Summary
Data on the relationship between the two genotypes of Giardia duodenalis that infect humans, assemblages A and B, their clinical presentation and intestinal inflammation are limited. We analyzed 108 stool samples previously collected for a diarrhoeal study among Brazilian children, representing 71 infections in 47 children. Assemblage B was most prevalent, accounting for 43/58 (74.1%) infections, while assemblage A accounted for 9/58 (15.5%) infections and 6/58 (10.3%) infections were mixed (contained both assemblage A and B). There was no significant difference in diarrhoeal symptoms experienced during assemblage A, B or mixed infections. Children with assemblage B demonstrated greater variability in G. duodenalis cyst shedding but at an overall greater level (n = 43, mean 3.6 × 105, range 5.3 × 102–2.5 × 106 cysts/ml) than children infected with assemblage A (n = 9, mean 1.4 × 105, range 1.5 × 104–4.6 × 105 cysts/ml; P = 0.009). Children with mixed infections shed more cysts (mean 8.3 × 105, range 3.1 × 104–2.8 × 106 cysts/ml) than children with assemblage A or B alone (P = 0.069 and P = 0.046 respectively). This higher rate of cyst shedding in children with assemblage B may propagate its spread, accounting for its increased incidence. Additionally, second and third infections had decreasing faecal lactoferrin, suggesting some protection against severity, albeit not against infection, by prior infection.
Keywords: Giardia, Diarrhoea, Lactoferrin, Cysts, Genotype, Brazil
1. Introduction
Giardia duodenalis is one of the most common infectious intestinal parasites worldwide. Each year in Asia, Africa and Latin America, 500 000 new cases are reported and about 200 million people develop symptomatic giardiasis (CDC, 1994). The prevalence of G. duodenalis in stools submitted for ova and parasite examination is between 2–5% in industrialized countries to 20–30% in developing countries (Ortega and Adam, 1997).
Clinical manifestations of G. duodenalis infection vary between asymptomatic infection, acute diarrhoea and persistent and life-threatening diarrhoea for malnourished children in developing countries. There are two distinct genotypes of G. duodenalis that infect humans, commonly referred to as assemblages A and B. Molecular analyses have shown that the genetic distance between the two assemblages is greater than that used to delineate other species of protozoa (Thompson et al., 2000). Additionally, it has been hypothesized that there may be phenotypic differences between assemblages; however, a limited number of studies looking at differences in clinical symptomatology between people infected with assemblage A and B of G. duodenalis have reported different results. One study showed a strong association between intermittent diarrhoea and assemblage A and between persistent diarrhoea and assemblage B (Homan and Mank, 2001). Others have found that children with assemblage A were more likely to be symptomatic (Haque et al., 2005, Read et al., 2002).
Also, limited numbers of studies genotyping G. duodenalis have found a predominance of assemblage A (Ponce-Macotela et al., 2002; Thompson et al., 2000) or B (Amar et al., 2002; Haque et al., 2005; Sulaiman et al., 2003) in human samples, depending on location. Small numbers of mixed assemblage infections have also been reported (El-Shazly et al., 2004; Haque et al., 2005).
We therefore re-analyzed specimens that were collected as part of a longitudinal study of diarrhoeal disease (including G. duodenalis) in northeastern Brazilian children (Newman et al., 2001) in order to look at the relationships between assemblage, clinical presentation and intestinal inflammation as evidenced by faecal lactoferrin in a larger cohort than in most previous studies.
2. Materials and methods
2.1. Study area and population
The complete description of the study area and population has been presented previously (Newman et al., 1994, 2001). The poor, urban shantytown (favela) of Gonçalves Dias with about 2000 residents is located within the capital city of Fortaleza, in the northeastern Brazilian state of Ceará. Gonçalves Dias is the site of an ongoing project initiated in 1989 by the University of Virginia (USA) and the Federal University of Ceará. Briefly, this study is part of a longitudinal evaluation of diarrhoeal disease, nutrition, fitness and cognitive development in a cohort of children.
All pregnant women in the community were identified by study nurses who offered enrolment to newborns beginning in 1989. From birth, children in the study were visited three times weekly on Monday, Wednesday and Friday by a study nurse who recorded all diarrhoeal and other illnesses, as well as dietary information. Children with diarrhoea were visited daily until 48 h after resolution of illness and mothers or guardians were asked to provide detailed clinical information about the diarrhoeal illnesses, including stool consistency and character and other symptoms of fever, vomiting or dehydration. Stool specimens were collected from children with diarrhoea and surveillance stools were collected at 4-month intervals. The surveillance period was from August 1989 to April 1993. Informed consent was obtained from parents or guardians of all study participants.
2.2. Definition of separate diarrhoea episodes, Giardia duodenalis infections and new/subsequent infection
WHO criteria to determine diarrhoea and diarrhoeal episodes were used. A total of three unformed stools in one 24 h period was defined as a day of diarrhoea, with distinct diarrhoeal episodes defined as being separated by ≥2 diarrhoea-free days (WHO, 1988). The beginning of a single G. duodenalis infection period was defined as the time at which the G. duodenalis positive stool was collected, or if symptomatic at stool collection, the date when diarrhoea began and was preceded by seven diarrhoea-free days. The end of an infection period was defined as the date after which the child experienced seven symptom-free days. A subsequent G. duodenalis positive stool in a child was identified as a new, second or third infection if there was at least one negative intervening stool since the prior G. duodenalis positive stool or if the stool was collected 6 months after the end of the infection period and initial positive stool. Four children went to a local clinic and were treated with metronidazole at the time of their infection.
2.3. Stool processing
Faecal samples were obtained from all children at 3-month intervals and during diarrhoea episodes. Stool samples were collected by parents or guardians and placed into disposable plastic cups. A portion of each sample was subsequently placed into a tube containing 10% formalin.
Fresh stools were transported on ice within 4 h to the laboratory for processing and microbiologic evaluation. All specimens were examined by microscopy for parasites, including Ascaris and Trichuris, as well as for leukocytes using iodine-stained and methylene blue-stained wet-mount preparations. Faecal smears were prepared on glass slides for modified acid-fast stain for Cryptosporidium. Stool samples that were found to contain G. duodenalis by initial microscopy were stored frozen at −22 °C until 2004, when they were thawed and G. duodenalis cysts subsequently quantified using direct fluorescence antibody staining and DNA was extracted.
As previously described (Newman et al., 2001) fresh stool was plated onto appropriate agar media for isolation of bacterial pathogens. One Escherichia coli isolate from each patient was examined for HEp-2 adherence and enterotoxigenicity using established methods (Fang et al., 1995). Adherence to HEp-2 cells was scored as aggregate (AA), localized (LA) or diffuse (DA) (Nataro et al., 1987). One E. coli strain per stool was also evaluated by oligonucleotide probe hybridization for the heat-labile (LT) and heat-stable (STa) toxins (Fang et al., 1995). Fragment probes were used to evaluate for EPEC (eae, E. coli attaching and effacing gene; Jerse et al., 1991), and EAF (EPEC adherence factor; Nataro et al., 1985), DAEC (diffusely adherent E. coli; Bilge et al., 1993), EIEC (enteroinvasive E. coli; Wood et al., 1986), EHEC (enterohaemorrhagic E. coli; Levine et al., 1987) and EAEC (enteroaggregative E. coli; Baudry et al., 1990).
Escherichia coli strains were classified into four virulence categories if one or more of these tests were positive. Therefore, E. coli strains were classified as EPEC if the eae probe, EAF probe or HEp-2 assay for localized adherence were positive; as enterotoxigenic (ETEC) if either STa or LT probes were positive; as EAEC if the HEp-2 assay or the AA probe were positive and DAEC if either the HEp-2 assay or DA probe were positive. No EIEC or EHEC strains were identified in the first 39 stools tested, so the remaining stools were not tested.
2.4. Lactoferrin latex agglutination assay
Stool supernatants were tested according to the manufacturer’s specifications including appropriate kit controls (LEUKO-TEST; Tech Lab, Blacksburg, VA, USA). Ten microlitres of stool supernatant (1:3 dilution) was added to 72.5 µl of diluent, yielding a 1:25 dilution. Twenty-five microlitres of the sample and 25 µl of sensitized latex (lactoferrin antibody-coated latex beads) were mixed and observed for agglutination after 3 min. Positive and negative controls provided with the test kits were also performed. Agglutination reaction was graded with the unaided eye from 0 (no agglutination) to 3+ (large agglutination with a clear background). For stools positive at a dilution of 1:25, two-fold serial dilutions were done until lactoferrin was negative. The highest dilution at which the test result was positive was recorded as the positive titre. Lactoferrin was considered high if titres were 1:400–1:3200, low if titres were 1:25–1:200, and negative if negative at a titre of 1:25. Stools from children who were breastfeeding were not tested for faecal lactoferrin.
2.5. Quantification of Giardia
Direct immunofluorescence assay (Merifluor Cryptosporidium and Giardia kits, Meridian Diagnostics, Cincinnati, OH, USA) was performed on faecal samples identified as containing G. duodenalis and quantification performed as previously described by Genta et al. (1993). Briefly, each stool sample was removed from storage at −22 °C and allowed to come to room temperature. Faecal samples were diluted 1:4 in 10% formalin (30 µl stool + 90 µl formalin), mixed and 5 µl spread evenly onto an etched 1 cm2 area of a glass slide and allowed to air dry. Samples were then incubated for 30 min with a flourescein isothiocyanate-labelled G. duodenalis and anti-Cryptosporidium monoclonal antibody and examined under a fluorescence microscope. The number of cysts in 10 fields at 40× magnification was counted. If no cysts were seen, the entire slide was examined and cysts counted at 10× magnification. This quantification procedure was duplicated three times on each sample.
2.6. DNA extraction
DNA was extracted from 200 mg stool samples stored at −22 °C using a QIAamp DNA Stool Mini Kit (QIAGEN, Valencia, CA, USA) per the manufacturer's instructions except that the suspension was incubated in the kit's stool lysis buffer at 95 °C, and a 3-min incubation with InhibitEx tablets was performed.
2.7. Multiplex real-time PCR assay
DNA was tested for G. duodenalis infection by quantitative PCR using the primers and validated protocol of Ng et al. (2005) and was carried out in their laboratory at the University of Virginia. The amplification product was the 18S rRNA gene, which differs between assemblage A and B, and was detected using gel electrophoresis. This method has a sensitivity to detect ≤20 trophozoites of G. duodenalis for assemblage A and ≤200 trophozoites assemblage B in stool.
2.8. Statistical analysis
SPSS version 14.0 (release 14.0.2; SPSS Inc., Chicago, IL, USA) was used to complete all data analyses. Simple t tests were used to compare mean differences and χ2 analyses were used to compare categorical variables. A P-value of less than 0.05 was considered statistically significant. Equal variances were not assumed for t test analyses comparing cyst counts by assemblage and corresponding P values were reported. EPEC, EIEC, DAEC, EHEC, EAEC, ETEC, Ascaris, Trichuris and Cryptosporidium co-infections were assessed as possibly confounding variables.
3. Results
During the study period 189 children were enrolled and 1054 stools in children with or without diarrhoea collected. One hundred and eight stool samples from 47 children were positive by microscopy for G. duodenalis. Using the definition of infection period defined earlier, the 108 stools represented 71 infections. Fifty-eight stools, from different infections, were genotyped and nine (15.5%) were found to be assemblage A, 43 (74.1%) were assemblage B, and six (10.3%) were mixed infections. Two samples had inadequate amounts of stool for DNA extraction, and 11 samples were negative by qPCR for assemblage A or B. Average age at first mixed infection was 13.0 ± 9.0 months (range 6.3–30.0), while age at first infection with assemblage A or B alone was 14.9 ± 5.6 months (range 4.43–20.6) and 16.2 ± 6.7 months (range 4.27–36.1) respectively. None of these differences were significant. Boys and girls were equally likely to be infected with either assemblage of G. duodenalis (data not shown). Forty-one percent of children were asymptomatic without diarrhoea at the time of positive Giardia stool collection and 59% were symptomatic with diarrhoea.
3.1. Sequence of multiple Giardia infections
Ten of 28 (35.7%) children with initial assemblage B infections, 3/7 (42.9%) with initial assemblage A infections and 5/6 (83.3%) with initial mixed infections went on to develop subsequent G. duodenalis infections (χ2: 4.6, P = 0.10). All subsequent infections were able to be genotyped in 36 children; in this group initial infections with assemblage A (n = 6) were followed by assemblage A (n = 1) and assemblage B (n = 1). Initial infections with assemblage B (n = 26) were followed by assemblage A (n = 1), but more commonly by assemblage B (n = 7). When mixed assemblage infections occurred they were always the first documented infection. Three of four children with initial mixed infections went on to develop a second infection, always with assemblage B. Third infections after initial mixed or assemblage B infections were always assemblage B (n = 1 and n = 2 respectively).
3.2. Cyst shedding and assemblage
Giardia duodenalis cysts were quantified in all 58 stool samples genotyped. During first infection, children with assemblage B shed more G. duodenalis cysts and over a greater range than children infected with assemblage A (P = 0.006). Children with mixed infections shed slightly more G. duodenalis cysts than children infected with either A or B alone, although these differences did not reach statistical significance (P = 0.09 and P = 0.109 respectively) (Table 1).
Table 1.
Cyst shedding by assemblage during Giardia duodenalis infections in northeastern Brazilian children
| n | Mean | SD | Min. | Max. | |
|---|---|---|---|---|---|
| cysts/ml | cysts/ml | cysts/ml | |||
| First infection | |||||
| Assemblage A | 7 | 8.7 × 104 | 6.8 × 104 | 1.5 × 104 | 2.1 × 105 |
| Assemblage Ba | 28 | 3.7 × 105 | 4.9 × 105 | 1.6 × 103 | 2.5 × 106 |
| Mixed (A+B)b,c | 6 | 8.3 × 105 | 1.1 × 106 | 3.1 × 104 | 2.8 × 106 |
| All infections | |||||
| Assemblage A | 9 | 1.4 × 105 | 1.4 × 105 | 1.5 × 104 | 4.6 × 105 |
| Assemblage Bd | 43 | 3.6 × 105 | 4.3 × 105 | 5.3 × 102 | 2.5 × 106 |
| Mixed (A+B)e,f | 6 | 8.3 × 105 | 1.1 × 106 | 3.1 × 104 | 2.8 × 106 |
Assemblage A vs. Assemblage B, t test (equal variances not assumed) P = 0.006.
Assemblage A vs. mixed infections, t test (equal variances not assumed) P = 0.09.
Assemblage B vs. mixed infections, t test (equal variances not assumed) P = 0.109.
Assemblage A vs. assemblage B, t test (equal variances not assumed) P = 0.009.
Assemblage A vs. mixed, t test (equal variances not assumed) P = 0.069.
Assemblage B vs. mixed, t test (equal variances not assumed) P = 0.046.
In all stool samples representing first, second and third infections, children with assemblage B again shed more cysts and over a greater range than children infected with assemblage A (P = 0.009). Children with mixed infections shed more G. duodenalis cysts than children infected with either A (P = 0.069) or B (P = 0.046) alone (Table 1).
Children with initial assemblage A shed more cysts during their second infection with G. duodenalis (1st infection (n = 7) 8.7 × 104 cysts/ml; 2nd infection (n = 3) 1.5 × 106 cysts/ml; SD 6.9 × 105; P = 0.073) than their first infection. There was no significant difference in cyst shedding between first, second and third infection after initial assemblage B (1st infection (n = 28) 3.7 × 105 cysts/ml; 2nd infection (n = 10) 5.3 × 105 cysts/ml; 3rd infection (n = 3) 5.0 × 105 cysts/ml; P = 0.710).
3.3. Giardia duodenalis assemblage and symptoms
There was no significant difference in the number of days of diarrhoea, length of infection period or number of diarrhoea episodes experienced by children infected with assemblage A, B or those with mixed infections (Table 2). Children with assemblage A, B and mixed infections were equally likely to be symptomatic or asymptomatic from their infection (assemblage A 77.8% symptomatic, assemblage B 67.4% symptomatic and mixed assemblages 60.0% symptomatic).
Table 2.
Days of diarrhoea, length of illness and episodes of diarrhoea associated with assemblage A, B and mixed infections in northeastern Brazilian children
| Assemblage A | Assemblage B | Mixed | P-value | |
|---|---|---|---|---|
| Days of diarrhoea | 7.2 ± 6.0 | 9.7 ± 16.2 | 13.0 ± 16.4 | 0.82 |
| Total illness days | 8.4 ± 7.0 | 12.1 ± 19.7 | 14.2 ± 17.9 | 0.79 |
| Episodes of | 0.89 ± 0.6 | 1.3 ± 1.5 | 0.80 ± 0.84 | 0.58 |
| diarrhoea |
Additionally, there was no significant difference in the number of days of diarrhoea (8.6 days vs. 2.5 days; P = 0.397), length of infection period (10.1 days vs. 2.5 days; P = 0.165) and episodes of diarrhoea (1.0 episode vs. 0.5 episode; P = 0.573) experienced between assemblage A first and second infections. There was also no significant difference in the number of days of diarrhoea (7.14 days vs. 15.8 days vs. 10.0 days; P = 0.314), length of illness period (8.9 days vs. 19.3 days vs. 13.3 days; P = 0.312) and episodes of diarrhoea (1.1 episode vs. 1.6 episodes vs. 2.0 episodes; P = 0.409) experienced between the first, second and third infections with assemblage B.
3.4. Giardia duodenalis assemblage, cyst shedding and diarrhoeal symptoms
Assemblage A, B and mixed infections were analyzed individually to look for a correlation between cyst shedding and diarrhoeal symptoms by assemblage. No significant correlation between diarrhoeal symptoms and cyst shedding was found. There was no difference in stool consistency, mucus, occult or visible blood or faecal leukocytes between assemblage A and assemblage B.
3.5. Giardia duodenalis assemblages and lactoferrin
Similar rates of lactoferrin positivity were seen with assemblage A, B and mixed infections (assemblage A 4/6 (66.7%) positive; assemblage B 22/33 (66.6%) positive; mixed assemblage 3/5 (60.0%) positive; χ2: 0.09, P = 0.96). Furthermore, among those who were positive, the rates of high and low levels of lactoferrin were similar (assemblage A 3/4 (75.0%) low, 1/4 (25.0%) high; assemblage B 15/22 (78.9%) low, 7/22 (31.8%) high; mixed assemblages 1/3 (33.3%) low, 2/3 (66.6%) high; χ2: 1.60, P = 0.45).
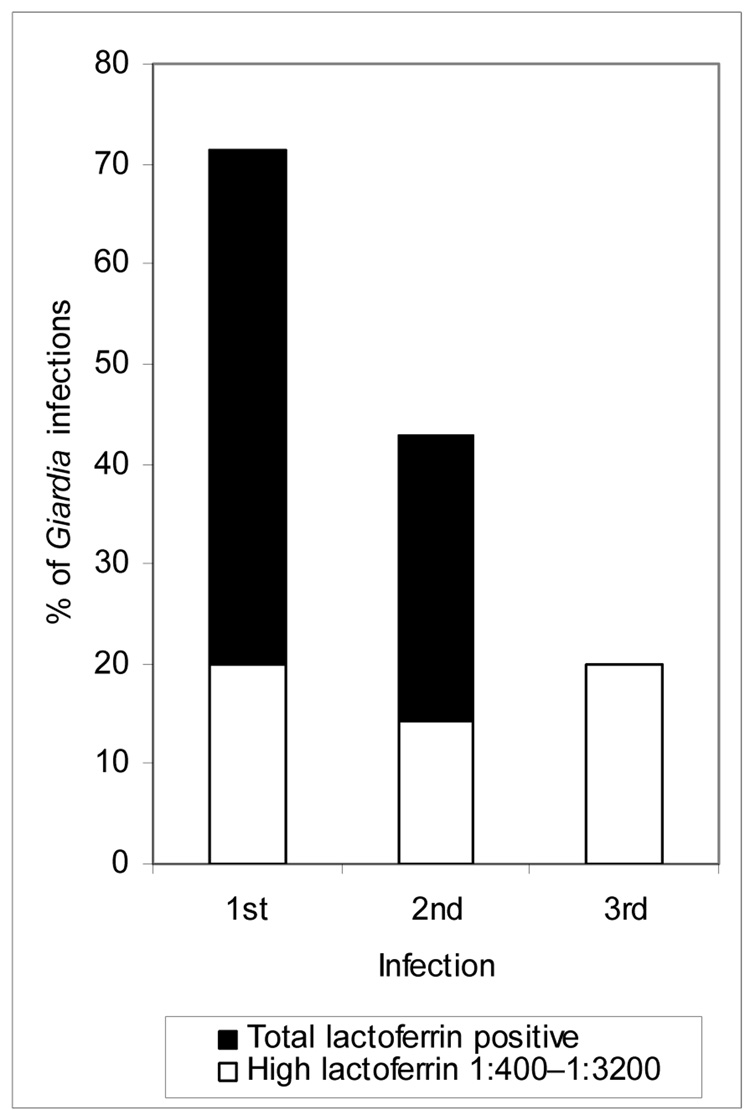
However, 74.0% (25/34) of first infections (7/34 (20.6%) high), 40.0% (6/15) of second infections (2/15 (13.3%) high), and only 20.0% (1/5) of third infections (1/5 (20.0%) high) were lactoferrin positive, with a significant decrease in the percent of lactoferrin-positive stools with subsequent G. duodenalis infections (χ2: 8.4, P = 0.015; Figure 1). This decrease in the percentage of lactoferrin-positive stools remained statistically significant when assemblage A was examined alone (1st infection: 3/4 (75%) low, 1/4 (25%) high; 2nd infection: 2/2 negative; χ2: 6.0, P = 0.05). This trend was seen for assemblage B, however, it was not statistically significant (1st infection: 6/21 (28.6%) negative, 11/21 (52.4%) low, 4/21 (19.0%) high; 2nd infection: 3/9 (33.3%) negative, 4/9 (44.4%) low, 2/9 (22.2%) high; 3rd infection: 2/3 (66.6%) negative, 1/3 (33.3%) high; χ2: 2.99, P = 0.560).
Figure 1.
Relationship between lactoferrin positivity and multiple Giardia infections in northeastern Brazilian children (χ2 analyses, P = 0.015).
At first infection both asymptomatic and symptomatic children were equally likely to experience intestinal inflammation as evidenced by the presence of lactoferrin (asymptomatic: 5/14 (35.7%) lactoferrin negative, 7/14 (50.0%) low lactoferrin, 2/14 (14.3%) high lactoferrin; symptomatic: 4/20 (20.0%) lactoferrin negative, 11/20 (55.0%) low lactoferrin, 5/20 (25.0%) high lactoferrin; P = 0.531). However, at second infection, symptomatic children were more likely to have intestinal inflammation (6/8 (75%) lactoferrin positive) than asymptomatic children (0/7 lactoferrin positive; P = 0.01). Elevated lactoferrin titre in stool during infection was associated with a significantly longer period of illness (negative lactoferrin 2.5 days; low lactoferrin 11.5 days; high lactoferrin 14.6 days; P = 0.017). In addition, higher titres of lactoferrin were associated with more days of diarrhoea (negative lactoferrin 2.2 days; low lactoferrin 9.7 days; high lactoferrin 14.6 days; P = 0.017), and more episodes of diarrhoea per Giardia infection period (negative lactoferrin 0.38 episodes; low lactoferrin 1.14 episodes; high lactoferrin 1.60 episodes; P = 0.013).
3.6. Lactoferrin and shedding
During assemblage A, B, and mixed infection, G. duodenalis cyst shedding did not vary with stool lactoferrin titres (assemblage A: negative lactoferrin 3.0 × 105 cysts/ml, low lactoferrin 9.1 × 104 cysts/ml, high lactoferrin 6.4 × 104 cysts/ml, P = 0.298; assemblage B: negative lactoferrin 3.1 × 105 cysts/ml, low lactoferrin 4.8 × 105 cysts/ml, high lactoferrin 3.5 × 105 cysts/ml, P = 0.659; mixed: negative lactoferrin 5.3 × 104 cysts/ml, low lactoferrin 8.4 × 105 cysts/ml, high lactoferrin 1.9 × 106 cysts/ml, P = 0.320).
3.7. Giardia duodenalis assemblage and co-infection with Cryptosporidium
One out of nine (11.1%) assemblage A, 8/43 (18.6%) assemblage B and 1/5 (20.0%) mixed G. duodenalis infections had co-infections with Cryptosporidium. Co-infection with Cryptosporidium was equally likely to occur with either assemblage of G. duodenalis or in mixed infections. Cyst shedding by genotype was not significantly different in children with and without Cryptosporidium co-infection (assemblage A only 1.3 × 105 cysts/ml; assemblage A with Cryptosporidium 1.4 × 105 cysts/ml; P = 0.960) (assemblage B only 3.5 × 105 cysts/ml; assemblage B with Cryptosporidium 3.6 × 105 cysts/ml; P = 0.996) (mixed only 8.3 × 105 cysts/ml; mixed with Cryptosporidium 8.3 × 105 cysts/ml; P = 0.992).
In addition for both assemblage A and B, co-infection with Cryptosporidium had no significant effect on the number of days of diarrhoea, total days ill or number of episodes of diarrhoea experienced by each child.
3.8. Giardia duodenalis assemblage and other co-infections
Four of 57 (7.1%) stools tested were co-infected with ETEC, 7 of 58 (12.1%) co-infected with EPEC, 21 of 42 (50%) co-infected with EAEC, 5 of 63 (7.9%) co-infected with DAEC, 12 of 69 (17.4%) co-infected with T. trichuris and 13 of 69 (18.8%) with A. lumbricoides. There was no difference in diarrhoeal symptoms (including days of diarrhoea, episodes of diarrhoea, and days of illness) experienced between G. duodenalis-infected children with or without any of the above co-infections. Additionally there was no significant difference in the proportion of lactoferrin-positive stools with or without co-infection with ETEC (χ2: 2.12, P = 0.29), EPEC (χ2: 0.13, P = 1.0), EAEC (χ2: 1.60, P = 0.23), DAEC (χ2: 0.015, P = 1.0), Trichuris (χ2: 0.982, P = 0.461) or Ascaris (χ2: 1.89, P = 0.285).
3.9. Giardia duodenalis assemblages and environmental factors
There was no significant difference in type of house, water source, water storage, toilet (pit vs. none), hand-washing habits, number and types of animals in the house (dogs, cats, other) or season of infection (rainy vs. non-rainy season) between children infected with assemblage A or B. One of 35 children with G. duodenalis lived in a house where the water was boiled, 34/35 lived in homes where water was not boiled or only filtered in clay pots.
4. Discussion
The majority of G. duodenalis infections in this northeastern Brazilian community were identified as being assemblage B. This is similar to findings by Haque et al. (2005), in Bangladesh; however it is in contrast to studies from Mexico, where assemblage A was more prevalent (Cedillo-Rivera et al., 2003). Children with assemblage B infections shed more cysts than children infected with assemblage A. In other settings, higher stool parasite DNA burdens have also been detected for assemblage B using RT-PCR (Haque et al., 2005). This increased shedding, in combination with the faecal-oral transmission of G. duodenalis, may account for the increased prevalence of assemblage B in the community.
Children with multiple G. duodenalis infections were more likely to be infected with assemblage B during subsequent infections. These may represent re-exposure to assemblage B in a community where it is highly prevalent. Additionally, Genta et al. (1993) showed that in children infected with Cryptosporidium, oocyst shedding reflected the extent of duodenal infection with the parasite. Children with assemblage B shed significantly more cysts than children infected with assemblage A, potentially representing higher intestinal cyst burden. Subsequent assemblage B infections may therefore represent asymptomatic infections, more difficult to treat or clear because of higher intestinal cyst burden. However, given reports of high rates of asymptomatic G. duodenalis infection and carriage which may take months to clear (Pickering et al., 1984), we used different criteria to define a subsequent G. duodenalis infection than in our earlier analyses (Newman et al., 2001). In this study more than 6 months or a negative stool was required before a subsequent infection was defined, thus making asymptomatic assemblage B carriage a less likely cause for the recurrent assemblage B infections seen.
Children with mixed infections shed more cysts than those with single infections and were more likely to develop subsequent infections than those with single genotype infections. These subsequent infections were always with assemblage B and again may represent indolent assemblage B G. duodenalis infections in children with higher intestinal cyst burden, or re-exposure.
Overall, there was no difference in diarrhoeal symptoms by genotype. This is different from other studies, some of which have shown increased diarrhoea, especially in children with assemblage A (Haque et al., 2005). However, amongst children with multiple assemblage A infections there was a decrease in days of diarrhoea and length of illness with repeated infections. This decrease, however, was not significant possibly due to limited statistical power from small numbers of assemblage A infections this study.
High rates of faecal lactoferrin were seen in both genotypes and mixed infections of G. duodenalis. This is reminiscent of Cryptosporidium in developing countries, which has been shown to stimulate an inflammatory response in malnourished children (Alcantara et al., 2003; Kirkpatrick et al., 2002). It was also independent of co-infections with EAEC infections, which have also been associated with increased faecal lactoferrin in this population (Steiner et al., 1998). Additionally, there was a significant decrease in the proportion of lactoferrin-positive stools with subsequent G. duodenalis infections. This declining inflammatory response was especially prominent in children with second or third infections and lactoferrin was negative in all repeat infections that were asymptomatic. We found that repeat illnesses tended to be shorter with assemblage A infections but longer with assemblage B infections. Whether this represents an acquired immunity or difficulty in clearing G. duodenalis is unclear.
Increased levels of faecal lactoferrin, a marker of intestinal inflammation (Guerrant et al., 1992), were associated with more diarrhoeal symptoms, length of illness period, days of diarrhoea and episodes of diarrhoea. Faecal lactoferrin can be used as an important tool to predict diarrhoeal morbidity in G. duodenalis-infected children in developing countries. Our findings confirm and extend previous reports of lactoferrin as a marker of intestinal inflammation and show how lactoferrin titres are associated with more days and episodes of diarrhoeal symptoms in patients with G. duodenalis infection.
Giardia duodenalis cyst shedding was not predictive of diarrhoea, similar to another RT-PCR study where G. duodenalis stool DNA was not predictive of diarrhoeal symptoms (Haque et al., 2005). This once again suggests that induction of diarrhoea by G. duodenalis is unrelated to parasite burden.
First infections with assemblage A or B of G. duodenalis occurred at similar ages in these children living in this Brazilian shantytown. Additionally, analysis of possible sources of exposure to G. duodenalis showed no difference between children infected with assemblage A and B. Exposure to unfiltered or unboiled water was a risk factor for infection with both assemblage A and B. Because of the lack of temporal or environmental exposure differences between children it is likely that the sources of assemblage A and B of G. duodenalis are similar.
This study had several limitations. Several samples were unable to be genotyped, probably because these samples had been frozen and thawed numerous times since initial collection, leading to the degradation of DNA. Another potential explanation for this is the multiplex PCR assay’s lower sensitivity for assemblage B infections, which may not have detected assemblage B in stools with lower trophozoite and cyst burden. Additionally, the PCR assay’s lower sensitivity for assemblage B may have lead to an underestimation of the number of infections which were mixed, that is, containing both assemblages A and B. The presence of non-A, non-B genotypes of G. duodenalis in these stools cannot be excluded; however, these genotypes have only been described in animals (Caccio et al., 2005). We were limited in our ability to assess for sources of assemblage A and B within the community, which would require environmental sampling in the future. Also, there were small numbers of assemblage A and mixed infections in this study, which again limits the power of this study for assessing differences in diarrhoea in these groups. We also found a high proportion of stools containing G. duodenalis that were positive for faecal lactoferrin. We cannot entirely exclude documented or undocumented concomitant co-infections as the aetiology of increased faecal lactoferrin in these children.
In summary, we found that assemblage B G. duodenalis infections are more common and associated with greater cyst shedding than assemblage A infections in favela children in this Northeast Brazilian community, that higher levels of inflammation as determined by faecal lactoferrin are associated with first and longer infections, as well as increased diarrhoeal symptoms, and that some protection against inflammation occurs with repeated infections. There does not appear to be a significant difference in overt diarrhoeal symptoms experienced by children with either assemblage.
In past studies we have found that G. duodenalis infections are associated with impaired cognitive function, even in the absence of overt diarrhoea (Berkman et al., 2002; Patrick et al., 2005) and we have found, with others, that G. duodenalis infections or intestinal inflammation may be associated with growth failure (Prado et al., 2005; Steiner et al., 1998). Further studies to assess the long term sequelae of G. duodenalis infection by assemblage would be useful to determine if deleterious long-term effects are assemblage-specific and if treatment prevents these sequelae. Children with mixed assemblage infections require further attention given their high probability of recurrent infections. Additionally, future studies to further elucidate differences in pathophysiology between G. duodenalis assemblages should help identify pathogen and host factors that are responsible for diarrhoea, inflammation, cognitive impairment and growth stunting. This will also guide the development of effective interventions, including novel anti-parasitic or mucosal repair therapies and vaccines to decrease G. duodenalis infections, which could improve the health of children in tropical developing areas.
Acknowledgements
We are grateful to Susan Stroup for help with the multiplex PCR, Leah Barrett for help with much of the laboratory work in the initial study and to the study nurses who collected the information on childhood diarrhoea and samples.
Funding: This work and its authors were supported in part by grants from the International Collaboration in Infectious Disease Research (ICIDR: U01-AI026512) USA; the Mid Atlantic Regional Centers of Excellence (MARCE U54-AI57168) USA; Infectious Disease Training Grant (T32-AI007046) USA; the Ellison Medical Foundation (ID-T-0019-03) USA and Pfizer Foundation to the Center for Global Health USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None declared.
Ethical approval: The study protocol and informed consent were approved by the human investigations committees of the Federal University of Ceará, Fortaleza, CE, Brazil and the University of Virginia, Charlottesville, VA, USA.
References
- Alcantara CS, Yang CH, Steiner TS, Barrett LJ, Lima AA, Chappell CL, Okhuysen PC, White AC, Jr, Guerrant RL. Interleukin-8, tumor necrosis factor-alpha, and lactoferrin in immunocompetent hosts with experimental and Brazilian children with acquired cryptosporidiosis. Am. J. Trop. Med. Hyg. 2003;68:325–328. [PubMed] [Google Scholar]
- Amar CF, Dear PH, Pedraza-Diaz S, Looker N, Linnane E, McLauchlin J. Sensitive PCR-restriction fragment length polymorphism assay for detection and genotyping of Giardia duodenalis in human feces. J. Clin. Microbiol. 2002;40:446–452. doi: 10.1128/JCM.40.2.446-452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry B, Savarino SJ, Vial P, Kaper JB, Levine MM. A sensitive and specific DNA probe to identify enteroaggregative Escherichia coli, a recently discovered diarrhoeal pathogen. J. Infect. Dis. 1990;161:1249–1251. doi: 10.1093/infdis/161.6.1249. [DOI] [PubMed] [Google Scholar]
- Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359:564–571. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- Bilge SS, Apostol JM, Fullner KJ, Moseley SL. Transcriptional organization of the F1845 fimbrial adhesion determinant of Escherichia coli. Mol. Microbiol. 1993;7:993–1006. doi: 10.1111/j.1365-2958.1993.tb01191.x. [DOI] [PubMed] [Google Scholar]
- Caccio SM, Thompson RC, McLauchlin J, Smith HV. Unravelling Cryptosporidium and Giardia epidemiology. Trends. Parasitol. 2005;21:430–437. doi: 10.1016/j.pt.2005.06.013. [DOI] [PubMed] [Google Scholar]
- CDC. Addressing emerging infectious disease threats: a prevention strategy for the United States. Atlanta, GA: Department of Health and Human Services; 1994. [Google Scholar]
- Cedillo-Rivera R, Darby JM, Enciso-Moreno JA, Ortega-Pierres G, Ey PL. Genetic homogeneity of axenic isolates of Giardia intestinalis derived from acute and chronically infected individuals in Mexico. Parasitol. Res. 2003;90:119–123. doi: 10.1007/s00436-002-0807-0. [DOI] [PubMed] [Google Scholar]
- El-Shazly AM, Mowafy N, Soliman M, El-Bendary M, Morsy AT, Ramadan NI, Arafa WA. Egyptian genotyping of Giardia lamblia. J. Egypt. Soc. Parasitol. 2004;34:265–280. [PubMed] [Google Scholar]
- Fang GD, Lima AA, Martins CV, Nataro JP, Guerrant RL. Etiology and epidemiology of persistent diarrhoea in northeastern Brazil: a hospital-based, prospective, case control study. J. Pediatr. Gastoenterol. Nutr. 1995;21:137–144. doi: 10.1097/00005176-199508000-00003. [DOI] [PubMed] [Google Scholar]
- Genta RM, Chappell CL, White AC, Jr, Kimball KT, Goodgame RW. Duodenal morphology and intensity of infection in AIDS-related intestinal cryptosporidiosis. Gastroenterology. 1993;105:1769–1775. doi: 10.1016/0016-5085(93)91075-s. [DOI] [PubMed] [Google Scholar]
- Guerrant RL, Araujo V, Soares E, Kotloff K, Lima AA, Cooper WH, Lee AG. Measurement of fecal lactoferrin as a marker of fecal leukocytes. J. Clin. Microbiol. 1992;30:1238–1242. doi: 10.1128/jcm.30.5.1238-1242.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R, Roy S, Kabir M, Stroup SE, Mondal D, Houpt ER. Giardia assemblage A infection and diarrhea in Bangladesh. J. Infect. Dis. 2005;192:2171–2173. doi: 10.1086/498169. [DOI] [PubMed] [Google Scholar]
- Homan WL, Mank TG. Human giardiasis: Genotype linked differences in clinical symptomatology. Int. J. Parasitol. 2001;31:822–826. doi: 10.1016/s0020-7519(01)00183-7. [DOI] [PubMed] [Google Scholar]
- Jerse AE, Gicquelais KG, Kaper JB. Plasmid and chromosomal elements involved in the pathogenesis of attaching and effacing Escherichia coli. Infect. Immun. 1991;59:3869–3875. doi: 10.1128/iai.59.11.3869-3875.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick BD, Daniels MM, Jean SS, Pape JW, Karp C, Littenberg B, Fitzgerald DW, Lederman HM, Nataro JP, Sears CL. Cryptosporidiosis stimulates an inflammatory intestinal response in malnourished Haitian children. J. Infect. Dis. 2002;186:94–101. doi: 10.1086/341296. [DOI] [PubMed] [Google Scholar]
- Levine MM, Xu J, Kaper JB, Lior H, Prado V, Tall B, Nataro J, Karch H, Wachsmuth K. A DNA probe to identify enterohemorrhagic Escherichia coli of O157:H7 and other serotypes that cause hemorrhagic colitis and hemolytic uremic syndrome. J. Infect. Dis. 1987;156:175–182. doi: 10.1093/infdis/156.1.175. [DOI] [PubMed] [Google Scholar]
- Nataro JP, Baldini MM, Kaper JB, Black RE, Bravo N, Levine MM. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J. Infect. Dis. 1985;152:560–565. doi: 10.1093/infdis/152.3.560. [DOI] [PubMed] [Google Scholar]
- Nataro JP, Kaper JB, Robins-Browne R, Prado V, Vial P, Levine MM. Patterns of adherence of diarrheagenic Escherichia coli to Hep-2 cells. Pediatr. Infect. Dis. J. 1987;6:829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- Newman RD, Zu SX, Wuhib T, Lima AA, Guerrant RL, Sears CL. Household epidemiology of Cryptosporidium parvum infection in an urban community in northeast Brazil. Ann. Intern. Med. 1994;120:500–505. doi: 10.7326/0003-4819-120-6-199403150-00009. [DOI] [PubMed] [Google Scholar]
- Newman RD, Moore SR, Lima AA, Nataro JP, Guerrant RL, Sears CL. A longitudinal study of Giardia lamblia infection in north-east Brazilian children. Trop. Med. Int. Health. 2001;6:624–634. doi: 10.1046/j.1365-3156.2001.00757.x. [DOI] [PubMed] [Google Scholar]
- Ng CT, Gilchrist CA, Lane A, Roy S, Haque R, Houpt ER. Multiplex real-time PCR assay using scorpion probes and DNA capture for genotype-specific detection of Giardia lamblia on fecal samples. J. Clin. Microbiol. 2005;43:1256–1260. doi: 10.1128/JCM.43.3.1256-1260.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega YR, Adam RD. Giardia: overview and update. Clin. Infect. Dis. 1997;25:545–549. doi: 10.1086/513745. [DOI] [PubMed] [Google Scholar]
- Patrick PD, Oria RB, Madhavan V, Pinkerton RC, Lorntz B, Lima AA, Guerrant RL. Limitations in verbal fluency following heavy burdens of early childhood diarrhea in Brazilian shantytown children. Child Neuropsychol. 2005;11:233–244. doi: 10.1080/092970490911252. [DOI] [PubMed] [Google Scholar]
- Pickering LK, Woodward WE, DuPont HL, Sullivan P. Occurrence of Giardia lamblia in children in day care centers. J. Pediatr. 1984;104:522–526. doi: 10.1016/s0022-3476(84)80540-5. [DOI] [PubMed] [Google Scholar]
- Ponce-Macotela M, Martinez-Gordillo MN, Bermudez-Cruz RM, Salazar-Schettino PM, Ortega-Pierres G, Ey PL. Unusual prevalence of the Giardia intestinalis A-II subtype amongst isolates from humans and domestic animals in Mexico. Int. J. Parasitol. 2002;32:1201–1202. doi: 10.1016/s0020-7519(02)00086-3. [DOI] [PubMed] [Google Scholar]
- Prado MS, Cairncross S, Strina A, Barreto ML, Oliveira-Assis AM, Rego S. Asymptomatic giardiasis and growth in young children; a longitudinal study in Salvador, Brazil. Parasitology. 2005;131:51–56. doi: 10.1017/s0031182005007353. [DOI] [PubMed] [Google Scholar]
- Read C, Walters J, Robertson ID, Thompson RC. Correlation between genotype of Giardia duodenalis and diarrhoea. Int. J. Parasitol. 2002;32:229–231. doi: 10.1016/s0020-7519(01)00340-x. [DOI] [PubMed] [Google Scholar]
- Steiner TS, Lima AA, Nataro JP, Guerrant RL. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J. Infect. Dis. 1998;177:88–96. doi: 10.1086/513809. [DOI] [PubMed] [Google Scholar]
- Sulaiman IM, Fayer R, Bern C, Gilman RH, Trout JM, Schantz PM, Das P, Lal AA, Xiao LL. Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg. Infect. Dis. 2003;9:1444–1452. doi: 10.3201/eid0911.030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RC, Hopkins RM, Homan WL. Nomenclature and genetic groupings of Giardia infecting mammals. Parasitol. Today. 2000;16:210–213. doi: 10.1016/s0169-4758(99)01624-5. [DOI] [PubMed] [Google Scholar]
- WHO. Persistent diarrhea in children in developing countries: Memorandum from a WHO meeting. World Health Organ; Bull. 1988;66:709. [PMC free article] [PubMed]
- Wood PK, Morris JG, Small PL, Sethabutr O, Toledo MR, Trabulsi L, Kaper JB. Comparison of DNA probes and the Sereny test for identification of invasive Shigella and Escherichia coli strains. J. Clin. Microbiol. 1986;24:498–500. doi: 10.1128/jcm.24.3.498-500.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]