Abstract
In amniotes, BMP signaling from lateral plate and dorsal neural tube inhibits differentiation of muscle precursors in the dermomyotome. Here, we show that BMPs are expressed adjacent to the dermomyotome during and after segmentation in zebrafish. In addition, downstream BMP pathway members are expressed within the somite during dermomyotome development. We also show that zebrafish dermomyotome is responsive to BMP throughout its development. Ectopic overexpression of Bmp2b increases expression of the muscle precursor marker pax3, and changes the time course of myoD expression. At later stages, overexpression increases the number of Pax7+ myogenic precursors, and delays muscle differentiation, as indicated by decreased numbers of MEF2+ nuclei, decreased number of multi-nucleated muscle fibers, and an increased myotome angle. In addition, we show that while BMP overexpression is sufficient to delay myogenic differentiation, inhibition of BMP does not detectably affect this process, suggesting that other factors redundantly inhibit myogenic differentiation.
Keywords: myogenesis, BMP4, zebrafish, pax7, dermomyotome, myotome
INTRODUCTION
In vertebrates, skeletal muscle is derived from reiterated epithelialized spheres of mesoderm on either side of the midline known as somites. During the differentiation of somites the external portion develops into the dermomyotome, in which reside skeletal muscle precursors for the trunk and limbs. Myogenic precursors in the dermomyotome express Pax3 and Pax7, two genes required for normal embryonic and postnatal myogenesis. Within the dermomyotome these Pax3/Pax7 expressing cells proliferate, transition into Muscle Regulatory Factor (MRF) expressing myoblasts as they translocate into the myotome, and finally differentiate into myosin expressing muscle fibers (for review, see Buckingham and Vincent, 2009).
Development of cells in the dermomyotome is primarily regulated by signals from surrounding tissues. Hedgehog (Hh) from the notochord and ventral neural tube promotes the differentiation of Pax3/7 expressing muscle precursors into Muscle Regulatory Factor (MRF) expressing myoblasts (Chiang et al., 1996; Feng et al., 2006). In amniotes, in addition to Hh, Wnts from the surface ectoderm promote the differentiation of muscle precursors in the dermomyotome, and BMP from the dorsal neural tube and ectoderm and lateral plate mesoderm inhibits the differentiation of dermomyotome into muscle (Munsterberg et al., 1995; Reshef et al., 1998). The balance of all of these signals regulates the timing of muscle development, and the amount of skeletal muscle formed.
In chick, inhibition of BMP signaling by overexpression of the secreted BMP inhibitor Noggin in the dermomyotome results in a decrease in the expression of Pax3 and an increase in the expression of MRFs (Reshef, et al. 1998). In addition, overexpression of BMP results in an increase in the expression of Pax3, and a decrease in the expression of MyoD. Thus, BMP may inhibit the differentiation of Pax3/7 expressing cells into MRF expressing myoblasts, perhaps by promoting their proliferation (Amthor et al., 1999). In axolotl, an anamniote tetrapod, BMP signaling has also been shown to regulate development of the dermomyotome, and inhibition of BMP signaling in the somite causes a down regulation of Pax7 within the dermomyotome, where overexpression of BMP results in upregulation of Pax7 (Epperlein et al., 2007).
The zebrafish dermomyotome generates lateral myotome fast muscle fibers that differentiate between the very early developing superficial slow muscle and the early developing deep fast muscle fibers (Stellabotte et al., 2007). Hedgehog, Fgf8, and Retinoic Acid signaling have all been implicated in dermomyotome development in zebrafish, but the role of BMP signaling has not been examined (Groves et al., 2005; Hamade et al., 2006; Hammond et al., 2007). BMP signaling regulates the development of slow muscle fibers in zebrafish, antagonizing the differentiation-promoting effects of Hedgehog signaling (Du et al., 1997; Kawakami et al., 2005).
Here, we show that BMP signaling regulates dermomyotome development in the zebrafish embryo. In 24h zebrafish, bmp2b and bmp4 are expressed dorsal and ventral to the dermomyotome, reminiscent of their expression domains in amniote embryos during dermomyotome differentiation. We find that Pax7+ dermomyotomal cells respond to BMP signaling, evident by the presence of phosphorylated Smad. We also show that Pax7+ dermomyotomal cells are responsive to BMP signaling, and that overexpression of Bmp2b is sufficient to delay the differentiation of these cells. However, we also demonstrate that BMP is not necessary for the differentiation of dermomyotomal cells by inhibiting BMP signaling via a heat shock inducible dnBMP receptor (to avoid the confounding effects of BMP inhibition during gastrulation found in BMP mutants), and that inhibition of BMP signaling during somite development does not lead to detectable changes in myogenesis.
RESULTS
Bmp signaling components are expressed within and adjacent to the developing dermomyotome
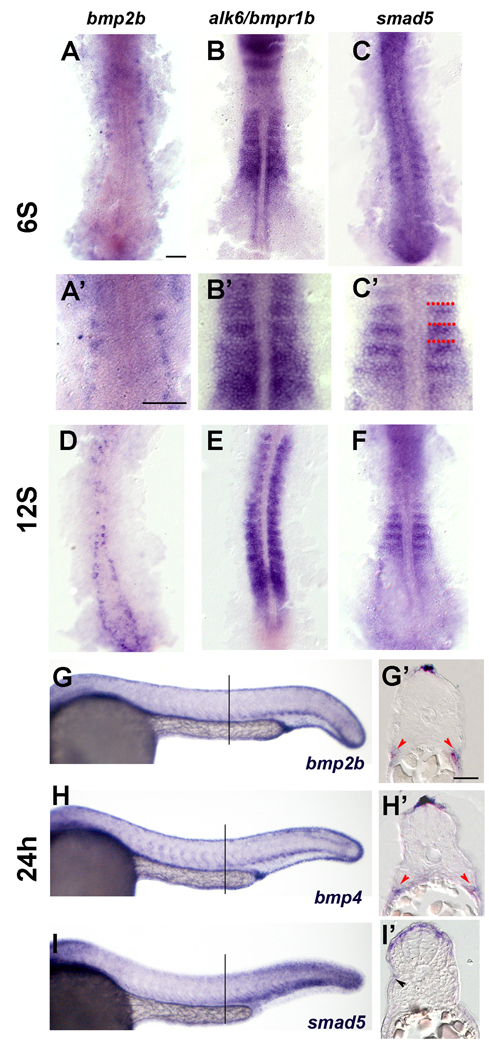
The expression pattern of bmp2b and bmp4 is consistent with a role in dermomyotome regulation (Stickney, et al., 2007; Dick, et al., 1999). During early somite patterning, at the 6S stage, bmp2b is expressed in cells adjacent to the myotome, which appear to be the presumptive Rohon-Beard neurons (Fig. 1A, A’). At the 12S stage, expression of bmp2b remains in this population of cells, which by this time have moved to a more medial position (Fig. 1D). By 24h, expression of bmp2b and bmp4 is in cells dorsal and ventral to the somite (Fig. 1G, G’, H, H’). The BMP expressing cells adjacent to the dorsal somite are within the dorsal ectoderm, immediately dorsal to the neural tube. In the ventral portion of the somite, the bmp2b and bmp4 expressing cells appeared to belong to the lateral plate mesoderm (Fig. 1G, G’, H, H’). These regions are adjacent to the dermomyotomal cells, and are in the appropriate position to signal to the dermomyotome.
Figure 1. Expression of BMP pathway members in the trunk at 24h.
(A–C). Expression of BMP pathway members at 6S. (D–F) Expression of BMP pathway members at 12S. (G–I’) Expression of BMP pathway members at 24h. A. Expression of bmp2b at the 6S stage. Expression is in Rohon-Beard neurons. A’. Close up of somites in A. B. Expression of alk6/bmpr1b is throughout the somite. B’ Close up of somites in B. C. Expression of smad5 is in the anterior border cells (ABCs). C’ Close up of somites in C, red dots indicate somite boundaries. D. bmp2b expression is found in Rohon-Beard cells, which have moved medially and dorsally. E. alk6/bmpr1b is expressed throughout the somites. F. Smad5 is expressed in the anterior most cells in the newly formed somites, and more diffusely in older somites. G. Whole mount expression of bmp2b at 24h. Bmp2b is expressed in the dorsal and ventral trunk. G’: Transverse section of embryo labeled in whole mount for bmp2b. Bmp2b is expressed in the dorsal most ectoderm, dorsal to the neural tube. Bmp2b is also expressed ventral to the myotome, in the presumptive lateral plate mesoderm (red arrowheads). H. Whole mount expression of bmp4 at 24h. Bmp4 is expressed in a pattern similar to the expression pattern of bmp2b. H’: Transverse section of embryo labeled in whole mount for bmp4. Bmp4 is expressed in the dorsal ectoderm and presumptive lateral plate (red arrowheads). I. Whole mount expression of Smad5 at 24h. Smad5 is expressed highly in the dorsal and ventral portions of the somite, especially in posterior somites. I’: Transverse section of embryo labeled in whole mount for smad5. High level of smad5 expression is evident in the dorsal somite, adjacent to the bmp expressing domain. Some fainter expressing cells are evident on the surface of the myotome (black arrowhead). Lines in G, H, and F indicate position of sections in each respective insert. Scale bars: 50µm in A (for A–C, A’-C’); 25µm in G’ (for G’–I’).
Downstream components of the BMP pathway, including the BMP receptors and Smads, are expressed in somite cells during the period of dermomyotome specification and myogenesis (Dick, et al., 1999). The BMP receptor, alk6/bmpr1b is expressed throughout the somite (Fig. 1B, E). During somitogenesis, smad5, one of the Smads responsible for transduction of the Bmp2b and Bmp4 signals, is expressed in the anterior portion of the somite, which contains the cells that give rise to the later lateral Pax7 expressing cells (Hollway et al., 2007; Stellabotte et al., 2007). At 12S, expression of smad5 remains in the anterior portion of the somite in the six most recently formed somites, and is more diffuse in older, more anterior somites (Fig. 1F). At later stages, smad5 expression is medial to the bmp2b and bmp4 expression domains, in cells on the surface of the somite (Fig. 1I, I’). This expression is deep to the skin, and external to the slow muscle fiber layer, in the same position as the Pax7 expressing dermomyotome (Barresi et al., 2001; Devoto et al., 2006).
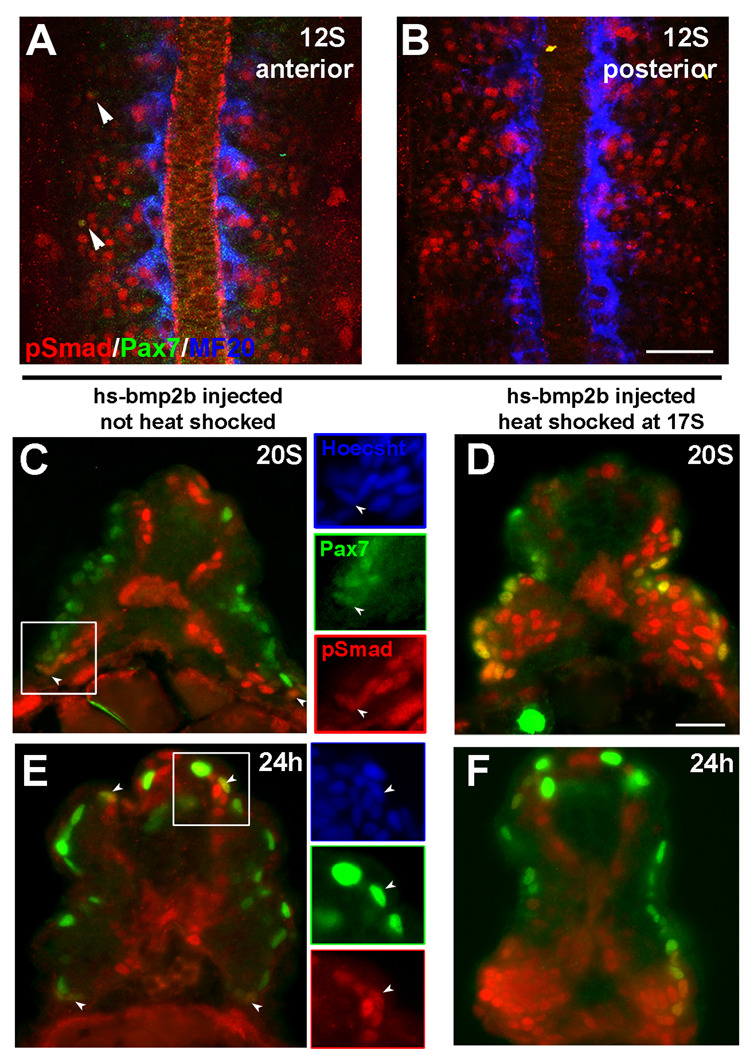
During BMP signaling, BMP binds to its receptors, which in turn phosphorylate the downstream effectors of the pathway, the Smad proteins. This allows the Smad complex to translocate to the nucleus and activate or repress transcription of BMP responsive genes. Therefore, expression of phosphorylated Smad (pSmad) indicates activated BMP signaling. We used an antibody that specifically recognizes the phosphorylated version of Smads 1, 5 and 8, to identify cells in the somite that have activated BMP signaling (Fig. 2). At 12S, there is pSmad labeling throughout the somite, including within some lateral cells in the anterior somites that also express Pax7 (Fig. 2A,B). In a 20S embryo, pSmad+ cells are in the medial portion of the somite, as well as at the dorsal and ventral extremes (Fig. 2C). A portion of these dorsal and ventral pSmad+ cells are also positive for Pax7, indicating that a subpopulation of the myogenic precursor population actively responds to BMP signaling. The distribution of pSmad at 24h is similar to the pattern at 20S, although there are fewer pSmad/Pax7 cells than at 20S, possibly indicating a decrease in the level of BMP signaling between 20S and 24h (Fig. 2E). This is also true at the 22S stage, where pSmad/Pax7 cells make up approximately 20% of the Pax7 expressing population within the somite (Fig. 6E).
Figure 2. Phospho-Smad 1, 5, 8 (pSmad) distribution.
(A–C, E) Normal pattern of pSmad labeling, (D, F) Pattern of pSmad following overexpression of Bmp2b. A. Pattern of phosphorylated Smad 1, 5, and 8 (red) in the anterior somites of 12S stage embryo. pSmad is distributed throughout the somite. A subset of lateral pSmad+ cells also express Pax7 (green, arrowheads). B. Pattern of pSmad (red) in the posterior somites of a 12S stage embryo. (C–F) Transverse sections labeled for pSmad (red) and Pax7 (green). C. At 20S pSmad is found in Pax7+ myogenic precursors at the dorsal and ventral regions of the somite (arrowheads). Inset panels show a Pax7+ nucleus (green) that is also pSmad+ (red). pSmad labeling is also seen in the medial portion of the somite. D. pSmad in a 20S embryo following heat shock induction of Bmp2b overexpression at 17S. Ectopic activation of BMP signaling is still evident at this time, including in the Pax7 expressing population (green). Because of the mosaic distribution of the plasmid DNA following injection, the pattern of ectopic activation can vary. In this case, high levels of ectopic pSmad (red) are seen in the ventral somite. E. pSmad labeling in a 24h embryo. A subset of Pax7+ myogenic precursors (green) at the dorsal and ventral regions of the somite are also pSmad+ (red). Inset panels indicate cells that are both Pax7+ and pSmad+. F. Distribution of pSmad at 24h following heat shock induction of Bmp2b overexpression at 17S. High level of ectopic BMP activation is evident throughout the somite (pSmad, red), including a high proportion of the Pax7 expressing myogenic precursors(green). Scale bars: 50µm in B (for A,B), 25µm in D (for C–F)
Figure 6. The effect of inhibition of BMP signaling on muscle differentiation.
A. Expression of Myogenin (MGN, red) and Pax7 (green) at 24h in an uninjected embryo. B. Expression of Pax7 (green) and MGN (red) at 24h following heat shock induction of Chordin at dome stage. Somitic Pax7 expression is still apparent. C. Normal Expression of myoD at 12S. D. Expression of myoD at 12S following heat shock induction of TBR at 3S. Inhibition of BMP signaling with TBR does not change the early expression of myoD compared to WT siblings. E. Quantification of pSmad labeling in 22S embryos following heat shock induction of TBR at 18S. The number of pSmad expressing cells in embryos containing the transgene (red, hatched) is reduced by approximately 40% compared to the WT siblings (red, solid). There is no significant change in the number of pSmad/Pax7 double (yellow, hatched) labeled cells, or the number of Pax7 (green, hatched) expressing cells compared to the WT siblings (solid bars). N=5 for all groups F. Quantification of Myogenin expressing cells at the dorsal and ventral thirds of the somite following heat shock of the TBR clutch analyzed in (E). There is no significant change in the number of cells expressing Myogenin (red, hatched), Pax7 (green, hatched), or both Myogenin and Pax7 (yellow, hatched) in transgenic embryos when compared to WT siblings (solid bars). N=5 for all groups. Scale bars: 50µm
Overexpression of Bmp2b leads to an increase in the number of Pax7+ myogenic precursors
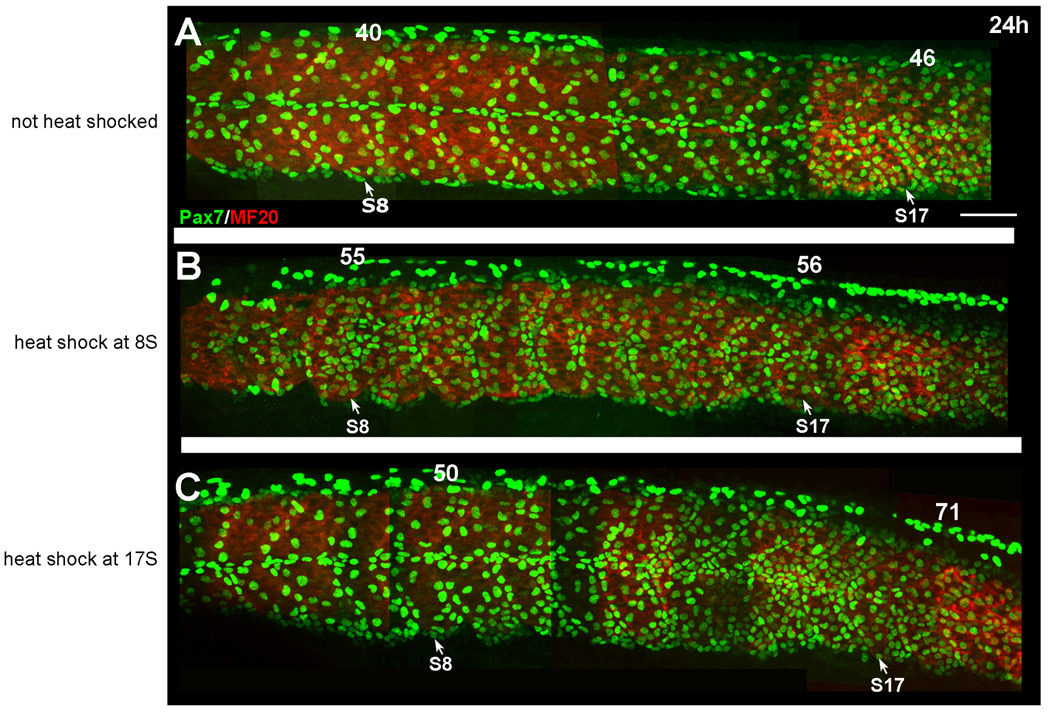
To activate BMP during dermomyotome development without affecting early dorsoventral patterning, we injected a construct containing Bmp2b driven by a heat shock inducible promoter (hs-bmp2b). Without heat shock, pSmad levels were the same as those in uninjected embryos (Fig. 2C, E, data not shown). Following heat shock at 17S, many more cells were pSmad+ at both the 20S stage (1.5 h. post heat shock, Fig. 2D) and at 24h (6.5 h. post heat shock, Fig. 2F). These additional pSmad+ cells included Pax7-positive cells and indicated that there was ectopic BMP signaling within the dermomyotome. When we ectopically overexpressed Bmp2b beginning at the 8S stage, we saw an increase in the number of Pax7+ myogenic precursors on the surface of the somite in somites along the anterior-posterior axis at 24h (staged by both eye pigmentation and number of somites; Fig. 3B) compared to non-heat shocked controls (Fig. 3A). In these embryos, the organization and differentiation of the myotome appeared to be delayed as well, suggesting that the increase in Pax7+ myogenic precursors may be at the expense of differentiated muscle fibers. We saw a similar pattern of Pax7 expression in embryos heat shocked at the 3S stage (data not shown). To determine if dermomyotomal cells are responsive to BMP signaling late in their development, we also overexpressed Bmp2b at 17S. When we induced overexpression of BMP at the 17S stage, we saw a large increase in the number of Pax7+ myogenic precursors in the posterior trunk and tail (Fig. 3C). The effect of BMP expression on the muscle precursor population in the anterior somites is less severe, but we found an increase in the number of Pax7 cells in these somites as well (Fig. 3C), suggesting that the dermomyotome in more mature somites can also respond to BMP overexpression. No difference was found in the level of cell proliferation between control embryos and embryos overexpressing BMP (data not shown), suggesting that the increase in Pax7+ cells is not due to an increase in cell proliferation in the whole somite or Pax7 expressing population.
Figure 3. Expression of Pax7 at 24h following heat shock induction of Bmp2b.
A. Expression of Pax7 (green) in embryos that were injected with the hs-bmp construct, but not heat shocked. Pax7 cells are distributed along the borders of the myotome and in the horizontal myosepta. Myosin is shown in red. B. Pax7 expression (green) in an embryo that was heat shocked at 8S to induce overexpression of BMP. The number of Pax7 expressing cells is increased in somites along the anterior-posterior axis. C. Pax7 expression in an embryo that was heat shocked at the 17S stage to induce overexpression of BMP. A strong increase in the number of Pax7 expressing cells is evident in the posterior somites, particularly around somite 17. There is also a smaller increase in the number of Pax7 expressing cells in the anterior portion of the somite. Numbers above somites indicate the number of Pax7+ nuclei in that somite. Scale bar: 50µm
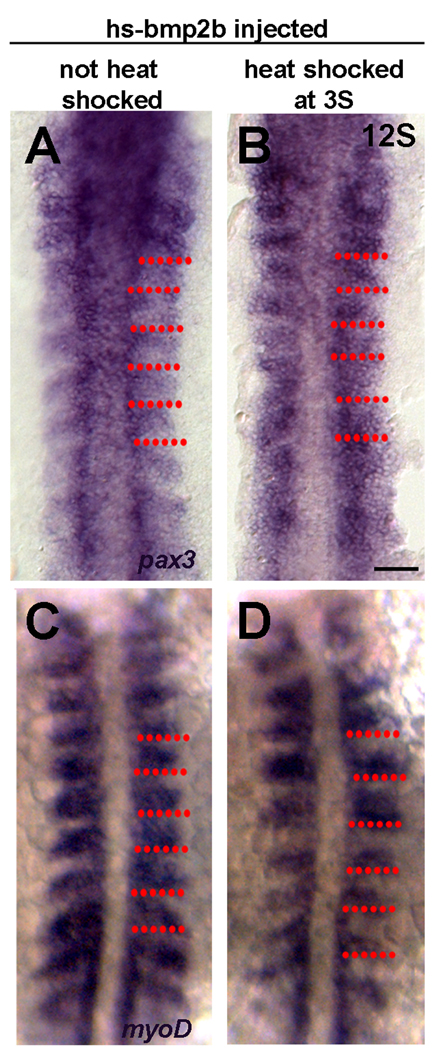
To determine if BMP overexpression affects the early development of dermomyotomal precursors, we examined the expression of pax3 during segmentation stages via in situ hybridization (an antibody to Pax3 is not available for zebrafish). At 12S, the dermomyotomal precursors are in the anterior domain of the somite and express pax3 (Fig. 4A, Feng et al., 2006). Following overexpression of BMP, there is an upregulation of pax3 in the posterior portion of the somite such that the domain of pax3 expression encompasses the majority of the somite, suggesting an increase in the number of myogenic precursors (Fig. 4B). The expansion of pax3 expression is consistent with the increase in Pax7+ myogenic precursors also noted above (Fig. 3).
Figure 4. Early changes in differentiation following overexpression of Bmp2b.
A. Expression of pax3 in a 12S non-heat shocked control embryo. Pax3 is expressed by the anterior border cells (ABCs). B. Expression of Pax3 at 12S following heat shock induction of Bmp2b overexpression at 3S. pax3 expression is no longer confined to the anterior cells, and is expressed throughout the somite. C. Expression of myoD in a 12S non-heat-shocked control embryo. Myod is expressed throughout the posterior somite, and excluded from the ABCs. D. Expression of myoD at 12S following heat shock induction of Bmp2b overexpression at 3S. The domain of myoD expression is decreased. Red dots indicate somite boundaries. Scale bar: 50µm
Overexpression of Bmp2b leads to a delay in differentiation of cells in the myotome
In amniotes, BMP inhibits the differentiation of myogenic precursors into MRF expressing myoblasts. To determine if the effect of Bmp2b on dermomyotomal development in zebrafish is also on the differentiation of myogenic precursors, we looked at the expression of MRFs following overexpression of Bmp2b. In uninjected embryos at 12S, myoD is expressed throughout the posterior somite, and excluded from the anterior border (Fig. 4C). In embryos ectopically overexpressing Bmp2b, we found a decrease in the expression domain of myoD in the somites (Fig. 4D). At the 18S stage myoD is expressed highly throughout the myotome, and dermomyotomal precursors have moved to the lateral surface of the anterior somites. In 18S embryos overexpressing Bmp2b, there was also a decrease in myoD expression in the somites compared to the non-heat shocked control embryos, suggesting a decrease in the differentiation of muscle precursors (Fig. 5A, B). At 24h, the domain of myoD expression in the somite was broader in embryos overexpressing Bmp2b than in non-heat shocked control embryos (Fig. 5C, D). This pattern of expression is similar to that seen in somites of younger embryos, where fewer cells have differentiated from MRF expressing cells into terminally differentiated muscle fibers (compare to Fig. 5A).
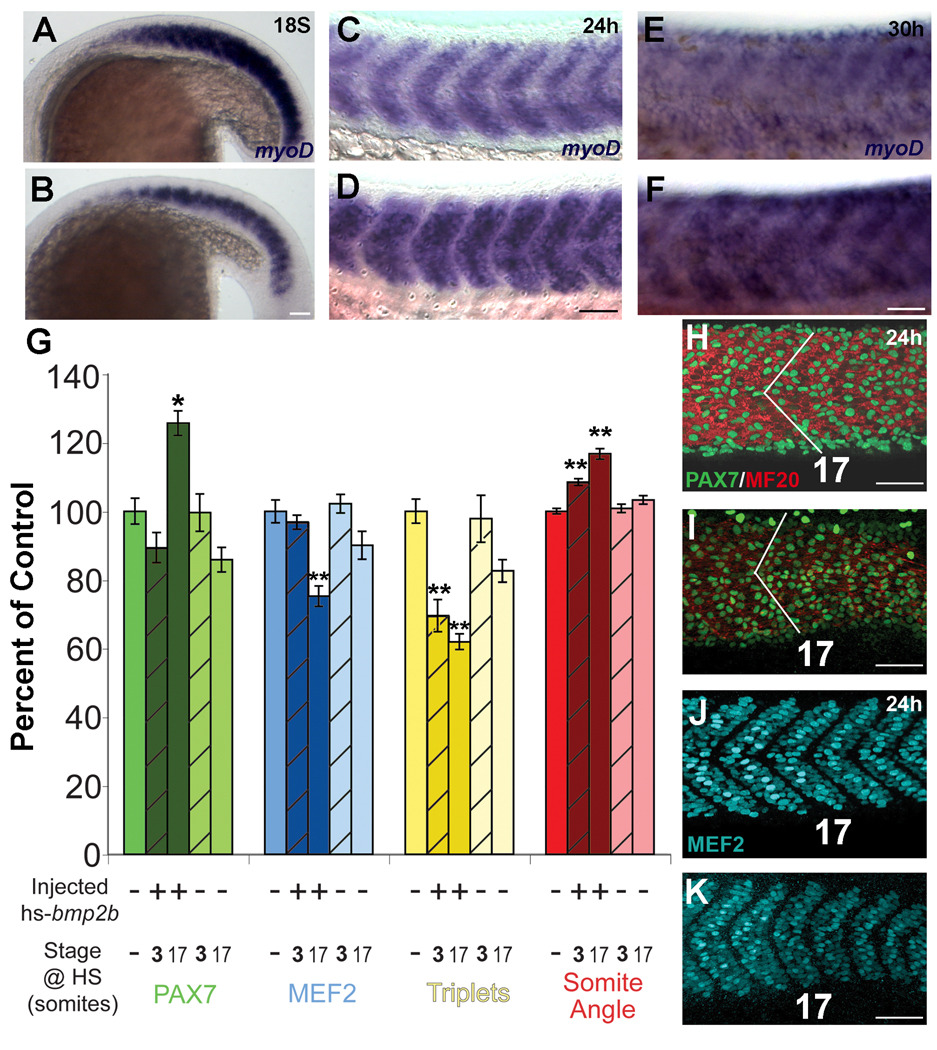
Figure 5. Differentiation of muscle following overexpression of Bmp2b.
Differentiation of muscle following ectopic overexpression of Bmp2b. A, C, E: Normal differentiation of muscle. B, D, F: Differentiation of muscle in the presence of ectopic Bmp2b. A. Expression of myoD at the 18S stage in an embryo that was not injected or heat shocked. B. Expression of myoD at the 18S stage following heat shock induction of Bmp2b at the 3S stage. The domain and expression level of myoD is reduced compared to the non-injected control embryos. C. Expression of myoD at the 24h stage in an embryo that was injected with the hs-bmp2b construct, but was not heat shocked. At 24h, myoD expression has begun to decrease in the somite as the cells differentiate into myosin expressing fibers, and is present in a stripe in the anterior-posterior middle of the myotome. D. Expression of myoD at the 24h stage following heat shock induction of Bmp2b at the 3S stage. The domain of myoD is increased compared to non-heat shocked control embryos and resembles the domain of expression in control 18S embryos. E. Expression of myoD at the 30h stage in an embryo that was injected with the hs-bmp2b construct, but was not heat shocked. F. Expression of myoD at the 30h stage following heat shock induction of Bmp2b at the 3S stage. The level of myoD expression is higher than in non-heat shocked control embryos, and appears similar to a 24h control embryo. G. Maturity assays for somite 17 after heat shock, compared to control. All measurements are expressed as a percentage of control (mean set to 100%). Measurements were: total number of Pax7+ cells (green bars), total number of MEF2+ cells (blue bars), total number of muscle fibers with triplet nuclei (yellow bars), and somite angle (red bars). Controls are medium color bars (first in each set), bmp2b-injected are dark bars, and non-injected are light bars. Bars for 3S heat shocked embryos are hatched. * = significant difference from control at p<0.001, ** = difference from control at p<0.0001; lack of an asterisk indicates no statistical difference from control. A significant increase (~25%) in Pax7+ cells was seen in bmp2b-injected 17S heat shocked embryos (compare H and I), while all others were not different from control. A significant decrease (~25%) was seen in MEF2+ cells in bmp2b-injected 17S heat shocked embryos, while all others were not different from control (compare J and K). A significant decrease (20–40%) in number of fibers with triplet nuclei was seen in bmp2b-injected embryos when heat shocked at either 3S or 17S. A significant increase in somite angle (10–20%) was seen in bmp2b-injected heat shocked embryos at either 3S and 17S (compare angle in H and I). All error bars = standard error. N=10 for each group within each category. H–K: Changes in cell composition and morphology of somite 17 at 24h stage in control (H,J) and hs-bmp2b-injected embryos heat shocked at stage 17 (number indicates somite number). H. Pax7 (green) and MF20 (red) antibody staining in a 24h stage control embryo. I. Pax7 (green) and MF20 (red) antibody staining in a hs-bmp2b-mCherry injected 17S heat shocked embryo. Note the increase in both number of Pax7+ cells and somite angle (white lines). J. MEF2 antibody staining in a 24h stage control embryo (same individual as H). K. MEF2 antibody staining in a hs-bmp2b-mCherry injected 17S heat shocked embryo (same individual as I). Note the decrease in the number of MEF2+ cells. Scale bars: 50 µm.
As a separate measure of the differentiation state of the myotome, we examined the expression of myosin (MF20) in 24h embryos that had been induced to express excess BMP beginning at the 3S stage. Embryos overexpressing Bmp2b appear to be less differentiated than uninjected control embryos. The pattern of myosin is similar to that of a younger embryo, consistent with the change in myoD expression in embryos with overexpression of Bmp2b. In the 24h embryos with ectopic overexpression of Bmp2b, the somites lack the characteristic V-shape, and the fibers are less well aligned than uninjected controls, consistent with a delay in differentiation. Similar effects on somite shape have been seen when disrupting BMP signaling (Stickney et al., 2007). To determine if the effect of Bmp2b overexpression at later stages was consistent with a delay in differentiation, we examined the expression of myoD in somites of 30h embryos with ectopic overexpression of Bmp2b beginning at 3S (Fig. 5E, F). In these embryos, myoD expression resembles that in a non-heat shocked 24h embryo, supporting our interpretation that Bmp2b overexpression causes a delay in the differentiation of the myotome.
To test our hypothesis that ectopic Bmp2b overexpression causes a delay in the differentiation of dermomyotome cells into myotome, we documented four different measures of maturation in somite 17. We used the number of Pax7+ cells to quantify the number of undifferentiated dermomyotome cells. We used the number of cells expressing MEF2, a transcription factor expressed in all nascent and mature muscle fibers (Hinits and Hughes, 2007), to quantify number of muscle nuclei. We measured somite angle, which decreases as the somite matures from the epithelialized sphere shape into the classic chevron shape (Kimmel, et al., 1995), as a measure of myotome maturation. Muscle fibers become multinucleated as they mature, and we used the number of fibers with three nuclei as the final measure of muscle maturation. Between 22h and 24h in wildtype, uninjected embryos, we found that as the relative number of Pax7+ cells decreased by 26%, the number of MEF2+ cells increased by 32%, the number of muscle fibers with three nuclei (a measure of fusion) increased by 70%, and the angle formed by the myotomes decreased by 6%. Bmp2b overexpressing embryos showed a delay in all indicators of myotome maturation when compared to controls (Fig. 5G). There were 25% more Pax7+ cells in hs-bmp2b-injected embryos heat shocked at 17S compared to both non-injected, non-heat shocked embryos (p<0.001, compare Fig. 5H, I) and to non-injected, heat shocked embryos (all p<0.001, Fig. 5G). Hs-bmp2b-injected embryos heat shocked at 17S had 25% fewer MEF2+ cells compared to control (compare Fig. 5J, K, p<0.0001), and had significantly fewer MEF2+ cells than all other treatment groups (Fig. 5G), while non-injected embryos heat shocked at 17S showed no significant decrease in the number of MEF2+ cells compared to control. The reduction in the number of MEF2+ cells and the apparent reduction of MF20 labeling is consistent with the shift in myoD expression (Fig. 4C, D). The number of muscle fibers with three nuclei decreased by ~40% in hs-bmp2b-injected embryos heat shocked at 17S and ~30% in hs-bmp2b-injected embryos heat shocked at 3S compared to control (both p<0.0001, Fig. 5G). Somite angle increased in hs-bmp2b-injected embryos heat shocked at 17S by 16% versus control (p<0.0001, compare angles in Fig. 5H, I), while the angle in hs-bmp2b-injected embryos heat shocked at 3S increased by 8% compared to control (p<0.0001, Fig. 5G). hs-bmp2b-injected embryos heat shocked at 17S had significantly wider somite angles than all other groups (p<0.001 compared to 3S heat shocked, p<0.0001 for all other comparisons).
Inhibition of BMP signaling does not effect the development of Pax7 cells of the dermomyotome
Since BMP overexpression results in an increase in the number of Pax7+ cells in the somites and a delay in the expression of MRFs, we predicted that inhibition of BMP signaling would cause a loss of Pax7+ cells, and an increase in expression of MRFs. We first looked for changes in Pax7 expression in embryos in which BMP signaling was globally inhibited beginning in gastrulation. For these experiments, we inhibited BMP signaling by injecting a construct with a heat shock promoter driving expression of Chordin, a secreted BMP inhibitor, and induced expression of this construct at dome stage (Tucker et al., 2008). At 24h, these embryos are severely dorsalized, missing much of the tail tissue (data not shown). Despite this dramatic dorsalization, Pax7+ cells are still present on the surface of the somites, as in uninjected controls (Fig. 6A, B), indicating that inhibition of BMP during gastrulation does not lead to a loss of the dermomyotome. However, because of the abnormal patterning of the embryos, it was not possible to quantify the effect of BMP inhibition on myogenic differentiation. In addition, the time between inhibition of BMP and labeling for dermomyotome may have allowed later BMP signaling to influence the dermomyotome.
To determine if BMP is required for the development of the dermomyotome later in development, we inhibited BMP signaling during somite patterning. We inhibited BMP signaling using a transgenic zebrafish line that has a dominant negative form of BMP receptor IA under the control of a heat shock inducible promoter (TBR) (Pyati et al., 2005; Pyati et al., 2006). We first tested whether BMP signaling is required for the development of precursors to the dermomyotome—while they are in the anterior portion of the somite. We saw no effect of heat shock induction of the dnBMP receptor at the 3S stage, on either the level or the distribution of myoD in the somites at 12S compared to WT siblings. (Fig. 6C, D).
We also looked at the effect of inhibition of BMP signaling on Pax7 expression at 24h after inhibiting BMP signaling by heat shocking TBR embryos at 10S. BMP overexpression at or close to this stage has a strong effect on Pax7 expression along the A–P axis (Fig. 3B). In contrast, BMP signaling inhibition caused no detectable effect on Pax7 expression (data not shown). As the somitic cells that are actively responding to BMP signal in normal embryos are exclusively in the dorsal and ventral regions of the somite, we quantified the number of Pax7+ cells in the dorsal- and ventral-most third of the somite. For these experiments we used a shorter window of time between heat shock induction and fixation, inducing expression of the dnBMP receptor at the 18S stage, and fixing at 22S, to ensure that BMP signal had not recovered fully at the time of analysis. In addition, we found 22S to be the period with the highest number of Pax7/pSmad double-labeled cells, suggesting that is the peak period of BMP effect on Pax7+ cells. In these embryos, although pSmad levels decreased following heat shock indicating a decrease in BMP signaling, we did not detect a significant change in the number of Pax7 cells in the somite, and overall proportion of Pax7, pSmad double-labeled cells did not change (Fig. 6E, N=5). There was also no significant increase in the number of Myogenin+ cells, suggesting that there is no change in the differentiation of myogenic precursors following inhibition of BMP signaling (Fig. 6F, N=5). A lack of effect on Pax7 expression could be a result of redundancies in the BMP pathway such that another pathway not affected by the dnBMP receptor is sufficient, or it may be that TBR allows a sufficient level of BMP signaling for dermomyotome differentiation to occur (see Discussion).
DISCUSSION
Here, we have demonstrated that cells in the zebrafish dermomyotome are responsive to BMP signaling throughout their early development, and that BMP can regulate the timing of differentiation of these cells. In the presence of ectopic, extra BMP, myogenic precursors are inhibited from differentiating into MRF expressing myoblasts. However, although BMP is sufficient to inhibit myogenic differentiation, it does not appear to be necessary. The inhibition of BMP signaling does not affect myogenesis, suggesting that there is redundancy in the inhibition of myogenic differentiation.
BMP regulates the development of myogenic precursors at multiple stages of their development
The progression of expression of BMP pathway members during somite development in zebrafish suggests that BMP may regulate the development of dermomyotomal cells throughout their development—including their spatial positioning in the anterior row of the epithelial somite and after they reach the lateral surface. We have shown that throughout muscle development, the domain of bmp2b expression is adjacent to the developing dermomyotome (Fig. 1). During early development of the dermomyotome, bmp2b is expressed in the presumptive Rohon-Beard neurons, adjacent to the developing paraxial mesoderm (Fig. 1A, A‘, D). At the end of segmentation, bmp2b and bmp4 are expressed in distinct domains adjacent to the dorsal and ventral aspects of the somite (Fig. 1G, H). Near the dorsal somite, the dorsal ectoderm expresses bmp2b and bmp4, and near the ventral somite, the lateral plate mesoderm also expresses bmp2b and bmp4.
The expression of BMP signaling components also supports the idea that BMP signaling regulates the development of myogenic precursors throughout their development. During somitogenesis, somitic cells express BMP signal transduction genes, including bmpr1b (Alk6; Fig. 1B, B‘, E) and smad5 (Fig. 1C, C‘, F). Interestingly, smad5 is expressed most abundantly by cells in the anterior portion of the somite, which have been shown to give rise to the Pax7 expressing myogenic precursors on the lateral surface of the somite during later stages of development. Smad1 is expressed in a wider domain, with expression throughout the somite during early somite development (Dick et al., 1999; Muller et al., 1999). Activated Smad is also distributed throughout the somite at the earliest detectable stages where dermomyotome precursors are present (Fig. 2A), and the pattern of Smad activation correlates with the combined expression of smad1 and smad5 mRNAs. At 24h, smad5 expressing cells are on the surface of the myotome, corresponding to the location of Pax7 expressing dermomyotomal cells, suggesting that cells much later in maturation can still respond to BMP signaling. Activated Smad is found in only a subset of the Pax7 expressing cells on the surface of the somite, similar to that found for chick (Faure et al., 2002), suggesting that BMP signaling is regulating the development of at least some of the dermomyotomal cells at any given moment, specifically at the dorsal and ventral extremes of the somite. Therefore, pSmad labeling shows a “snapshot” of which cells are responding at that moment to BMP. As this population is continuously replenished as they differentiate (i.e. not static), a significant proportion of dermomyotome cells will be influenced by BMP signaling throughout development. Our work corroborates and extends previous findings on the expression of BMPs and Smads in the developing zebrafish somite (Dick et al., 1999).
The effect of overexpression of BMP on the dermomyotome supports our interpretation that the zebrafish dermomyotome is responsive to BMP signaling throughout its development. BMP overexpression at the 3S stage led to an expansion of the pax3 expressing anterior domain (Fig. 4A, B), and a reduction in the myoD expressing posterior domain (Fig. 4C, D). As the anterior domain gives rise to dermomyotome, these effects suggest that BMP promotes the development or maintenance of dermomyotome precursors at the expense of myotome, while they are still at the anterior border of newly formed somites. While both Pax7 and MEF2 cell numbers in hs-bmp2b 3S heat shocked embryos returned to control levels by 24h (Fig. 5G), the continued reduction of multinucleated fibers and increase in somite angle indicate a lasting effect of ectopic bmp2b overexpression. BMP overexpression at late segmentation stages led to a similar expansion of the dermomyotome (Fig. 3). The expansion of dermomyotome in the anterior somites of these embryos, while not as extensive as in posterior somites, indicates that dermomyotomal cells remain responsive to BMP quite late in their development.
Embryos injected with hs-bmp2b and heat shocked at various stages display marked changes from control that cannot be accounted for by heat shock alone (Fig. 5G). With respect to somite 17, hs-bmp2b-injected embryos heat shocked at the 17S stage show more dramatic changes than those heat shocked at the 3S stage, which we interpret as a partial recovery of 3S heat shocked embryos to near control levels during the post-heat shock interval before fixation at 24h. Embryos heat-shocked at the 17S stage apparently do not have sufficient time to recover before fixation at 24h. Taken together, the maturity assays, lack of an effect on proliferation, shifted myoD expression patterns, and increase in dermomyotome domain suggest that bmp2b is likely causing a delay in differentiation of dermomyotome cells (Pax7+) into muscle fibers (as determined by MEF2 labeling). This hypothesis is supported by the 25% increase in Pax7+ cells after 17S heat shock combined with a concomitant 25% decrease in MEF2+ cells (Fig. 5G). While we cannot exclude the possibility that overexpression of BMP may cause an increase in the number of cells being directed down the dermomyotome route, our data strongly suggest that a developmental delay in muscle differentiation is the most likely action of BMP overexpression. This hypothesis also closely mirrors the pattern found in amniotes (Reshef et al., 1998; Amthor et al., 2006)
Other morphological indicators of somite maturity (multinucleated fibers and somite angle) were also greatly affected by ectopic bmp2b overexpression. The number of triplet fibers was the only measure where hs-bmp2b-injected embryos heat shocked at either the 3S or 17S stages were not significantly different from each other, indicating the recovery of these embryos is not complete, and that somite differentiation is affected long after bmp2b exposure, even though Pax7+ and MEF2+ numbers had returned to control levels. While there was a significant increase in somite angle in both 3S and 17S hs-bmp2b-injected embryos, little is known regarding the underlying developmental mechanism ultimately responsible for regulating somite angle, and the significance of a change in somite angle (shape) requires further investigation.
Regulation of muscle development by BMP signaling
There are similarities and differences between our results and those from previous studies in amniotes, in which the role of BMP signaling in somite patterning has been extensively investigated. In both zebrafish and amniotes, BMP2 and BMP4 are expressed in tissues dorsal (dorsomedial) and ventral (ventrolateral) to the dermomyotome, during the time when the dermomyotome is undergoing both proliferation and differentiation into muscle fibers (Sela-Donenfeld and Kalcheim, 2002), and in both amniotes and zebrafish, the effect of BMP signaling is to delay or inhibit the differentiation of dermomyotome cells into muscle fibers. Moreover, in both amniotes and zebrafish, BMP inhibits premature differentiation of myogenic precursors both in the epithelial somite and later in the fully formed epithelial dermomyotome. In chick, ectodermal BMP expression during segmentation prevents premature expression of MRFs in the somite (Linker et al., 2003). After formation of the dermomyotome, BMP from the lateral plate mesoderm inhibits the differentiation of myogenic precursors in the hypaxial dermomyotome (Pourquié et al., 1996; Reshef et al., 1998; Amthor et al., 1999). In zebrafish, we have shown that ectopic BMP signaling during segmentation increases pax3 expression while dermomyotomal precursors are still in the anterior domain of the somite. After the dermomyotomal cells reach the lateral surface of the somite, ectopic BMP expression can also delay the differentiation of myogenic precursors into MRF expressing myoblasts.
One distinct difference between the function of BMP in myogenesis in zebrafish and amniotes is the spatial requirement of BMP. In amniotes, BMP signaling represses the differentiation of cells mainly in the ventrolateral hypaxial dermomyotome, which provides cells to the limb musculature and body wall. This is presumably to delay the differentiation of cells that migrate into the limb, ensuring that there are enough precursors to support muscle growth there. In the epaxial somite, BMP signaling to the dermomyotome is blocked by the secreted inhibitor Noggin, which allows for earlier differentiation of the epaxial dermomyotome compared to the hypaxial dermomyotome. However, this inhibition can be overcome by overexpression of BMP, which lateralizes the epaxial somite and causes a delay in the differentiation of myogenic precursors, indicating that both the epaxial and hypaxial somite are responsive to BMP signaling (Pourquié et al., 1996; Reshef et al., 1998). In zebrafish, the spatial contribution of the dermomyotome to the epaxial and hypaxial musculature is still unclear, and cells in both the dorsal and ventral dermomyotome appear to be actively responding to BMP signaling. In axolotl, an anamniote tetrapod, BMP inhibition results in decreased Pax7 throughout the lateral surface. In addition, overexpression of BMP leads to upregulation of Pax7, similar to zebrafish (Epperlein et al., 2007). Therefore, the function of BMP signaling in inhibition of myogenic differentiation may be basal, and may have been modified in the amniote lineage to regulate development of mainly the lateral dermomyotome.
Inhibition of BMP signaling does not affect the development of the zebrafish dermomyotome
Inhibition of BMP signaling using a dominant negative BMP receptor has no apparent effect on the development of myogenic precursors in zebrafish (Fig. 6E, F). The lack of an effect of inhibiting BMP signaling on dermomyotomal cells was surprising given the strong effect of BMP overexpression. Inhibition of BMP signaling during gastrulation, at a level high enough to perturb dorsoventral patterning and phenocopy a known bmp4 mutant (Stickney et al., 2007), did not significantly reduce Pax7 expression in the somites, although it did appear that embryos with overexpression of Chordin had increased Myogenin expression (Fig. 6A). This is consistent with our hypothesis that BMP inhibits the transition of myogenic precursors into MRF expressing myoblasts, but it leaves unexplained why there is no effect on Pax7 expression.
We also examined the expression of Pax7 and MRFs in embryos in which the inhibition of BMP signaling was restricted to the segmentation period, and found no significant change. One possibility is that dermomyotome cells are still receiving sufficient BMP signaling, even when the dominant negative BMP receptor is expressed. This is supported by the lack of a significant decrease in the number of cells expressing both Pax7 and pSmad following heat shock induction of the dominant negative BMPR1a transgene (both between 20–25% of all Pax7+ cells). Although the overall level of pSmad is reduced by approximately 40%, this change is not reflected in the Pax7 population. Therefore, it is possible that the level of BMP signaling remaining after heat shock induction of the dominant negative receptor is sufficient for development and maintenance of the dermomyotome.
A second possibility is that there is redundancy in the regulation of differentiation of cells in the dermomyotome. Because of the importance of precisely regulating the timing of development of myogenic precursors, there may be multiple mechanisms ensuring that muscle differentiation occurs at the correct time and place. Fewer factors have been identified that negatively regulate muscle differentiation, compared to the number of factors identified that promote this process. In addition to BMP, Notch signaling negatively regulates the differentiation of muscle precursors in mouse and chick (Holowacz et al., 2006; Schuster-Gossler et al., 2007). Myostatin, a TGF-beta family member, is also required for the proper regulation of myogenic proliferation in chick, mouse and zebrafish, and in the absence of myostatin, muscle develops to 2 or 3 times its normal size (McPherron et al., 1997; Xu et al., 2003; Amthor et al., 2006). If there were redundancy in the regulation of differentiation of muscle precursors, we would still expect to see an effect of overexpression of BMP, as BMP would be sufficient to repress the differentiation of muscle precursors. However, removing the BMP signal would not necessarily have an effect, as the secondary signal would be capable of appropriately regulating the differentiation of muscle precursors in the absence of BMP.
Molecular regulation of the zebrafish dermomyotome
Despite the relatively small size, rapid development, and simple organization of the zebrafish somite, multiple signals regulate somite patterning, as in amniotes. Fgf8 signaling promotes the differentiation of myogenic precursors into myoblasts. The inhibition of Fgf8 signaling results in an upregulation of pax3 and Pax7, and a downregulation of MRFs (Groves et al., 2005; Hammond et al., 2007). The expression of Fgf8 in the somite is regulated by Retinoic Acid (RA), and exogenous RA also results in an upregulation of myoD and a downregulation of pax3 (Hamade et al., 2006; Hammond et al., 2007).
Hedgehog (Hh) also positively regulates the differentiation of myogenic precursors. Loss of Hh signaling leads to an increase in pax3 and Pax7 expression in the somites, and a decrease in differentiation (Feng et al., 2006; Hammond et al., 2007). Hh and BMP act in opposing ways in various developmental contexts, including in patterning of the somite (Marcelle et al., 1997). In zebrafish, BMP and Hh have been proposed to act in opposing ways in the development of slow muscle fibers, through the action of Scube2 (Du et al., 1997; Kawakami et al., 2005). The effect on muscle precursors following overexpression of BMP closely resembles the effect on muscle precursors following inhibition of Hh signaling. Based on the similarities between the effect of removing Hh and adding BMP, it appears that these signals may also be regulating the differentiation of myogenic precursors in opposite ways. However, it is not clear if BMP and Hh are acting in opposing ways at the same time.
Understanding how BMP, Hedgehog, Fgf8, and Retinoic Acid signaling integrate to regulate the dermomyotome will be critical to understanding how a balance between proliferation and differentiation is maintained during muscle development and growth.
EXPERIMENTAL PROCEDURES
Fish strains
Embryos were obtained from wild-type (AB) zebrafish and Tg (hs:dnBMPR1a) [TBR] zebrafish maintained by standard procedures in the Wesleyan University Zebrafish Facility. Tg (hs:dnBMPR1a) (Pyati et al., 2005) fish were generously provided by David Kimelman. Staging of embryos was done by counting somite number or by pigmentation as in Kimmel, et al. (Kimmel et al., 1995).
DNA injection and overexpression
Plasmids injected were hsp70-bmp2b, hsp70-chd, hsp70-eGFP, and hsp70-bmp2b-mCherry. The hsp70-bmp2b and hsp70-chd plasmids were constructed in the lab of Mary Mullins at University of Pennsylvania, and contain the coding sequence of zebrafish bmp2b or chordin downstream of the HSP70/4 promoter (Tucker et al., 2008). The hsp70-eGFP plasmid was developed by Jim Warren and leads to high levels of GFP expression in zebrafish upon heat shock (Halloran et al., 2000). The hsp70-bmp2b-mCherry plasmid was constructed in our lab (Rosemarie Doris), and contains the same bmp2b coding sequence found in the hsp70-bmp2b plasmid. Plasmid DNA was microinjected at a concentration of 25ng/µl into the yolk of 1-cell zebrafish embryos. To identify embryos with high levels of gene expression in embryos injected with hsp70-bmp2b following heat shock, a plasmid containing GFP under the control of a heat shock inducible promoter was co injected with the hsp70-bmp2b or hsp70-chd plasmids. Following heat shock, embryos were sorted based on the level of GFP expression, and embryos with high GFP expression were assumed to be expressing BMP at high levels as well. Embryos injected with hsp70-bmp2b-mCherry were screened for high mCherry expression following heat shock.
Embryos were heat shocked either in approximately 100µl of embryo medium in PCR tubes using a PCR machine, or in a water bath filled with embryo medium. Heat shock conditions were either 30 minutes at 37°C (hsp70-bmp2b, hsp70-chd, hsp70-eGFP) or 1h at 37°C (hsp70-bmp2b-mCherry, Tg(hsp70:dnbmpr1), Pyati et al., 2005). GFP expression following heat shock was evident by 1h post heat shock, while mCherry expression was evident as early as 10 minutes post heat shock.
Immunohistochemistry and in situ hybridization
Antibodies used were MF20, a mouse monoclonal antibody obtained from the Developmental Studies Hybridoma Bank (DSHB) that labels differentiated muscle fibers in all species examined (Bader et al., 1982); Pax7, a mouse monoclonal antibody obtained from DSHB that specifically recognizes Pax7 protein in chicken, and labels myogenic precursors in zebrafish (Kawakami et al., 1997); Myogenin, a rabbit antiserum from Santa Cruz Biotechnology, and MEF2, a rabbit antiserum from Santa Cruz Biotechnology. The pSmad antibody recognizes the phosphorylated form of Smads 1, 5, and 8 (Ed Laufer, pers. comm.). This antibody was produced as a collaborative effort between the labs of Ed Laufer and Tom Jessell and generously supplied to us. Anti-pSmad was used at a dilution of 1:1000. Rabbit antibody against MEF2 was used at a dilution of 1:100. Antibodies against Pax7, MF20, Myogenin were used at 5µg/ml. Alexa fluor-conjugated secondary antibodies (Invitrogen) were used at a dilution of 1:400 (anti-Mouse IgG1 and anti- Rabbit IgG) or 1:800 (anti-mouse IgG2b, anti-Mouse IgG1 and anti- Rabbit IgG).
Antibody labeling was performed in whole mount as in Feng et al. (2006). For antibody labeling on cryostat sections, embryos were fixed overnight at 4°C. Fixative was washed out, and embryos were embedded in agarose blocks and cryoprotected overnight in 30% sucrose. Blocks were sectioned at 12µm. Antibody labeling on cryosections was performed as in Devoto et al. (1996).
In situ hybridization was done in whole-mount as in Feng et al (2006).
Somite maturity assays
In order to ascertain the overall effect of ectopic bmp2b expression on somite maturity, four measures were quantified in somite 17 (anal somite) at the 24h stage using several combinations of heat shock and hs-bmp2b-mCherry injection (Fig. 5G). Ten individuals in each of four heat shock treatments (3S heat shock injected/non-injected, 17S heat shock injected/non-injected) were randomly selected from a pool of individuals. In addition, ten control embryos (non-injected and non-heat shocked) staged to 24h were also randomly selected for analysis. All embryos were processed using triple immunohistochemistry for Pax7, MEF2, and MF20 (described above). Confocal stacks of each embryo were obtained for somite 17, for which all Pax7 (Fig. 5H, I) and MEF2+ (Fig. 5J, K) cells were counted, as well as the number of fibers with triplet nuclei (via MEF2/MF20 staining) and the angle of somite 17 (as determined by MF20 staining at the boundary of somites 16 and 17, Fig. 5H, I).
One-way ANOVAs were calculated, after which either Tukey’s HSD or Dunnett’s test were utilized to determine how groups differed (Fig. 5G). Tests were performed in Microsoft Excel.
ACKNOWLEDGEMENTS
We thank Dan Vasiliauskas, Susan Morton, Tom Jessell and Ed Laufer for the Psmad antibody, Mary Mullins and Mary Halloran for gifts of plasmids, and David Kimelman for the Tg (hs:dnBMPR1a) fish. We would also like to thank Ron Gordon, Sera Brown, and Angela Lentini for animal care. We give special thanks to Rosemarie Doris for construction of the hsp70-bmp2b-mcherry plasmid.
NIH: R01 HD37509.
NIH: R01 HD044929.
References
- Amthor H, Christ B, Patel K. A molecular mechanism enabling continuous embryonic muscle growth - a balance between proliferation and differentiation. Development. 1999;126:1041–1053. doi: 10.1242/dev.126.5.1041. [DOI] [PubMed] [Google Scholar]
- Amthor H, Otto A, Macharia R, McKinnell I, Patel K. Myostatin imposes reversible quiescence on embryonic muscle precursors. Dev Dyn. 2006;235:672–680. doi: 10.1002/dvdy.20680. [DOI] [PubMed] [Google Scholar]
- Bader D, Masaki T, Fischman DA. Immunochemical Analysis of Myosin Heavy-Chain During Avian Myogenesis In vivo and In vitro. Journal of Cell Biology. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barresi MJ, D'Angelo JA, Hernandez LP, Devoto SH. Distinct mechanisms regulate slow-muscle development. Curr Biol. 2001;11:1432–1438. doi: 10.1016/s0960-9822(01)00428-6. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Vincent SD. Distinct and dynamic myogenic populations in the vertebrate embryo. Curr Opin Genet Dev. 2009 doi: 10.1016/j.gde.2009.08.001. doi:10.1016/j.gde.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Devoto SH, Melancon E, Eisen JS, Westerfield M. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development. 1996;122:3371–3380. doi: 10.1242/dev.122.11.3371. [DOI] [PubMed] [Google Scholar]
- Devoto SH, Stoiber W, Hammond CL, Steinbacher P, Haslett JR, Barresi MJ, Patterson SE, Adiarte EG, Hughes SM. Generality of vertebrate developmental patterns: evidence for a dermomyotome in fish. Evol Dev. 2006;8:101–110. doi: 10.1111/j.1525-142X.2006.05079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick A, Meier A, Hammerschmidt M. Smad1 and Smad5 have distinct roles during dorsoventral patterning of the zebrafish embryo. Dev Dyn. 1999;216:285–298. doi: 10.1002/(SICI)1097-0177(199911)216:3<285::AID-DVDY7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Du SJ, Devoto SH, Westerfield M, Moon RT. Positive and negative regulation of muscle cell identity by members of the hedgehog and TGF-beta gene families. J Cell Biol. 1997;139:145–156. doi: 10.1083/jcb.139.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperlein HH, Vichev K, Heidrich FM, Kurth T. BMP-4 and Noggin signaling modulate dorsal fin and somite development in the axolotl trunk. Dev Dyn. 2007;236:2464–2474. doi: 10.1002/dvdy.21247. [DOI] [PubMed] [Google Scholar]
- Faure S, de Santa Barbara P, Roberts DJ, Whitman M. Endogenous patterns of BMP signaling during early chick development. Dev Biol. 2002;244:44–65. doi: 10.1006/dbio.2002.0579. [DOI] [PubMed] [Google Scholar]
- Feng X, Adiarte EG, Devoto SH. Hedgehog acts directly on the zebrafish dermomyotome to promote myogenic differentiation. Dev Biol. 2006;300:736–746. doi: 10.1016/j.ydbio.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Groves JA, Hammond CL, Hughes SM. Fgf8 drives myogenic progression of a novel lateral fast muscle fibre population in zebrafish. Development. 2005;132:4211–4222. doi: 10.1242/dev.01958. [DOI] [PubMed] [Google Scholar]
- Halloran MC, Sato-Maeda M, Warren JT, Su F, Lele Z, Krone PH, Kuwada JY, Shoji W. Laser-induced gene expression in specific cells of transgenic zebrafish. Development. 2000;127:1953–1960. doi: 10.1242/dev.127.9.1953. [DOI] [PubMed] [Google Scholar]
- Hamade A, Deries M, Begemann G, Bally-Cuif L, Genet C, Sabatier F, Bonnieu A, Cousin X. Retinoic acid activates myogenesis in vivo through Fgf8 signalling. Dev Biol. 2006;289:127–140. doi: 10.1016/j.ydbio.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Hammond CL, Hinits Y, Osborn DP, Minchin JE, Tettamanti G, Hughes SM. Signals and myogenic regulatory factors restrict pax3 and pax7 expression to dermomyotome-like tissue in zebrafish. Dev Biol. 2007;302:504–521. doi: 10.1016/j.ydbio.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinits Y, Hughes SM. Mef2s are required for thick filament formation in nascent muscle fibers. Development. 2007;134:2511–2519. doi: 10.1242/dev.007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollway GE, Bryson-Richardson RJ, Berger S, Cole NJ, Hall TE, Currie PD. Whole-somite rotation generates muscle progenitor cell compartments in the developing zebrafish embryo. Dev Cell. 2007;12:207–219. doi: 10.1016/j.devcel.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Holowacz T, Zeng L, Lassar AB. Asymmetric localization of numb in the chick somite and the influence of myogenic signals. Dev Dyn. 2006;235:633–645. doi: 10.1002/dvdy.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami A, Kimura-Kawakami M, Nomura T, Fujisawa H. Distributions of PAX6 and PAX7 proteins suggest their involvement in both early and late phases of chick brain development. Mech Dev. 1997;66:119–130. doi: 10.1016/s0925-4773(97)00097-x. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Nojima Y, Toyoda A, Takahoko M, Satoh M, Tanaka H, Wada H, Masai I, Terasaki H, Sakaki Y, Takeda H, Okamoto H. The zebrafish-secreted matrix protein you/scube2 is implicated in long-range regulation of hedgehog signaling. Curr Biol. 2005;15:480–488. doi: 10.1016/j.cub.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Linker C, Lesbros C, Stark MR, Marcelle C. Intrinsic signals regulate the initial steps of myogenesis in vertebrates. Development. 2003;130:4797–4807. doi: 10.1242/dev.00688. [DOI] [PubMed] [Google Scholar]
- Marcelle C, Stark MR, Bronner-Fraser M. Coordinate actions of BMPs, Wnts, Shh and noggin mediate patterning of the dorsal somite. Development. 1997;124:3955–3963. doi: 10.1242/dev.124.20.3955. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- Muller F, Blader P, Rastegar S, Fischer N, Knochel W, Strahle U. Characterization of zebrafish smad1, smad2 and smad5: the amino-terminus of smad1 and smad5 is required for specific function in the embryo. Mech Dev. 1999;88:73–88. doi: 10.1016/s0925-4773(99)00173-2. [DOI] [PubMed] [Google Scholar]
- Munsterberg AE, Kitajewski J, Bumcrot DA, McMahon AP, Lassar AB. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev. 1995;9:2911–2922. doi: 10.1101/gad.9.23.2911. [DOI] [PubMed] [Google Scholar]
- Pourquié O, Fan CM, Coltey M, Hirsinger E, Watanabe Y, Bréant C, Francis West P, Brickell P, Tessier Lavigne M, Le Douarin NM. Lateral and axial signals involved in avian somite patterning: a role for BMP4. Cell. 1996;84:461–471. doi: 10.1016/s0092-8674(00)81291-x. [DOI] [PubMed] [Google Scholar]
- Pyati UJ, Cooper MS, Davidson AJ, Nechiporuk A, Kimelman D. Sustained Bmp signaling is essential for cloaca development in zebrafish. Development. 2006;133:2275–2284. doi: 10.1242/dev.02388. [DOI] [PubMed] [Google Scholar]
- Pyati UJ, Webb AE, Kimelman D. Transgenic zebrafish reveal stage-specific roles for Bmp signaling in ventral and posterior mesoderm development. Development. 2005;132:2333–2343. doi: 10.1242/dev.01806. [DOI] [PubMed] [Google Scholar]
- Reshef R, Maroto M, Lassar AB. Regulation of dorsal somitic cell fates: BMPs and Noggin control the timing and pattern of myogenic regulator expression. Genes Dev. 1998;12:290–303. doi: 10.1101/gad.12.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster-Gossler K, Cordes R, Gossler A. Premature myogenic differentiation and depletion of progenitor cells cause severe muscle hypotrophy in Delta1 mutants. Proc Natl Acad Sci U S A. 2007;104:537–542. doi: 10.1073/pnas.0608281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela-Donenfeld D, Kalcheim C. Localized BMP4-noggin interactions generate the dynamic patterning of noggin expression in somites. Dev Biol. 2002;246:311–328. doi: 10.1006/dbio.2002.0672. [DOI] [PubMed] [Google Scholar]
- Stellabotte F, Dobbs-McAuliffe B, Fernandez DA, Feng X, Devoto SH. Dynamic somite cell rearrangements lead to distinct waves of myotome growth. Development. 2007;134:1253–1257. doi: 10.1242/dev.000067. [DOI] [PubMed] [Google Scholar]
- Stickney HL, Imai Y, Draper B, Moens C, Talbot WS. Zebrafish bmp4 functions during late gastrulation to specify ventroposterior cell fates. Dev Biol. 2007;310:71–84. doi: 10.1016/j.ydbio.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JA, Mintzer KA, Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell. 2008;14:108–119. doi: 10.1016/j.devcel.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Wu G, Zohar Y, Du SJ. Analysis of myostatin gene structure, expression and function in zebrafish. J Exp Biol. 2003;206:4067–4079. doi: 10.1242/jeb.00635. [DOI] [PubMed] [Google Scholar]