiatr Scand. 2007;115:295–303. doi: 10.1111/j.1600-0447.2006.00901.x. [DOI] [PubMed] [Google Scholar]
- Bolton D, Gibb W, Lees A, et al. Neurological soft signs in obsessive compulsive disorder: standardized assessment and comparison with schizophrenia. Behav Neurol. 1998;11:197–204. doi: 10.1155/1999/639045. [DOI] [PubMed] [Google Scholar]
- Braun CMJ, Lapierre D. Neurological soft signs in schizophrenia: are they related to negative or positive symptoms, neuropsychological performance, and violence? Arch Clin Neuropsychol. 1995;10:489–509. [PubMed] [Google Scholar]
- Buchanan RW, Heinrichs DW. The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res. 1989;27:335–350. doi: 10.1016/0165-1781(89)90148-0. [DOI] [PubMed] [Google Scholar]
- Chan RCK, Wang L, Chen EE, et al. Neurological soft signs and their relationships to neurocognitive functions: a re-visit with the structural equation modeling design. Submitted. doi: 10.1371/journal.pone.0008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R, Chen E. Executive dysfunctions and neurological manifestations in schizophrenia. Hong Kong J Psychiatry. 2004;14:2–6. [Google Scholar]
- Chan RCK, Wang Y, Ma Z, et al. Neurological soft signs and neurocognitive deficits along the continuum of schizophrenia spectrum disorders. Submitted [Google Scholar]
- Chen EYH, Shapleske J, Luque R, et al. The Cambridge Neurological Inventory, a clinical instrument for assessment of soft neurological signs. Psychiatry Res. 1995;56:183–204. doi: 10.1016/0165-1781(95)02535-2. [DOI] [PubMed] [Google Scholar]
- Chen EYH, Lam LCW, Chen RYL, Nguyen DGH. Neurological signs, age, and illness duration in schizophrenia. J Nerv Ment Dis. 1996;184:339–345. doi: 10.1097/00005053-199606000-00002. [DOI] [PubMed] [Google Scholar]
- Chen EYH, Kwok CL, Au JWY, Chen RYL, Lau BST. Progressive deterioration of soft neurological signs in chronic schizophrenia patients. Acta Psychiatr Scand. 2000;102:342–349. doi: 10.1034/j.1600-0447.2000.102005342.x. [DOI] [PubMed] [Google Scholar]
- Chen YLR, Chen YHE, Mak FL. Soft neurological signs in schizophrenic patients and their nonpsychotic siblings. J Nerv Ment Dis. 2000;188:84–89. doi: 10.1097/00005053-200002000-00004. [DOI] [PubMed] [Google Scholar]
- Chen EYH, Chan CK. The Cambridge Neurological Inventory; clinical, demographic, and ethnic correlates. Psychiatr Ann. 2003;33:202–210. [Google Scholar]
- Chen EYH, Hui CLM, Chan RCK, et al. A 3-year prospective study of neurological soft signs in first-episode schizophrenia. Schizophr Res. 2005;75:45–54. doi: 10.1016/j.schres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Compton MT, Bollini AM, Mack LM, et al. Neurological soft signs and minor physical anomalies in patients with schizophrenia and related disorders, their first degree biological relatives, and non-psychiatric controls. Schizophr Res. 2007;94:64–73. doi: 10.1016/j.schres.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Cuesta MJ, Peralta V, de Leon J. Neurological frontal signs and neuropsychological deficits in schizophrenic patients. Schizophr Res. 1996;20:15–20. doi: 10.1016/0920-9964(95)00110-7. [DOI] [PubMed] [Google Scholar]
- Cuesta MJ, Peralta V, Zarzuela A, Calvo R, García M, Serrano F. Neurological soft-signs in psychosis: threshold criteria for discriminating normal controls and for predicting cognitive impairment. Schizophr Res. 2002;58:263–271. doi: 10.1016/s0920-9964(01)00390-5. [DOI] [PubMed] [Google Scholar]
- Dazzan P, Morgan KD, Orr KG, et al. The structural brain correlates of neurological soft signs in AESOP first-episode psychoses study. Brain. 2004;127:143–153. doi: 10.1093/brain/awh015. [DOI] [PubMed] [Google Scholar]
- Egan MF, Hyde TM, Bonomo JB, et al. Relative risk of neurological signs in siblings of patients with schizophrenia. Am J Psychiatry. 2001;158:1827–1834. doi: 10.1176/appi.ajp.158.11.1827. [DOI] [PubMed] [Google Scholar]
- Flashman LA, Flaum M, Gupta S, Andreasen NC. Soft signs and neuropsychological performance in schizophrenia. Am J Psychiatry. 1996;153:526–532. doi: 10.1176/ajp.153.4.526. [DOI] [PubMed] [Google Scholar]
- Gourion D, Goldberger C, Bourdel MC, et al. Neurological soft-signs and minor physical anomalies in schizophrenia: differential transmission within families. Schizophr Res. 2003;63:181–187. doi: 10.1016/s0920-9964(02)00333-x. [DOI] [PubMed] [Google Scholar]
- Gourion D, Goldberger C, Olie JP, Lôo H, Krebs MO. Neurological and morphological anomalies and the genetic liability to schizophrenia: a composite phenotype. Schizophr Res. 2004;67:23–31. doi: 10.1016/s0920-9964(03)00099-9. [DOI] [PubMed] [Google Scholar]
- Jahn T, Hubmann W, Karr M, et al. Motoric neurological soft signs and psychopathological symptoms in schizophrenic psychoses. Psychiatry Res. 2006;142:191–199. doi: 10.1016/j.psychres.2002.12.003. [DOI] [PubMed] [Google Scholar]
- John JP, Arunachalam V, Ratnam B, Isaac MK. Expanding the schizophrenia phenotype: a composite evaluation of neruodevelopmental markers. Compr Psychiatry. 2008;49:78–86. doi: 10.1016/j.comppsych.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Sanders RD, Sweeney JA, et al. Diagnostic specificity and neuroanatomical validity of neurological abnormalities in first-episode psychoses. Am J Psychiatry. 2003;160:1298–1304. doi: 10.1176/appi.ajp.160.7.1298. [DOI] [PubMed] [Google Scholar]
- Krebs MO, Fayand AG, Bourdel MC, Dischamp J, Olie JP. Validity and factorial structure of a standardized examination for neurological soft-signs in schizophrenia. Schizophr Res. 2000;45:245–260. doi: 10.1016/s0920-9964(99)00206-6. [DOI] [PubMed] [Google Scholar]
- Lindberg N, Tani P, Stenberg JH, Appelberg B, Heiskanen TP, MattiVirkkunen Neurological soft signs in homicidal men with antisocial personality disorder. Eur Psychiatry. 2004;19:433–437. doi: 10.1016/j.eurpsy.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Merriam AE, Kay SR, Opler LA, Kushner SF, van Praag HM. Neurological Signs and the Positive-Negative Dimension in Schizophrenia. Biol Psychiatry. 1990;28:181–192. doi: 10.1016/0006-3223(90)90573-k. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Hasenkamp W, Sanfilipo M, et al. Relation of neurological soft signs to psychiatric symptoms in schizophrenia. Schizophr Res. 2007;94:37–44. doi: 10.1016/j.schres.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Mohr F, Hubmann W, Albus M, et al. Neurological soft signs and neuropsychological performance in patients with first episode schizophrenia. Psychiatry Res. 2003;121:21–30. doi: 10.1016/s0165-1781(03)00203-8. [DOI] [PubMed] [Google Scholar]
- Picchioni MM, Toulopoulou T, Landau S, Davies N, Ribchester T, Murray RM. Neurological abnormalities in schizophrenic twins. Biol Psychiatry. 2006;59:341–348. doi: 10.1016/j.biopsych.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Poole JH, Ober BA, Shenautb GK, Vinogradov S. Independent frontal-system deficits in schizophrenia: cognitive, clinical, and adaptive implications. Psychiatry Res. 1999;85:161–176. doi: 10.1016/s0165-1781(98)00146-2. [DOI] [PubMed] [Google Scholar]
- Prikryl R, Ceskova E, Kasparek T, Kucerova H. Neurological soft signs, clinical symptoms and treatment reactivity in patients suffering from first episode schizophrenia. J Psychiatr Res. 2006;40:141–146. doi: 10.1016/j.jpsychires.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Quitkin F, Rifkin A, Klein DF. Organicity in schizophrenia with premorbid asociality and emotionally unstable character disorders. Arch Gen Psychiatry. 1976;33:845–853. doi: 10.1001/archpsyc.1976.01770070075008. [DOI] [PubMed] [Google Scholar]
- Rossi A, Cataldo SD, Michele VD, et al. Neurological soft signs in schizophrenia. Br J Psychiatry. 1990;157:735–739. doi: 10.1192/bjp.157.5.735. [DOI] [PubMed] [Google Scholar]
- Sanders RD, Keshavan MS, Forman SD, et al. Factor structure of neurologic examination abnormalities in unmedicated schizophrenia. Psychiatry Res. 2000;95:237–243. doi: 10.1016/s0165-1781(00)00176-1. [DOI] [PubMed] [Google Scholar]
- Sanders RD, Schuepbach D, Goldstein G, Haas GL, Sweeney JA, Keshavan MS. Relationships between cognitive and neurological performance in neuroleptic-naïve psychosis. J Neuropsychiatry Clin Neurosci. 2004;16:480–487. doi: 10.1176/jnp.16.4.480. [DOI] [PubMed] [Google Scholar]
- Scheffer RE. Abnormal neurological signs at the onset of psychosis. Schizophr Res. 2004;70:19–26. doi: 10.1016/j.schres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Schröder J, Niethammer R, Geider FJ, et al. Neurological soft signs in schizophrenia. Schizophr Res. 1992;6:25–30. doi: 10.1016/0920-9964(91)90017-l. [DOI] [PubMed] [Google Scholar]
- Schröder J, Essig M, Baudendistel K, et al. Motor dysfunction and sensorimotor cortex activation changes in schizophrenia: a study with functional magnetic resonance imaging. Neuroimage. 1999;9:81–89. doi: 10.1006/nimg.1998.0387. [DOI] [PubMed] [Google Scholar]
- Sevincok L, Akoglu A, Topaloglu B, Aslantas H. Neurological soft signs in schizophrenic patients with obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2004;58:274–279. doi: 10.1111/j.1440-1819.2004.01231.x. [DOI] [PubMed] [Google Scholar]
- Sevincok L, Topaloglu B. Neurological soft signs and positive treatment response to olanzapine in chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:141–143. doi: 10.1016/j.pnpbp.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Shibre T, Kebede D, Alem A, et al. Neurological soft signs (NSS) in 200 treatment-naive cases with schizophrenia: a community-based study in a rural setting. Nord J Psychiatry. 2002;56:425–431. doi: 10.1080/08039480260389343. [DOI] [PubMed] [Google Scholar]
- Smith RC, Kadewari RP. Neurological soft signs and response to risperidone in chronic schizophrenia. Biol Psychiatry. 1996;40:1056–1059. doi: 10.1016/S0006-3223(96)00251-X. [DOI] [PubMed] [Google Scholar]
- Smith RC, Hussain MI, Chowdhrury SA, Stearns A. Stability of neurological soft signs in chronically hospitalized schizophrenic patients. J Neuropsychiatry Clin Neurosci. 1999;11:91–96. doi: 10.1176/jnp.11.1.91. [DOI] [PubMed] [Google Scholar]
- Stedman TJ, Clair AL. Neuropsychological, neurological and symptom correlates of impaired olfactory identification in schizophrenia. Schizophr Res. 1998;32:23–30. doi: 10.1016/s0920-9964(98)00021-8. [DOI] [PubMed] [Google Scholar]
- Szendi In, Kissb M, Racsmányd Ml, et al. Correlations between clinical symptoms, working memory functions and structural brain abnormalities in men with schizophrenia. Psychiatry Res. 2006;147:47–55. doi: 10.1016/j.pscychresns.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Varambally S, Venkatasubramanian G, Thirthalli J, Janakiramaiah N, Gangadhar BN. Cerebellar and other neurological soft signs in antipsychotic-naive schizophrenia. Acta Psychiatr Scand. 2006;114:352–356. doi: 10.1111/j.1600-0447.2006.00837.x. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Ritch JL, Sui D, DiCocco M, Huntzinger CD. Frontal systems behavior scale in schizophreia: relationships with psychiatric symptomatology, cognition and adaptive function. Psychiatry Res. 2002;113:227–236. doi: 10.1016/s0165-1781(02)00264-0. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian G, Latha V, Gangadhar BN, et al. Neurological soft signs in never-treated schizophrenia. Acta Psychiatr Scand. 2003;108:144–146. doi: 10.1034/j.1600-0447.2003.00113.x. [DOI] [PubMed] [Google Scholar]
- Whitty P, Clarke M, Browne S, et al. Prospective evaluation of neurological soft signs in first-episode schizophrenia in relation to psychopathology: state versus trait phenomena. Psychol Med. 2003;33:1479–1484. doi: 10.1017/s0033291703008225. [DOI] [PubMed] [Google Scholar]
- Wong AHC, Voruganti LNP, Heslegrave RJ, Awad AG. Neurocognitive deficits and neurological signs in schizophrenia. Schizophr Res. 1997;23:139–146. doi: 10.1016/S0920-9964(96)00095-3. [DOI] [PubMed] [Google Scholar]
- Yazici AH, Demir B, Yazici KM, Gogus A. Neurological soft signs in schizophrenic patients and their nonpsychotic siblings. Schizophr Res. 2002;58:241–246. doi: 10.1016/s0920-9964(01)00338-3. [DOI] [PubMed] [Google Scholar]
References
- 1.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 2.de Wilde OM, Bour LJ, Dingermans PM, Koelman JHTM, Linszen DH. A meta-analysis of the P50 studies in patients with schizophrenia and relatives: differences in methodology between research groups. Schizophr Res. 2007;97:137–151. doi: 10.1016/j.schres.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 3.Heinrichs DW, Buchanan RW. The significance and meaning of neurological signs in schizophrenia. Am J Psychiatry. 1988;145:11–18. doi: 10.1176/ajp.145.1.11. [DOI] [PubMed] [Google Scholar]
- 4.Tsuang MT, Gilberson MW, Faraone SV. The genetics of schizophrenia: current knowledge and future directions. Schizophr Res. 1991;4:157–171. doi: 10.1016/0920-9964(91)90031-l. [DOI] [PubMed] [Google Scholar]
- 5.Tsuang MT, Faraone SV. The concept of target features in schizophrenia research. Acta Psychiatr Scand Suppl. 1999;395:2–11. doi: 10.1111/j.1600-0447.1999.tb05977.x. [DOI] [PubMed] [Google Scholar]
- 6.Schröder J, Wenz F, Schad LR, Baudendistel K, Knopp MV. Sensorimotor cortex and supplementary motor area changes in schizophrenia: a study with functional magnetic resonance imaging. Br J Psychiatry. 1995;167:197–201. doi: 10.1192/bjp.167.2.197. [DOI] [PubMed] [Google Scholar]
- 7.Chan RCK, Rao H, Chen EE, Ye B, Zhang C. The neural basis of motor sequencing: an fMRI study of healthy subjects. Neurosci Lett. 2006;398:189–194. doi: 10.1016/j.neulet.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Rao H, Di X, Chan RCK, Ding Y, Ye B, Gao D. A regulation role of the prefrontal cortex in the fist-edge-palm task: evidence from functional connectivity analysis. Neuroimage. 2008;41:1345–1351. doi: 10.1016/j.neuroimage.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Mohr F, Hubmann W, Cohen R, et al. Neurological soft signs in schizophrenia: assessment and correlates. Eur Arch Psychiatry Clin Neurosci. 1996;246:240–248. doi: 10.1007/BF02190275. [DOI] [PubMed] [Google Scholar]
- 10.Cuesta MJ, Peralta V, de Leon J. Neurological frontal signs and neuropsychological deficits in schizophrenic patients. Schizophr Res. 1996;20:15–20. doi: 10.1016/0920-9964(95)00110-7. [DOI] [PubMed] [Google Scholar]
- 11.Wong AHC, Voruganti LNP, Heslegrave RJ, Awad AG. Neurocognitive deficits and neurological signs in schizophrenia. Schizophr Res. 1997;23:139–146. doi: 10.1016/S0920-9964(96)00095-3. [DOI] [PubMed] [Google Scholar]
- 12.Chen EYH, Lam LCW, Chen RYL, Nguyen DGH, Kwok CL, Au JWY. Neurological signs and sustained attention impairment in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2001;251:1–5. doi: 10.1007/s004060170059. [DOI] [PubMed] [Google Scholar]
- 13.Mohr F, Hubmann W, Albus M, et al. Neurological soft signs and neuropsychological performance in patients with first episode schizophrenia. Psychiatry Res. 2003;121:21–30. doi: 10.1016/s0165-1781(03)00203-8. [DOI] [PubMed] [Google Scholar]
- 14.Merriam AE, Kay SR, Opler LA, Kushner SF, van Praag HM. Neurological signs and the positive-negative dimension in schizophrenia. Biol Psychiatry. 1990;28:181–192. doi: 10.1016/0006-3223(90)90573-k. [DOI] [PubMed] [Google Scholar]
- 15.Flashman LA, Flaum M, Gupta S, Andreasen NC. Soft signs and neuropsychological performance in schizophrenia. Am J Psychiatry. 1996;153:526–532. doi: 10.1176/ajp.153.4.526. [DOI] [PubMed] [Google Scholar]
- 16.Arango C, Bartko JJ, Gold JM, Buchannan RW. Prediction of neuropsychological performance by neurological signs in schizophrenia. Am J Psychiatry. 1999;156:1349–1357. doi: 10.1176/ajp.156.9.1349. [DOI] [PubMed] [Google Scholar]
- 17.Sanders RD, Schuepbach D, Goldstein G, Haas GL, Sweeney JA, Keshavan MS. Relationships between cognitive and neurological performance in neuroleptic-naïve psychosis. J Neuropsychiatry Clin Neurosci. 2004;16:480–487. doi: 10.1176/jnp.16.4.480. [DOI] [PubMed] [Google Scholar]
- 18.Bombin I, Arango C, Buchanan RW. Significance and meaning of neurological signs in schizophrenia: two decades later. Schizophr Bull. 2005;31:962–977. doi: 10.1093/schbul/sbi028. [DOI] [PubMed] [Google Scholar]
- 19.Chan RCK, Gottesman II. Neurological soft signs as candidate endophenotypes for schizophrenia: a shooting star or a Northern star? Neurosci Biobehav Rev. 2008;32:957–971. doi: 10.1016/j.neubiorev.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Bombin I, Arango C, Buchanan RW. Assessment tools for soft signs. Psychiatr Ann. 2003;33:170–176. [Google Scholar]
- 21.Heinrichs RW. In Search of Madness: Schizophrenia and Neuroscience. New York, NY: Oxford University Press; 2001. [Google Scholar]
- 22.Heinrichs RW. Meta-analysis and the science of schizophrenia: variant evidence or evidence of variants? Neurosci Biobehav Rev. 2004;28:379–394. doi: 10.1016/j.neubiorev.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Lipsey MW, Wilson DB. The way in which intervention studies have “personality″ and why it is important to meta-analysis. Eval Health Prof. 2001;24:236–254. doi: 10.1177/016327870102400302. [DOI] [PubMed] [Google Scholar]
- 24.Heinrichs RW. The primacy of cognition in schizophrenia. Am Psychol. 2005;60:229–242. doi: 10.1037/0003-066X.60.3.229. [DOI] [PubMed] [Google Scholar]
- 25.Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: integration of performance-based and brain imaging findings. Psychol Bull. 2007;133:833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- 26.Buchannan RW, Heinrichs DW. The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res. 1989;27:335–350. doi: 10.1016/0165-1781(89)90148-0. [DOI] [PubMed] [Google Scholar]
- 27.Chen EYH, Shapleske J, Luque R, et al. The Cambridge Neurological Inventory, a clinical instrument for assessment of soft neurological signs. Psychiatry Res. 1995;56:183–204. doi: 10.1016/0165-1781(95)02535-2. [DOI] [PubMed] [Google Scholar]
- 28.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 29.Kay S, Fiszbein A, Opler L. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 30.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, Iowa: The University of Iowa; 1984. [Google Scholar]
- 31.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, Iowa: The University of Iowa; 1984. [PubMed] [Google Scholar]
- 32.Wechsler D. Wechsler Adult Intelligence Scale—Revised: Manual. New York, NY: The Psychological Corporation; 1981. [Google Scholar]
- 33.Wechsler D. WMS-III Manual. New York, NY: The Psychological Corporation; 1997. [Google Scholar]
- 34.Reitan RM. Trail Making Test: Manual for Administration, Scoring and Interpretation. Bloomington: Section of Neuropsychology, Department of Neurology, Indiana University Medical Centre; 1956. [Google Scholar]
- 35.Heaton RK. Wisconsin Card Sorting Test Manual. Odessa, Fla: Psychological Assessment Resources; 1981. [Google Scholar]
- 36.Rossi A, Cataldo SD, Michele VD, et al. Neurological soft signs in schizophrenia. Br J Psychiatry. 1990;157:735–739. doi: 10.1192/bjp.157.5.735. [DOI] [PubMed] [Google Scholar]
- 37.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-analysis. Englewood, NJ: Biostat, Inc.; 2005. [Google Scholar]
- 38.Rosenthal R. The “file drawer problem” and tolerance for null results. Psychol Bull. 1979;86:638–641. [Google Scholar]
- 39.Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. Br Med J. 2000;320:1574–1577. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenberg MS. The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis. Evolution. 2005;59:464–468. [PubMed] [Google Scholar]
- 41.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duval SJ, Tweedie RL. Practical estimates of the effect of publication bias in meta-analysis. Australas Epidemiol. 1998;5:14–17. [Google Scholar]
- 43.Ismail B, Cantor-Graae E, McNeil TF. Minor physical anomalies in schizophrenia, cognitive, neurological and other clinical correlates. J Psychiatr Res. 2000;34:45–56. doi: 10.1016/s0022-3956(99)00034-5. [DOI] [PubMed] [Google Scholar]
- 44.Ismail B, Cantor-Graae E, McNeil TF. Neurological abnormalities in schizophrenia, clinical, etiological and demographic correlates. Schizophr Res. 1998;30:229–238. doi: 10.1016/s0920-9964(97)00150-3. [DOI] [PubMed] [Google Scholar]
- 45.Cohen . Statistical Power Analysis for the Behavioral Sciences (2nd ed) New York, NY: Academic Press; 1988. [Google Scholar]
- 46.Braun CMJ, Lapierre D. Neurological soft signs in schizophrenia: are they related to negative or positive symptoms, neuropsychological performance, and violence? Arch Clin Neuropsychol. 1995;10:489–509. [PubMed] [Google Scholar]
- 47.Chen EYH, Chan CK. The Cambridge Neurological Inventory: clinical, demographic, and ethnic correlates. Psychiatr Ann. 2003;33:202–210. [Google Scholar]
- 48.Jahn T, Hubmann W, Karr M, et al. Motoric neurological soft signs and psychopathological symptoms in schizophrenic psychoses. Psychiatry Res. 2006;142:191–199. doi: 10.1016/j.psychres.2002.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Quitkin F, Rifkin A, Klein DF. Organicity in schizophrenia with premorbid asociality and emotionally unstable character disorders. Arch Gen Psychiatry. 1976;33:845–853. doi: 10.1001/archpsyc.1976.01770070075008. [DOI] [PubMed] [Google Scholar]
- 50.Sanders RD, Keshavan MS, Forman SD, et al. Factor structure of neurologic examination abnormalities in unmedicated schizophrenia. Psychiatry Res. 2000;95:237–243. doi: 10.1016/s0165-1781(00)00176-1. [DOI] [PubMed] [Google Scholar]
- 51.Lane A, Colgan K, Moynihan F, et al. Schizophrenia and neurological soft signs, gender differences in clinical correlates and antecedent factors. Psychiatry Res. 1996;64:105–114. doi: 10.1016/0165-1781(96)02602-9. [DOI] [PubMed] [Google Scholar]
- 52.Griffiths TD, Sigmundsson T, Takei N, Rowe D, Murray RM. Neurological abnormalities in familial and sporadic schizophrenia. Brain. 1998;121:191–203. doi: 10.1093/brain/121.2.191. [DOI] [PubMed] [Google Scholar]
- 53.O'Reilly RL, Lane A, Cernovsky ZZ, O'Callaghan E. Neurological soft signs, minor physical anomalies and handedness in schizophrenia. Eur J Psychiatry. 2001;15:189–192. [Google Scholar]
- 54.Browne S, Clarke M, Gervin M, et al. Determinants of neurological dysfunction in first episode schizophrenia. Psychol Med. 2000;30:1433–1441. doi: 10.1017/s003329179900286x. [DOI] [PubMed] [Google Scholar]
- 55.Bachmann S, Bottmer C, Schröder J. Neurological soft signs in first-episode schizophrenia: a follow-up study. Am J Psychiatry. 2005;162:2337–2343. doi: 10.1176/appi.ajp.162.12.2337. [DOI] [PubMed] [Google Scholar]
- 56.Whitty P, Clarke M, McTigue O, et al. Diagnostic specificity and predictors of neurological soft signs in schizophrenia, bipolar disorder and other psychoses over the first 4 years of illness. Schizophr Res. 2006;86:110–117. doi: 10.1016/j.schres.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 57.Obiols JE, Serrano F, Caparrós B, Subirá S, Barrantes N. Neurological soft signs in adolescents with poor performance on the continuous performance test: markers of liability for schizophrenia spectrum disorders? Psychiatry Res. 1999;86:217–228. doi: 10.1016/s0165-1781(99)00039-6. [DOI] [PubMed] [Google Scholar]
- 58.King DJ, Wilson A, Cooper SJ, Waddington JL. The clinical correlates of neurological soft signs in chronic schizophrenia. Br J Psychiatry. 1991;158:770–775. doi: 10.1192/bjp.158.6.770. [DOI] [PubMed] [Google Scholar]
- 59.Bartko G, Zador G, Horvath S, Herczeg I. Neurological soft signs in chronic schizophrenic patients: clinical correlates. Soc Biol Psychiatry. 1988;24:458–460. doi: 10.1016/0006-3223(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 60.Liddle PF. Schizophrenic syndromes, cognitive performance and neurological dysfunction. Psychol Med. 1987;17:49–57. doi: 10.1017/s0033291700012976. [DOI] [PubMed] [Google Scholar]
- 61.Cox SM, Ludwig AM. Neurological soft signs and psychopathology: I. Findings in schizophrenia. J Nerv Ment Dis. 1979;167:161–165. doi: 10.1097/00005053-197903000-00004. [DOI] [PubMed] [Google Scholar]
- 62.Schröder J, Niethammer R, Geider F-J, et al. Neurological soft signs in schizophrenia. Schizophr Res. 1992;6:25–30. doi: 10.1016/0920-9964(91)90017-l. [DOI] [PubMed] [Google Scholar]
- 63.Flyckt L, Sydowb O, Bjerkenstedta L, Edmana G, Rydinb E, Wieselc F-A. Neurological signs and psychomotor performance in patients with schizophrenia, their relatives and healthy controls. Psychiatry Res. 1999;86:113–129. doi: 10.1016/s0165-1781(99)00027-x. [DOI] [PubMed] [Google Scholar]
- 64.Arango C, Kirkpatrick B, Buchannan RW. Neurological signs and the heterogeneity of schizophrenia. Am J Psychiatry. 2000;157:560–565. doi: 10.1176/appi.ajp.157.4.560. [DOI] [PubMed] [Google Scholar]
- 65.Chen EYH, Kwok CL, Au JWY, Chen RYL, Lau BST. Progressive deterioration of soft neurological signs in chronic schizophrenia patients. Acta Psychiatr Scand. 2000;102:342–349. doi: 10.1034/j.1600-0447.2000.102005342.x. [DOI] [PubMed] [Google Scholar]
- 66.Biswas P, Malhotra S, Malhotra A, Gupta N. Comparative study of neurological soft signs in schizophrenia with onset in childhood, adolescence. Acta Psychiatr Scand. 2007;115:295–303. doi: 10.1111/j.1600-0447.2006.00901.x. [DOI] [PubMed] [Google Scholar]
- 67.Das M, Kumari V, Soni W, et al. Neurological soft signs and their relationship to cognitive and clinical efficacy of atypical antipsychotics in schizophrenia. Schizophr Bull. 2004;30:241–253. doi: 10.1093/oxfordjournals.schbul.a007075. [DOI] [PubMed] [Google Scholar]
- 68.Bersani G, Clemente R, Gherardelli S, Pancheri P. Deficit of executive functions in schizophrenia: relationship to neurological soft signs and psychopathology. Psychopathology. 2004;37:118–123. doi: 10.1159/000078610. [DOI] [PubMed] [Google Scholar]
- 69.Dazzan P, Morgan KD, Orr KG, et al. The structural brain correlates of neurological soft signs in AESOP first-episode psychoses study. Brain. 2004;127:143–153. doi: 10.1093/brain/awh015. [DOI] [PubMed] [Google Scholar]
- 70.Boks MPM, Selten J-P, Leask S, Van den Bosch RJ. The 2-year stability of neurological soft signs after a first episode of non-affective psychosis. Eur Psychiatry. 2006;21:288–290. doi: 10.1016/j.eurpsy.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 71.Gureje O. Neurological soft signs in Nigerian schizophrenics: a controlled study. Acta Psychiatr Scand. 1988;78:505–509. doi: 10.1111/j.1600-0447.1988.tb06374.x. [DOI] [PubMed] [Google Scholar]
- 72.Aydemir C, Goka E, Kisa C, Kurt A, Yuksel FV. Dyskinesia and soft neurological signs in schizophrenia: a comparative study. Int J Psychiatry Clin Pract. 2005;9:238–243. doi: 10.1080/13651500500329150. [DOI] [PubMed] [Google Scholar]
- 73.Chen EY, Hui CL, Chan RC, et al. A 3-year prospective study of neurological soft signs in first-episode schizophrenia. Schizophr Res. 2005;75:45–54. doi: 10.1016/j.schres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 74.Scheffer RE. Abnormal neurological signs at the onset of psychosis. Schizophr Res. 2004;70:19–26. doi: 10.1016/j.schres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 75.Sevincok L, Topaloglu B. Neurological soft signs and positive treatment response to olanzapine in chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:141–143. doi: 10.1016/j.pnpbp.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 76.Emsley R, Turnera HJ, Oosthuizena PP, Carr J. Neurological abnormalities in first-episode schizophrenia: temporal stability and clinical and outcome correlates. Schizophr Res. 2005;75:35–44. doi: 10.1016/j.schres.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 77.Mittal VA, Hasenkamp W, Sanfilipo M, et al. Relation of neurological soft signs to psychiatric symptoms in schizophrenia. Schizophr Res. 2007;94:37–44. doi: 10.1016/j.schres.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 78.Picchioni MM, Toulopoulou T, Landau S, Davies N, Ribchester T, Murray RM. Neurological abnormalities in schizophrenic twins. Biol Psychiatry. 2006;59:341–348. doi: 10.1016/j.biopsych.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 79.Bersani G, Gherardelli S, Clemente R, et al. Neurologic soft signs in schizophrenic patients treated with conventional and atypical antipsychotics. J Clin Psychopharmacol. 2005;25:372–375. doi: 10.1097/01.jcp.0000169268.44421.cf. [DOI] [PubMed] [Google Scholar]
- 80.Boks MPM, Liddle PF, Burgerhof JGM, Knegtering R, Bosch R-Jvd. Neurological soft signs discriminating mood disorders from first episode schizophrenia. Acta Psychiatr Scand. 2004;110:29–35. doi: 10.1111/j.1600-0447.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- 81.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 82.Davidson L, Heinrichs RW. Quantification of brain imaging findings on the frontal and temporal lobes in schizophrenia: a meta-analysis. Psychiatry Res. 2003;122:69–87. doi: 10.1016/s0925-4927(02)00118-x. [DOI] [PubMed] [Google Scholar]
- 83.Hallmayer JF, Kalaydjieva L, Badcock J, et al. Genetic evidence for a distinct subtype of schizophrenia characterized by pervasive cognitive deficit. Am J Hum Genet. 2005;77:468–476. doi: 10.1086/432816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen EYH, Lam LCW, Chen RYL, Nguyen DGH. Neurological signs, age, and illness duration in schizophrenia. J Nerv Ment Dis. 1996;184:339–345. doi: 10.1097/00005053-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 85.Bjorck RV, Sjalin M, Nordin C. Neurological soft signs in schizophrenic patients: influence of age, age at onset, sex, and family history of schizophrenia. Nord J Psychiatry. 2000;54:437–440. [Google Scholar]
- 86.Duggal HS, Muddasani S, Keshavan MS. Insular volumes in first-episode schizophrenia: gender effect. Schizophr Res. 2005;73:113–120. doi: 10.1016/j.schres.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 87.Sevincok L, Akoglu A, Topaloglu B, Aslantas H. Neurological soft signs in schizophrenic patients with obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2004;58:274–278. doi: 10.1111/j.1440-1819.2004.01231.x. [DOI] [PubMed] [Google Scholar]
- 88.Bersani G, Orlandi V, Gherardelli S, Pancheri P. Cannabis and neurological soft signs in schizophrenia: absence of relationship and influence on psychopathology. Psychopathology. 2002;35:289–295. doi: 10.1159/000067064. [DOI] [PubMed] [Google Scholar]
- 89.Krebs MO, GutFayand A, Bourdel MC, Dischamp, Olie JP. Validity and factorial structure of a standardized examination for neurological soft-signs in schizophrenia. Schizophr Res. 2000;45:245–260. doi: 10.1016/s0920-9964(99)00206-6. [DOI] [PubMed] [Google Scholar]