Abstract
Objective: Our aim was to review recent studies and estimate the rate of cannabis use disorders (CUDs) in schizophrenia, as well as to examine the factors affecting this rate. Methods: We conducted an electronic search of 3 literature databases and a manual search of articles from 1996 to 2008. The key words used were “schizophreni*,” “psychos*s,” “psychotic,” “cannabis abuse,” “cannabis dependence,” “cannabis use disorder,” “substance use disorder,” “substance abuse,” “substance dependence,” and “dual diagnosis.” Articles that reported diagnoses according to the Diagnostic and Statistical Manual of Mental Disorders or International Classification of Diseases were included. Regression analysis was used to examine how estimated rates of CUDs are affected by various study characteristics such as the classification system, inpatient vs outpatient status, study location, proportion of males, age of the sample, or duration of illness. Results: Thirty-five studies met our search criteria. The median current rate of CUDs was 16.0% (interquartile range [IQR] = 8.6–28.6, 10 studies), and the median lifetime rate was 27.1% (IQR = 12.2–38.5, 28 studies). The median rate of CUDs was markedly higher in first-episode vs long-term patients (current 28.6%/22.0%, lifetime 44.4%/12.2%, respectively) and in studies where more than two-thirds of the participants were males than in the other studies (33.8%/13.2%). CUDs were also more common in younger samples than in the others (current 38.5%/16.0%, lifetime 45.0%/17.9%). Conclusions: Approximately every fourth schizophrenia patient in our sample of studies had a diagnosis of CUDs. CUDs were especially common in younger and first-episode patient samples as well as in samples with a high proportion of males.
Keywords: schizophrenia spectrum, dual diagnosis, cannabis abuse, cannabis dependence, comorbidity, substance use disorders
Introduction
Cannabis is one of the most commonly used addictive substances among patients with schizophrenia. Previous systematic reviews have reported a wide range (13%–45%) in the rates of cannabis use disorders (CUDs) in schizophrenia.1,2 Studies published from 1960–1989 were reviewed by Mueser et al1 and those from 1990–2001 by Cantor-Graae et al.2 Mueser and colleagues noted that many studies have suffered from methodological problems. They concluded that a history of cannabis abuse was related to fewer symptoms of schizophrenia and fewer hospitalizations, suggesting that more socially competent schizophrenia patients were prone to cannabis use.1 According to Cantor-Graae et al,2 alcohol was generally the most frequently used substance among schizophrenia patients, with cannabis use frequent among younger patients. Both reviews presented results on the use of several substances, and the reviewed studies varied greatly in their methodological, classification, and sample characteristics. However, the overall rate of CUDs in schizophrenia patients remains unclear, as do the factors affecting this rate. In addition, the earlier reviews included older studies. Because cannabis use in schizophrenia is currently an active research area, there is a need for an updated review, and this article presents the first meta-analysis on this topic.
Cannabis use may induce psychotic symptoms; eg, the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) includes a diagnosis of cannabis-induced psychotic disorder. However, the role of cannabis use in the onset, course, and clinical expression of schizophrenia is unclear.3 Schizophrenia patients who use cannabis are younger4 and have more psychotic relapses.5 It has also been suggested that schizophrenia patients are more vulnerable to the effects of tetrahydrocannabinol, which is the main psychotropic compound of cannabis.6 Cannabis use has been reported to increase positive symptoms in schizophrenia,6–8 while the findings regarding negative symptoms have been contradictory.6,7,9 In a recent systematic review, Zammit et al10 examined the effects of cannabis use on outcomes of psychotic disorders. They noted an association with increased relapse and nonadherence but little evidence that associations were specifically due to cannabis use. They also highlighted that only a few previous studies had adjusted for factors such as the baseline severity of illness. The reported findings were more variable concerning the severity of symptoms.
This meta-analysis focused on studies reporting rates of CUDs according to the DSM (by the American Psychiatric Association) or International Classification of Diseases (ICD by the World Health Organization) classification systems. According to the Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised) (DSM-III-R), cannabis dependence includes both physiological and behavioral symptoms. However, cannabis abuse is a residual category for diagnosing those who do not meet the criteria for dependence but who use cannabis despite substance-related physical, social, psychological, or occupational problems or who use cannabis in risky situations. In the DSM-IV, the criteria for cannabis abuse also include substance use despite recurrent social, interpersonal, and legal problems as a result of cannabis use, and the criteria for dependence are stricter. The DSM-IV and International Classification of Diseases, Tenth Revision (ICD-10), have been developed into more compatible classification systems for substance use disorders, although differences still exist. The ICD-10 differs little from DSM-IV diagnostics in terms of the diagnosis of dependence. Cannabis use that causes either physical or mental damage in the absence of dependence is categorized in the ICD-10 as harmful use. This category highlights the somatic problems related to cannabis use more than the abuse diagnosis of the DSM-IV.
The aims of this study were as follows.
To systematically collect and review studies published from 1996 to 2008 on CUDs in schizophrenia and contrast the estimated rates with previous reviews.
To estimate the current overall rate of CUDs in schizophrenia by pooling the existing data and carrying out a meta-analysis.
To determine how study characteristics affect the estimated rate of CUDs in schizophrenia.
Methods
The recommendations of Meta-analysis of Observational Studies in Epidemiology (MOOSE)11 were used as guidelines when conducting this meta-analysis. In order to find articles reporting the rate of CUDs in schizophrenia published from 1996 to 2008, we conducted searches of 3 electronic databases (PsycINFO, PubMed, and Web of Science), the most recent being in January 2009. The key words used were “schizophreni*,” “psychosis,” “psychoses,” and “psychotic” to locate studies on schizophrenic psychoses, as well as “cannabis abuse,” “cannabis dependence,” “cannabis use disorder,” “substance use disorder,” “substance abuse,” “substance dependence,” and “dual diagnosis.” A similar search was carried out at the same time for alcohol use disorders. Altogether 3323 articles were retrieved, and their abstracts and titles were analyzed by both J.K. and J.M. Of these, 611 were identified as possibly relevant, and their full texts were analyzed in detail by J.K. and J.M. The inclusion of each article was independently evaluated and agreed upon.
In addition, a manual literature search was performed for the same time period from the journals Acta Psychiatrica Scandinavica, American Journal of Psychiatry, Archives of General Psychiatry, British Journal of Psychiatry, Journal of Clinical Psychiatry, Psychiatry Research, Schizophrenia Bulletin, Schizophrenia Research, and Social Psychiatry and Psychiatric Epidemiology. These journals were selected because they were available, and each had published a considerable proportion of the articles (approximately 40%) in our systematic database search. We also contacted approximately 30 authors to obtain unpublished information.
The inclusion criteria published in studies in our collection were that (1) at least 80% of the participants were individuals with a schizophrenia-spectrum diagnosis (schizophrenia, schizophreniform disorder, schizoaffective disorder, delusional disorder). Results from studies reporting findings from several psychiatric classifications were also included if they had determined the rate of CUDs in schizophrenia-spectrum patients alone. Other inclusion criteria were the following: (2) the study reported on the rate of cannabis abuse or dependence, (3) the subjects were older than 16 years, and (4) the study sample included more than 15 participants. Only articles (5) reporting schizophrenia and CUD diagnoses according to the DSM or ICD criteria and (6) written in English were included. We excluded studies with samples that might have biased the presented rates of CUDs in the study (eg, samples recruited from prisons, forensic psychiatry units, or homeless shelters). Trials and intervention studies were also excluded.
Information was collected on the classification system used. The terminology for CUDs in this article is adopted from the DSM classification system (abuse and dependence). We examined whether the classification system used (ICD-10, DSM-III-R, or DSM-IV) affected the presented CUD rates. For schizophrenia, the classification criteria mainly differ in terms of the duration of psychotic symptoms: In the ICD, the symptoms should last 1 month and in the DSM 6 months before making the diagnosis. The diagnostic criteria for CUDs in ICD and DSM classification systems are presented earlier.
CUD rates were compared between first-episode and long-term schizophrenia patient samples. The average duration of illness was determined from the studies. In samples other than first-episode patients, the minimum reported average duration was 9 years, and all these studies were categorized as having long-term patient samples. In addition, we determined the study location and whether the sample consisted of inpatients or outpatients. Information on the gender distribution, the distribution of schizophrenia diagnoses, mean age, and age range were collected where reported. CUD rates were for patients with abuse, and dependence diagnoses were combined in studies in which the diagnoses were not overlapping.
In this article, we present the number of studies as well as the mean, SD, median, interquartile range (IQR), and range for estimates of the rate of CUDs in each of the categories of interest. Due to the significant heterogeneity in rate estimates12 (Cochran Q = 6900, P < .001), we present random mean estimates, which is a conservative weighting method giving the same weight to all studies. When evidence is found of heterogeneity among studies in rate estimates, linear regression analysis applying the bootstrap resampling technique (or meta-regression12) with the z test can be used to analyze associations between rates and study characteristics. Bootstrap methods were used because they make fewer assumptions about the distribution of the rates.13 We created 1000 bootstrap samples by randomly resampling with replacement from the original data. Regression analysis was used to compare the effect of classification systems (DSM-III-R vs DSM-IV), study setting (first-episode vs long-term sample, inpatients vs outpatients), and location (North America vs Europe) on the rate estimates. Both the gender distribution (proportion of males) and mean age were analyzed as continuous variables. For reasons of presentation, these were categorized into 2 groups. The results of regression analysis were adjusted for the method of diagnosing cannabis use, so that variables for abuse (no/yes), dependence (no/yes), and the time period (lifetime/current) were included in the regression models. We also applied more traditional meta-regression to check whether the results remained statistically significant. The data were analyzed with Stata 9.0.14
Results
Of the 611 articles evaluated in detail, 35 studies met the inclusion criteria (table 1). These provided data from 16 countries (1 study each from Finland, Germany, Greece, Ireland, Lebanon, Sweden, Switzerland, Turkey, and the United Kingdom; 2 studies from Canada, Israel, Italy, and The Netherlands; 4 from France; 6 from Australia; and 9 from the United States). The total number of cases was 5540. As can be seen from table 1, the characteristics of the studies varied considerably.
Table 1.
Studies Presenting Results on Cannabis Use Disorders in Schizophrenia Patients
| Reference | Country, Study Period | Diagnosisa | Duration of Schizophrenia, Mean Years (Setting)b | Mean Age (y) (Range) | Sample Size (Males/Females) | Rate of Cannabis Use Disorders (%) | Comments |
| Addington and Addington15 | Canada | DSM-IV (B, 79% N), SCID I | First episode (I) | 25.0 (—) | 300 (∼210/90) | Lifetime abuse/dependence (13.7) | Males and younger patients used more. Cannabis misuse was associated with increased positive symptoms. |
| Akvardar et al 16 | Turkey | DSM-IV (N), SCID I | 16.0 (I/O) | 39.3 (—) | 49 (26/23) | Lifetime abuse (2.0) and dependence (0) | Only one patient diagnosed with cannabis use disorder. |
| Altamura et al17 | Italy, 1992–1997 | DSM-III-R (B, 92% N), interview and clinical charts | 18.8 (O) | 38.0 (—) | 81 (55/26) | Lifetime abuse/dependence (4.9) | Suicide attempters more commonly had nicotine or cannabis abuse. |
| Barnett et al18,c | United Kingdom, 2002–2005 | DSM-IV (N), SCID | First episode (O) | — | 36 (—) | Lifetime abuse (16.7) and dependence (36.1) | Cannabis use associated with earlier age of onset of first psychotic symptoms. |
| Bersani et al19 | Italy | DSM-IV (N), clinical interviews | 10.3 (I) | 32.4 (—) | 125 (125/—) | Current abuse (26.0) | Cannabis users were younger and had fewer negative symptoms. |
| Cantor-Graae et al2 | Sweden, 1998 | DSM-IV (N), SCID, interviews, case records | 21.0 (I/O) | 48.0 (—) | 87 (54/33) | Lifetime abuse (17.2) | Both an extensive review and an original study (no results on factors associated with cannabis use). |
| Carr et al20 | Australia, 1997 | ICD-10 (B), DIP, clinical charts | Laske (I) | 37.8 (18–64) | 537 (350/187) | Lifetime abuse/dependence (30.0) | Somewhat more abuse among outpatients (34.3%) than inpatients (25.3%). |
| Compton et al21 | United States, 2002 | DSM-IV (B, 67% N), SCID | First episode (I) | 20.0 (18–29) | 18 (16/2) | Lifetime dependence (44.4) | Dependent subjects had fewer negative symptoms. |
| Compton et al22 | United States, 1999 | DSM-IV (B, 64% N), clinical charts | — (I/O) | 42.9 (—) | 248 (153/95) | Lifetime abuse/dependence (6.3) | Subjects with any substance use diagnosis were younger and more commonly males. |
| Compton et al23 | United States, 2004–2005 | DSM-IV (B, 40% N), SCID I, clinical charts | First episode (I) | 22.8 (18–40) | 25 (19/6) | Lifetime abuse (8.0) and dependence (40.0) | African American urban sample, no results on associative factors for cannabis. |
| Dervaux et al24 | France | DSM-III-R (B, 91% N), CIDI | — (I/O) | 34.7 (—) | 100 (68/32) | Lifetime abuse/dependence (27.0) | High level of impulsivity and sensation seeking associated with any substance abuse. |
| Deshmukh et al25 | United States | DSM-III-R or DSM-IV (N), SCID | — (I/O) | 31.6 (—) | 34 (34/—) | Lifetime abuse (11.8) and dependence (23.5) and both (35.3) | Cerebellar dysfunction was seen more often in schizophrenia patients abusing alcohol compared with drugs. |
| Dubertret et al26 | France | DSM-IV (N), DIGS | 11.3 (I) | — | 205 (139/66) | Lifetime abuse/dependence (32.2) | Schizophrenia patients with cannabis abuse were younger, their illness duration was shorter, and age at onset was younger. Males abused cannabis more often. |
| Farrelly et al27 | Australia, 1989–1992 | DSM-III-R (B, 46% N), SCID I/P | First episode (I) | 21.7 (16–29) | 91 (—) | Current abuse/dependence (28.6) | Patients with current or lifetime substance abuse were younger and more often male, not married, and from nonurban areas. |
| Fowler et al28 | Australia, 1993 | DSM-III-R (N), SCID-R | 12.7 (O) | 36.3 (18–60) | 194 (141/53) | Lifetime (7.7) and current (4.1) abuse, lifetime (28.3) and current (8.8) dependence | Schizophrenia-spectrum patients with substance use were more often male. |
| Gearon and Bellack29 | United States | DSM-IV (N), SCID-P | 16.2 (O) | 38.3 (18–55) | 67 (38/29) | Lifetime abuse/dependence (41.0) | Schizophrenia patients with substance use disorder were younger at the onset of illness. Women with schizophrenia and substance use disorders had a more severe course of illness, eg, more frequent hospitalizations and poorer overall functioning. They experienced more severe positive and general psychiatric symptoms compared with nonabusing women. |
| Gut-Fayand et al30 | France | DSM-III-R (B, 96% N), SADS-LA-R | 33.2 (I/O) | 33.2 (—) | 50 (32/18) | Lifetime abuse (6.0) and dependence (8.0) | Substance-abusing schizophrenia patients were younger, more often male, and single. They also had more positive symptoms. On average, prodromal symptoms preceded the onset of substance abuse. |
| De Haan et al31 | The Netherlands, 1996–1998 | DSM-IV (B, 86% N), all available information, research diagnosis | First episode (I/O) | 20.9 (—) | 119 (96/23) | Lifetime abuse/dependence (31.9) | Cannabis abuse or dependence was strongly related to medication nonadherence. |
| Hides et al32 | Australia, 1998–2000 | DSM-IV (B), CIDI | ≈9.4 (I) | 30.0 (—) | 142 (102/40) | Current dependence (53.5) | Cannabis dependence was more common in schizophrenia-spectrum compared with mood disorders with psychotic features. |
| Holthausen et al33 | The Netherlands | DSM-IV (B), CIDI | First admission (I) | 24.8 (—) | 118 (87/31) | Current abuse (48.3) | No statistically significant difference was found in terms of the presence of cognitive deficits within cannabis-abusing patients. |
| Kamali et al34 | Ireland | DSM-IV (B), SCID | 15.1 (I) | 38.4 (—) | 102 (68/34) | Lifetime (16.7) and current (17.6) abuse/dependence | Patients with current substance use disorders were younger and more often had suicidal ideation. |
| Karam et al35 | Lebanon, Israel, 1979–1992 | DSM-III-R (N), chart review | — (I) | 34.5 (—) | 18 (12/6) | Lifetime abuse (44.4) | Alcohol and cannabis were the most commonly abused substances among schizophrenia patients. Schizophrenia patients abused cannabis more often than patients with depression, anxiety, or bipolar disorder. |
| Katz et al36 | Israel, 2003–2006 | DSM-IV, (B), SCID | — (I/O) | — (18–70) | 227 (—) | Lifetime abuse (11.9) | Only little information on the schizophrenia patients group. |
| Kavanagh et al37 | Australia, 1997–1998 | DSM-III-R (N), clinical interview | — (I/O) | — | 430 (—) | Lifetime abuse/dependence (26.5) | Psychotic cannabis-abusing patients often had co-occurring tobacco use and a lifetime diagnosis of alcohol and other substance abuse or dependence. |
| Kirkpatrick et al38 | United States | DSM-IV (N), CASH | 11.3 (I/O) | 33.8 (current), 34.4 (lifetime) (—) | 154 (103/51) (current), 122 (84/38) (lifetime) | Current (1.9) and lifetime (12.3) abuse/dependence | No significant difference between deficit and nondeficit groups in current cannabis use, whereas current use of alcohol or other drugs was less severe in deficit patients. |
| Margolese et al39 | Canada | DSM-IV (B, 62% N), SCID-P, interviews, clinical charts | First episode (O) | 38.8 (—) | 207 (120/87) | Current abuse/dependence (8.2) | Alcohol and cannabis were the most commonly used drugs. |
| Modestin et al40 | Switzerland, 1993–1995 | ICD-10 (N), interview, hospital charts | 13.2 (I/O) | 40.6 (—) | 525 (∼278/247) | Lifetime harmful use/dependence (12.0) | Patients with cannabis use disorders were younger, with an earlier age at first psychiatric hospitalization |
| Moilanen et al41 | Finland, 1982–1997 | DSM-III-R (N), diagnoses rechecked from clinical charts | 9.3 (I/O) | 31.0 (31–31) | 96 (63/33) | Lifetime abuse/dependence (0.0) | Unpublished data (only results on alcohol). Comorbid substance use diagnoses did not show a statistically significant difference in discordance between clinical and research diagnoses of schizophrenia. |
| Mueser et al42 | United States | DSM-III-R (B, 51% N), SCID, clinical charts, all available information | — (I) | 38.8 (—) | 173 (154/19) | Lifetime abuse/dependence (27.2) | Cannabis use disorders are more common in schizophrenia and schizoaffective and bipolar disorder than in major depression. |
| Rabinowitz et al43 | United States, 1989–1995 | DSM-III-R, SCID, clinical charts | First episode (I) | — | 224 (146/78) | Lifetime abuse/dependence (31.3) | Males abused cannabis and other substances more often. |
| Verdoux et al44,c | France | DSM-IV (B, 44% N), clinical charts, MINI, interviews | First episode (I) | 31.0 (16–59) | 27 (15/12) | Lifetime abuse /dependence (18.5) | In early psychosis, suicidal behavior is more common in substance-abusing subjects. |
| Wade et al45,c | Australia, 1997 | DSM-III-R, CUAD, all available information | First episode (I) | 21.3 | 93 (68/25) | Lifetime abuse/dependence (65.6) | Persons with lifetime cannabis use disorders more often had another lifetime substance use disorder, lifetime polysubstance use disorder, or lifetime daily tobacco use. |
| Wobrock et al46 | Germany | ICD-10, EuropASI | First episode (I) | 25.7 (—) | 68 (∼45/23) | Lifetime harmful use (45.6) | Substance abuse patients were younger, more often males, unmarried, and unemployed and had a family history of severe mental illness and substance use disorder. No significant difference in cognitive domains. |
| Xafenias et al47 | Greece, 2004–2005 | DSM-IV, EuropASI, semistructured interview | — (I) | — | 105 (—) | Current abuse/dependence (8.6) | Comorbid substance use disorder did not affect the length of hospitalization. |
| Ziedonis and Trudeau48 | United States, 1995 | DSM-III-R (B, 64% N), clinical assessment, chart review | — (O) | 44.0 (—) | 497 (253/244) | Current abuse (14.3) | Alcohol, cannabis, and cocaine were the most commonly abused substances. Polydrug use was common. |
Diagnostic criteria and distribution of schizophrenia diagnoses: B = broad schizophrenia (schizophrenia spectrum), N = narrow schizophrenia. ICD-10: International Classification of Diseases, Tenth Revision; DSM-IV: Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition); SCID: Structured Clinical Interview for DSM-IV (I = Axis I disorders) (I/P = Axis I disorders, patient edition); SCID-R = SCID for Diagnostic and Statistical Manual of Mental Disorders Third Edition); DIP: Diagnostic Interview for Psychoses; SADS-LA-R: Schedule for Affective Disorders and Schizophrenia (Lifetime Axis I Diagnostic and Statistical Manual of Mental Disorders [Third Edition Revised]); CIDI: Composite International Diagnostic Interview; CASH: Comprehensive Assessment of Symptoms and History; CUAD: Chemical Use, Abuse, and Dependence Scale; EuropASI: European Addiction Severity Index; MINI: Mini-International Neuropsychiatric Interview; DIGS: Diagnostic Interview for Genetic Studies.
Setting: I = inpatients, O = outpatients, and I/O = both in- and outpatients.
Also unpublished data from the authors.
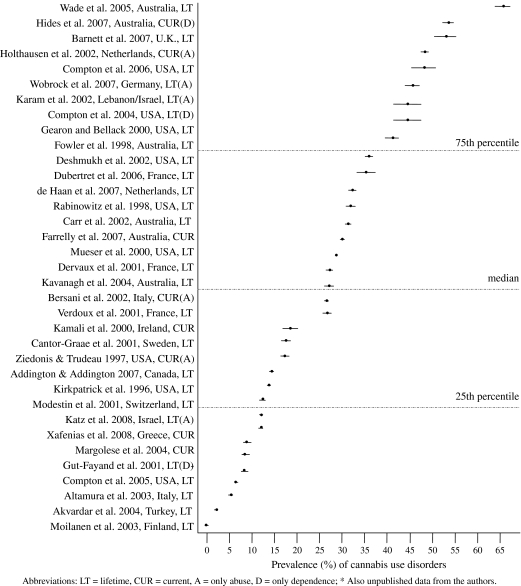
The total median rate of CUDs in schizophrenia was 27.0% (range = 0.0–65.6, 35 studies). The median rate of lifetime CUDs was 27.1% (IQR = 12.2–38.5, 28 studies) and that of current CUDs was 16.0% (IQR = 8.6–28.6, 10 studies). Figure 1 presents rate estimates for CUDs in all the samples. The results are presented using forest plots with 95% confidence intervals (CIs). The studies are sorted according to the CUD rate estimate, and the median and lower and upper quartiles for the rate estimates are also presented.
Fig. 1.
Rate of Cannabis Use Disorders in Schizophrenia Samples (1996–2008).
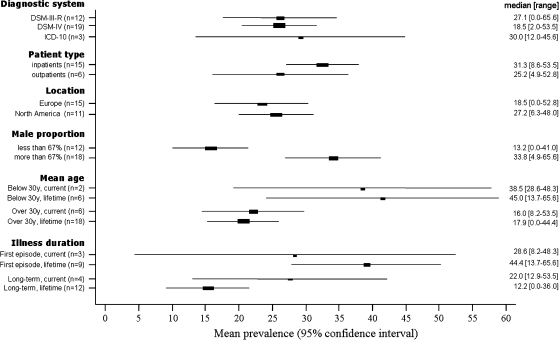
Figure 2 presents the studies categorized according to different study characteristics. Mean CUD rate estimates (with 95% CI), medians, and ranges are shown for each study characteristic. For mean age and duration of illness, we separately present current and lifetime diagnoses. CUDs were more common in younger (<30 y) than older (≥30 y) patient samples: The median estimated rates of CUDs for the 2 age groups were 38.5% vs 16.0% for current and 45.0% vs 17.9% for lifetime schizophrenia diagnoses. In bootstrapped regression analyses, these differences were also statically significant for current (z = −2.26, P = .02) and lifetime (z = −2.35, P = .02) schizophrenia diagnoses. The median rate of CUDs was also higher in samples in which more than two-thirds of the subjects were males compared with the other samples (33.8% vs 13.2%). In regression analysis, the finding was also statistically significant (z = 3.08, P = .002). In studies presenting results from first-episode samples, the median CUD rate was higher than for the others (28.6% vs 22.0% for current and 44.4% vs 12.2% for lifetime diagnoses). However, this difference was only statistically significant in patients with a lifetime diagnosis (current z = 1.72, P = .09; lifetime z = 3.46, P = .001). All the statistically significant results remained significant in meta-regression analyses. The rate of CUDs was not statistically significantly affected by other study characteristics. The results are presented in figure 2 with the mean CUD rate (95% CI), as well as the median and range. When comparing studies according to the recruitment strategy, the median estimated CUD rate was 26.7% (range = 0–53.5) in studies with consecutive patient samples (n = 20) and 12.3% (range = 6.3–35.3) in those with convenience samples.
Fig. 2.
Rate of Cannabis Use Disorders in Relation to Study Characteristics.
Discussion
Main Results
CUDs were found to be common in schizophrenia patients, approximately 16% of whom had a current diagnosis and 27% a lifetime diagnosis of CUDs. However, there was wide variation among studies in the rate of CUDs. CUDs were especially common in younger and first-episode patient samples, as well as in samples with a high proportion of males. The CUD rate was not affected by the study location (Europe vs North America), classification system used (DSM-III-R vs DSM-IV vs ICD-10), or patient type (inpatient vs outpatient).
Rate of CUDs in Schizophrenia
Most individuals suffering from schizophrenia undergo treatment,49 and the studies in our sample therefore mainly presented results from in- and outpatient samples. The rate of CUDs estimated in this meta-analysis therefore mainly relates to these patient groups. In our results, the median rate of current cannabis abuse was 20% and that of cannabis dependence 31%. The lifetime rates were 12% for abuse and 26% for dependence. In general, patients diagnosed with cannabis abuse are not also cannabis dependent, whereas patients with a diagnosis of cannabis dependence may include both those with abuse and dependence. Most studies reported combined abuse and dependence rates. We combined the rates for studies separately reporting diagnoses of cannabis abuse or dependence.
To avoid bias, we excluded studies presenting results solely from samples recruited from prisons, forensic psychiatry units, or homeless shelters. In these samples, substance use disorders tend to be significantly more common than in the general population.49–51 A schizophrenia diagnosis is also relatively common in these samples.52 However, exclusion of these studies is unlikely to have significantly affected our results. In addition, some studies included in our dataset may have included patients from prisons. The fact that cannabis is an illegal substance in most countries may also lead to underreporting of cannabis use. On the other hand, it is also possible that low rates of CUDs are not reported. This may especially be the case in articles reporting findings that are not considered to be affected by substance use.
Previous systematic reviews have reported the rate of CUDs in schizophrenia to vary over a wide range (13%–45%).1,2 Studies published from 1960 to1989 were reviewed by Mueser et al1 and those from 1990 to 2001 by Cantor-Graae et al.2 The median of pooled rates of cannabis abuse or dependence in these reviews was 35.4% (4 studies) and 26.0% (6 studies), respectively. These rates do not differ markedly from our results, but the relatively small number of studies makes the interpretation of a possible trend difficult.
Classification
A diagnosis of cannabis dependence in the ICD and DSM includes both physiological and behavioral symptoms, whereas cannabis abuse in the DSM and harmful use of cannabis in the ICD indicate cannabis use irrespective of substance-related physical, social, psychological, or occupational problems or cannabis use in risky situations. Although clinicians are trained to recognize dual diagnosis patients, underdiagnosing of substance use disorders still undoubtedly exists.53–55 In most studies included in our sample, CUD diagnoses were determined from several sources, such as structured or semistructured interviews, hospital charts, case notes, and registers.
Several articles used the term “abuse” to indicate heavy or continuous use rather than the definition according to DSM criteria. This confusing use of terminology made it challenging to evaluate the studies. Only articles using DSM or ICD classification criteria were therefore included. In our meta-analysis, there was no statistically significant difference in estimated CUD rates between classification systems. No studies used the International Classification of Diseases, Ninth Revision, classification system, and only 3 studies used the ICD-10.
Most of the studies in which information about the study sample was available reported results from schizophrenia samples with narrow diagnostic criteria (n = 12), but there were also several studies from schizophrenia-spectrum samples with broader criteria, including schizophrenia, schizophreniform disorder, schizoaffective disorder, and delusional disorder (n = 16). In the analysis, the rate of CUDs was similar in both groups: 26% for narrow schizophrenia and 23% for a schizophrenia spectrum.
Patient Characteristics
CUDs were more common among inpatients than outpatients; however, this difference was not statistically significant. Schizophrenia patients with CUDs were reported to have more positive symptoms15 and fewer negative symptoms.19,21 Kirkpatrick et al38 recorded no significant difference between deficit and nondeficit groups in current cannabis use, whereas the current use of alcohol or other drugs was less severe in deficit patients.
Substance use disorders have been reported to be more common in male schizophrenia patients than females.47 A significant gender difference was also seen in our dataset: The rate of CUDs was significantly higher in samples in which more than two-thirds of the subjects were male. All studies comparing CUDs in relation to gender reported higher rates among males.15,26,43 In addition, several studies reported that schizophrenia patients with CUDs were younger.15,18,26,40
A number of studies reported that alcohol and cannabis were the most commonly abused substances among schizophrenia patients.35,39–41,48 Cannabis-abusing patients also often had co-occurring tobacco use and a lifetime diagnosis of alcohol and other substance abuse or dependence.37,45 The presented CUD rates were higher than our unpublished meta-analysis on alcohol use disorders in schizophrenia, in which the median rate of current alcohol use disorder was 9.4% and the median rate of lifetime alcohol use disorder 20.6%. In addition, when comparing the rate of alcohol use disorders and CUDs, it seems that younger (<30 y) schizophrenia patient samples (7 studies) more often had CUDs, whereas alcohol use disorders were more common in older samples (18 studies).
Studies with consecutive patient samples had higher median rates of CUDs (26.7%) compared with convenience samples (12.3%). Consecutive samples may better represent those schizophrenia patients with substance use disorders. However, in our dataset, the number of convenience samples was relatively small (n = 5), and comparison with consecutive patient samples was therefore difficult.
Location of the Study
The substance use profile is greatly affected by cultural factors. Unfortunately, there were only a few non-Western studies in our dataset, and we were therefore only able to compare North American and (Western) European studies. There was no significant difference in the rate of CUDs between these 2 sets of samples.
We compared the CUD rate in schizophrenia with cannabis consumption (mainly in the age range 16–65 y) in the respective countries reported by the United Nations.56 There were examples of countries with a high consumption of cannabis in the general population and in schizophrenia patients (eg, Australia), as well as those with a low consumption in both the general population and schizophrenia patients (eg, Finland, Turkey). However, in some countries, the consumption in the general population was relatively high compared with that among schizophrenia patients (eg, Italy, Switzerland) and vice versa (eg, Germany, The Netherlands). However, general populations in the report of the United Nations and the patient samples in the studies included in our dataset differed in factors such as age and gender distribution, which makes the comparison challenging.
Direction of Causality of Cannabis Use and Schizophrenia
It is generally accepted that cannabis use can cause different psychotic states, ranging from toxic delirium to acute paranoia. In the DSM-IV classification, this is termed cannabis-induced psychosis. However, the role of cannabis use in the onset, course, and clinical expressions of schizophrenia is less clear. According to the recent meta-analysis by Moore et al,3 a study on 50 000 Swedish conscripts is still the only one in which a schizophrenia diagnosis has been used as an outcome for cannabis use.57,58 The authors concluded that “high consumers” (more than 50 occasions) of cannabis had a relative risk of 6.7 (95% CI = 2.1–21.7) for inpatient schizophrenia care in a 15-year follow-up period after conscription.
Moore et al3 reported in their systematic review that cannabis use increases the incidence of any psychotic outcome; the risk was approximately 40% higher for those who had ever used cannabis. However, a substantial confounding effect was present for psychotic outcomes. No robust evidence was presented indicating that earlier use of cannabis would have more harmful effects. In a recent systematic review, the same research group examined the effects of cannabis use on the outcomes of psychotic disorders.10 An association with increased relapse and nonadherence was observed, whereas the reported findings on the severity of symptoms were more variable.3 On the whole, the direction of causality of cannabis use and schizophrenia needs further studying.
Strengths and Weaknesses of the Study
This meta-analysis of recent studies presenting results on CUDs in schizophrenia patients was based on a comprehensive literature search and is also the first meta-analysis on this topic. We conducted a systematic database search and an extensive manual search from a range of scientific journals. In addition, we contacted several authors to obtain unpublished information. However, it is possible that some studies presenting results on the rates of CUDs in patients with schizophrenia were not included. For example, we may have overlooked studies in which CUDs were not the main focus of the article.
Because our search covered a long time period (1996–2008), a large number of studies (n = 35) met the inclusion criteria. These studies included a wide variety of methods and results, and this heterogeneity made pooling of the results challenging. When conducting meta-analyses on observational studies, inherent biases and differences in study design complicate the analysis. We followed the recommendations of MOOSE11 as guidelines when conducting this study. In reporting the variation in CUD rates in our data, the recommendations of Saha et al59 were used. Specific inclusion criteria can be applied to exclude studies with methodological problems. For instance, in the current study, only articles reporting results on diagnoses based on the criteria of the ICD-10, DSM-III-R, or DSM-IV classification systems (both in schizophrenia and in CUDs) were included. In the majority of the studies, the diagnoses of schizophrenia and CUDs were determined from several sources.
We used bootstrapped regression analysis and meta-regression to test the differences in CUD rates among studies according to various study characteristics. We examined the effect of differences in classification systems, first-episode vs long-term patients, study location, gender distribution, the distribution of schizophrenia diagnosis, and age. Due to possible differences in other patient and study characteristics (not controlled for here), meta-analysis with regression analysis is not necessarily an efficient method. We focused on estimating the rate of CUDs in schizophrenia patients, not on separately examining specific patterns (eg, gender differences). Few original studies have specifically examined study characteristics. Here, these studies are discussed separately. Study characteristics could be examined more efficiently in a separate meta-analysis with specially developed inclusion and exclusion criteria, which would be an interesting topic of future research. However, there is a lack of original studies comparing study characteristics between CUDs and other patients. Another limitation is that the CUD rates presented here are mainly derived from Western samples. Due to the limited resources, we were only able to include articles published in English. Without language limitations, a more comprehensive overview of global CUD rates in schizophrenia patients could perhaps be obtained.
In summary, our results demonstrate that CUDs are rather common in schizophrenia patients, particularly among young, male, and first-episode patients. Our results support the hypothesis that cannabis may now be a more commonly abused substance among young schizophrenia patients than alcohol. However, this hypothesis needs further testing. It is important to report information on substance use disorders in schizophrenia patient samples because it may affect the findings of studies in various research areas.
Funding
Sigrid Juselius Foundation (to J.K., M.I.); Resarch Foundation of Orion Corporation (to J.K.); Finnish Medical Foundation (to J.K.); Academy of Finland (113760 to H.K., 212848 to M.I., 125853 to J.M.); National Alliance for Research on Schizophrenia and Depression (to M.I.).
Acknowledgments
We thank the following researchers who helped in the search for data: Suzanne Archie, Jennifer Barnett, Susy Harrigan, Rosemary Purcell, Hélène Verdoux, and Darryl Wade.
References
- 1.Mueser KT, Yarnold PR, Levinson DF, et al. Prevalence of substance abuse in schizophrenia: demographic and clinical correlates. Schizophr Bull. 1990;16:31–55. doi: 10.1093/schbul/16.1.31. [DOI] [PubMed] [Google Scholar]
- 2.Cantor-Graae E, Nordström LG, McNeil TF. Substance abuse in schizophrenia: a review of the literature and a study of correlates in Sweden. Schizophr Res. 2001;48:69–82. doi: 10.1016/s0920-9964(00)00114-6. [DOI] [PubMed] [Google Scholar]
- 3.Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;28:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 4.Hambrecht M, Häfner H. Cannabis, vulnerability, and the onset of schizophrenia: an epidemiological perspective. Aust N Z J Psychiatry. 2000;34:468–475. doi: 10.1080/j.1440-1614.2000.00736.x. [DOI] [PubMed] [Google Scholar]
- 5.Dixon L. Dual diagnosis of substance abuse in schizophrenia: prevalence and impact on outcomes. Schizophr Res. 1999;35:S93–S100. doi: 10.1016/s0920-9964(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 6.Gregg L, Barrowclough C, Haddock G. Reasons for increased substance use in psychosis. Clin Psychol Rev. 2007;27:494–510. doi: 10.1016/j.cpr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Talamo A, Centorrino F, Tondo L, Dimitri A, Hennen J, Baldessarini RJ. Comorbid substance-use in schizophrenia: relation to positive and negative symptoms. Schizophr Res. 2006;86:251–255. doi: 10.1016/j.schres.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Stefanis NC, Delespaul P, Henquet C, Bakoula C, Stefanis CN, Van Os J. Early adolescent cannabis exposure and positive and negative dimensions of psychosis. Addiction. 2004;99:1333–1341. doi: 10.1111/j.1360-0443.2004.00806.x. [DOI] [PubMed] [Google Scholar]
- 9.Compton MT, Whicker NE, Hochman KM. Alcohol and cannabis use in urban, African American, first-episode schizophrenia-spectrum patients: associations with positive and negative symptoms. J Clin Psychiatry. 2007;68:1939–1945. doi: 10.4088/jcp.v68n1215. [DOI] [PubMed] [Google Scholar]
- 10.Zammit S, Moore THM, Lingford-Hughes A, et al. Effects of cannabis use on outcomes of psychotic disorders: systematic review. Br J Psychiatry. 2008;193:357–363. doi: 10.1192/bjp.bp.107.046375. [DOI] [PubMed] [Google Scholar]
- 11.Stroup D, Berlin J, Morton S, et al. Meta-analysis of observational studies in epidemiology. A proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 12.Sterne JAC, Bradburn MJ, Egger D. Meta-analysis in Stata. In: Egger M, Davey Smith G, Altman D, editors. Systematic Reviews in Health Care: Meta-analysis in Context. London, UK: BMJ Publishing Group; 2001. [Google Scholar]
- 13.Delucchi KL, Bostrom A. Methods for analysis of skewed data distributions in psychiatric clinical studies: working with many zero values. Am J Psychiatry. 2004;161:1159–1168. doi: 10.1176/appi.ajp.161.7.1159. [DOI] [PubMed] [Google Scholar]
- 14.Stata Corporation. Stata User's Guide. College Station, Tex: Stata Press; 2001. [Google Scholar]
- 15.Addington J, Addington D. Patterns, predictors and impact of substance use in early psychosis: a longitudinal study. Acta Psychiatr Scand. 2007;115:304–309. doi: 10.1111/j.1600-0447.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- 16.Akvadar Y, Turnuklu M, Akdede BB, Ulas H, Kitis A, Alptekin K. Substance use among patients with schizophrenia in a University Hospital. Bull Clin Psychopharmacol. 2004;14:191–197. [Google Scholar]
- 17.Altamura AC, Bassetti R, Bignotti S, Pioli R, Mundo E. Clinical variables related to suicide attempts in schizophrenic patients: a retrospective study. Schizophr Res. 2003;1:47–55. doi: 10.1016/s0920-9964(02)00164-0. [DOI] [PubMed] [Google Scholar]
- 18.Barnett JH, Werners U, Secher SM, et al. Substance use in a population-based clinic sample of people with first-episode psychosis. Br J Psychiatry. 2007;190:515–520. doi: 10.1192/bjp.bp.106.024448. [DOI] [PubMed] [Google Scholar]
- 19.Bersani G, Orlandi V, Kotzalidis GD, Pancheri P. Cannabis and schizophrenia: impact on onset, course, psychopathology and outcomes. Eur Arch Psychiatry Clin Neurosci. 2002;252:86–92. doi: 10.1007/s00406-002-0366-5. [DOI] [PubMed] [Google Scholar]
- 20.Carr VJ, Lewin TJ, Barnard RE, et al. Comparisons between schizophrenia patients recruited from Australian general practices and public mental health services. Acta Psychiatr Scand. 2002;105:346–355. doi: 10.1034/j.1600-0447.2002.1o156.x. [DOI] [PubMed] [Google Scholar]
- 21.Compton MT, Furman AC, Kaslow NJ. Lower negative symptom scores among cannabis-dependent patients with schizophrenia-spectrum disorders: preliminary evidence from an African American first-episode sample. Schizophr Res. 2004;71:61–64. doi: 10.1016/j.schres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Compton MT, Weiss PS, West JC, Kaslow NJ. The associations between substance use disorders, schizophrenia-spectrum disorders, and axis IV psychosocial problems. Soc Psychiatry Psychiatr Epidemiol. 2005;40:939–946. doi: 10.1007/s00127-005-0964-4. [DOI] [PubMed] [Google Scholar]
- 23.Compton MT, Esterberg ML, Druss BG, Walker EF, Kaslow NJ. A descriptive study of pathways to care among hospitalized urban African American first-episode schizophrenia-spectrum patients. Soc Psychiatry Psychiatr Epidemiol. 2006;41:566–573. doi: 10.1007/s00127-006-0065-z. [DOI] [PubMed] [Google Scholar]
- 24.Dervaux A, Bayle FJ, Laqueille X, et al. Is substance abuse in schizophrenia related to impulsivity, sensation seeking, or anhedonia? Am J Psychiatry. 2001;158:492–494. doi: 10.1176/appi.ajp.158.3.492. [DOI] [PubMed] [Google Scholar]
- 25.Deshmukh A, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Clinical signs of cerebellar dysfunction in schizophrenia, alcoholism, and their comorbidity. Schizophr Res. 2002;57:281–291. doi: 10.1016/s0920-9964(01)00300-0. [DOI] [PubMed] [Google Scholar]
- 26.Dubertret C, Bidard I, Ades J, Gorwood P. Lifetime positive symptoms in patients with schizophrenia and cannabis abuse are partially explained by co-morbid addiction. Schizophr Res. 2006;86:284–290. doi: 10.1016/j.schres.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Farrelly S, Harris MG, Henry LP, et al. Prevalence and correlates of comorbidity 8 years after a first psychotic episode. Acta Psychiatr Scand. 2007;116:62–70. doi: 10.1111/j.1600-0447.2006.00922.x. [DOI] [PubMed] [Google Scholar]
- 28.Fowler IL, Carr VJ, Carter NT, Lewin TJ. Patterns of current and lifetime substance use in schizophrenia. Schizophr Bull. 1998;24:443–455. doi: 10.1093/oxfordjournals.schbul.a033339. [DOI] [PubMed] [Google Scholar]
- 29.Gearon J, Bellack A. Sex differences in illness presentation, course, and level of functioning in substance-abusing schizophrenia patients. Schizophr Res. 2000;43:65–70. doi: 10.1016/s0920-9964(99)00175-9. [DOI] [PubMed] [Google Scholar]
- 30.Gut-Fayand A, Dervaux A, Olie JP, Lôo H, Poirier MF, Krebs MO. Substance abuse and suicidality in schizophrenia: a common risk factor linked to impulsivity. Psychiatry Res. 2001;102:65–72. doi: 10.1016/s0165-1781(01)00250-5. [DOI] [PubMed] [Google Scholar]
- 31.de Haan L, van Amelswoort T, Dingemans P, Linszen D. Risk factors for medication non-adherence in patients with first episode schizophrenia or related disorders; a prospective five year follow–up. Pharmacopsychiatry. 2007;40:264–268. doi: 10.1055/s-2007-992141. [DOI] [PubMed] [Google Scholar]
- 32.Hides L, Dawe S, Young R, Kavanagh DJ. The reliability and validity of the Severity of Dependence Scale for detecting cannabis dependence in psychosis. Addiction. 2006;102:35–40. doi: 10.1111/j.1360-0443.2006.01669.x. [DOI] [PubMed] [Google Scholar]
- 33.Holthausen EA, Wiersma D, Sitskoorn MM, et al. Schizophrenic patients without neuropsychological deficits: subgroup, disease severity or cognitive compensation? Psychiatry Res. 2002;15:1–11. doi: 10.1016/s0165-1781(02)00184-1. [DOI] [PubMed] [Google Scholar]
- 34.Kamali M, Kelly L, Gervin M, Browne S, Larkin C, O'Callaghan E. The prevalence of comorbid substance misuse and its influence on suicidal ideation among in-patients with schizophrenia. Acta Psychiatr Scand. 2000;101:452–456. doi: 10.1034/j.1600-0447.2000.101006452.x. [DOI] [PubMed] [Google Scholar]
- 35.Karam EG, Yabroudi PF, Melhem NM. Comorbidity of substance abuse and other psychiatric disorders in acute general psychiatric admissions: a study from Lebanon. Compr Psychiatry. 2002;43:463–468. doi: 10.1053/comp.2002.35910. [DOI] [PubMed] [Google Scholar]
- 36.Katz G, Durst R, Shufman E, Bar-Hamburger R, Grunhaus L. Substance abuse in hospitalized psychiatric patients. Isr Med Assoc J. 2008;10:672–675. [PubMed] [Google Scholar]
- 37.Kavanagh DJ, Waghorn G, Jenner L, et al. Demographic and clinical correlates of comorbid substance use disorders in psychosis: multivariate analyses from an epidemiological sample. Schizophr Res. 2004;66:115–124. doi: 10.1016/s0920-9964(03)00130-0. [DOI] [PubMed] [Google Scholar]
- 38.Kirkpatrick B, Amador XF, Flaum M, et al. The deficit syndrome in the DMS-IV field trial: I. Alcohol and other drug abuse. Schizophr Res. 1996;20:69–77. doi: 10.1016/0920-9964(95)00102-6. [DOI] [PubMed] [Google Scholar]
- 39.Margolese HC, Malchy L, Negrete JC, Tempier R, Gill K. Drug and alcohol use among patients with schizophrenia and related psychoses: levels and consequences. Schizophr Res. 2004;67:157–166. doi: 10.1016/S0920-9964(02)00523-6. [DOI] [PubMed] [Google Scholar]
- 40.Modestin J, Gladen C, Christen S. A comparative study on schizophrenic patients with dual diagnosis. J Addict Dis. 2001;29:41–51. doi: 10.1300/j069v20n04_05. [DOI] [PubMed] [Google Scholar]
- 41.Moilanen K, Veijola J, Läksy K, et al. Reasons for the diagnostic discordance between clinicians and researchers in schizophrenia in the Northern Finland 1966 Birth Cohort. Soc Psychiatry Psychiatr Epidemiol. 2003;38:305–310. doi: 10.1007/s00127-003-0638-z. [DOI] [PubMed] [Google Scholar]
- 42.Mueser KT, Yarnold PR, Rosenberg SD, Swett C, Miles KM, Hill D. Substance use disorder in hospitalized severely mentally ill psychiatric patients: prevalence, correlates, and subgroups. Schizophr Bull. 2000;26:179–192. doi: 10.1093/oxfordjournals.schbul.a033438. [DOI] [PubMed] [Google Scholar]
- 43.Rabinowitz J, Bromet EJ, Lavelle J, Carlson G, Kovasznay B, Schwartz JE. Prevalence and severity of substance use disorders and onset of psychosis in first-admission psychotic patients. Psychol Med. 1998;28:1411–1419. doi: 10.1017/s0033291798007399. [DOI] [PubMed] [Google Scholar]
- 44.Verdoux H, Liraud F, Gonzales B, Assens F, Abalan F, van Os J. Predictors and outcome characteristics associated with suicidal behaviour in early psychosis: a two-year follow-up of first-admitted subjects. Acta Psychiatr Scand. 2001;103:347–354. doi: 10.1034/j.1600-0447.2001.00202.x. [DOI] [PubMed] [Google Scholar]
- 45.Wade D, Harrigan S, Harris MG, Edwards J, McGorry PD. Pattern and correlates of inpatient admission during the initial acute phase of first-episode psychosis. Aust N Z J Psychiatry. 2006;40:429–436. doi: 10.1080/j.1440-1614.2006.01819.x. [DOI] [PubMed] [Google Scholar]
- 46.Wobrock T, Sittinger H, Behrendt B, D'Amelio R, Falkai P, Caspari D. Comorbid substance abuse and neurocognitive function in recent-onset schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2007;257:203–210. doi: 10.1007/s00406-006-0707-x. [DOI] [PubMed] [Google Scholar]
- 47.Xafenias A, Diakogiannis I, Iacovides A, Fokas K, Kaprinis G. Factors affecting hospital length of stay: is substance use disorder one of them? A study in a Greek public psychiatric hospital. Am J Addict. 2008;17:447–451. doi: 10.1080/10550490802269106. [DOI] [PubMed] [Google Scholar]
- 48.Ziedonis M, Trudeau K. Motivation to quit using substances among individuals with schizophrenia: implications for a motivation-based treatment model. Schizophr Bull. 1997;23:229–238. doi: 10.1093/schbul/23.2.229. [DOI] [PubMed] [Google Scholar]
- 49.Perälä J, Suvisaari J, Saarni SI, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64:19–28. doi: 10.1001/archpsyc.64.1.19. [DOI] [PubMed] [Google Scholar]
- 50.Shaw J, Hunt IM, Flynn S, et al. The role of alcohol and drugs in homicides in England and Wales. Addiction. 2006;101:1117–1124. doi: 10.1111/j.1360-0443.2006.01483.x. [DOI] [PubMed] [Google Scholar]
- 51.Kanato M. Drug use and health among prison inmates. Curr Opin Psychiatry. 2008;21:252–254. doi: 10.1097/YCO.0b013e3282fc985c. [DOI] [PubMed] [Google Scholar]
- 52.Folsom D, Jeste DV. Schizophrenia in homeless persons: a systematic review of the literature. Acta Psychiatr Scand. 2002;105:404–413. doi: 10.1034/j.1600-0447.2002.02209.x. [DOI] [PubMed] [Google Scholar]
- 53.Drake RE, Mueser KT. Alcohol-use disorder and severe mental illness. Alcohol Health Res World. 1996;2:87–93. [PMC free article] [PubMed] [Google Scholar]
- 54.Frances RJ. Schizophrenia and substance abuse. Psychiatr Ann. 1996;26:523–527. [Google Scholar]
- 55.Buckley P. Prevalence and consequences of the dual diagnosis of substance abuse and severe mental illness. J Clin Psychiatry. 2006;67:S5–S9. [PubMed] [Google Scholar]
- 56.United Nations. World Drug Report 2008. Office on Drugs and Crime, United Nations; 2008. [Google Scholar]
- 57.Andréasson S, Allebeck P, Engström A, Rydberg U. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;2:1483–1486. doi: 10.1016/s0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- 58.Zammit S, Allebeck P, Andréasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ. 2002;325:1199. doi: 10.1136/bmj.325.7374.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saha S, Chant D, McGrath J. Meta-analyses of the incidence and prevalence of schizophrenia: conceptual and methodological issues. Int J Methods Psychiatr Res. 2008;17:55–61. doi: 10.1002/mpr.240. [DOI] [PMC free article] [PubMed] [Google Scholar]