Abstract
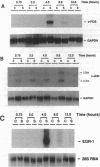
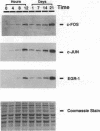
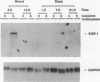
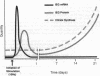
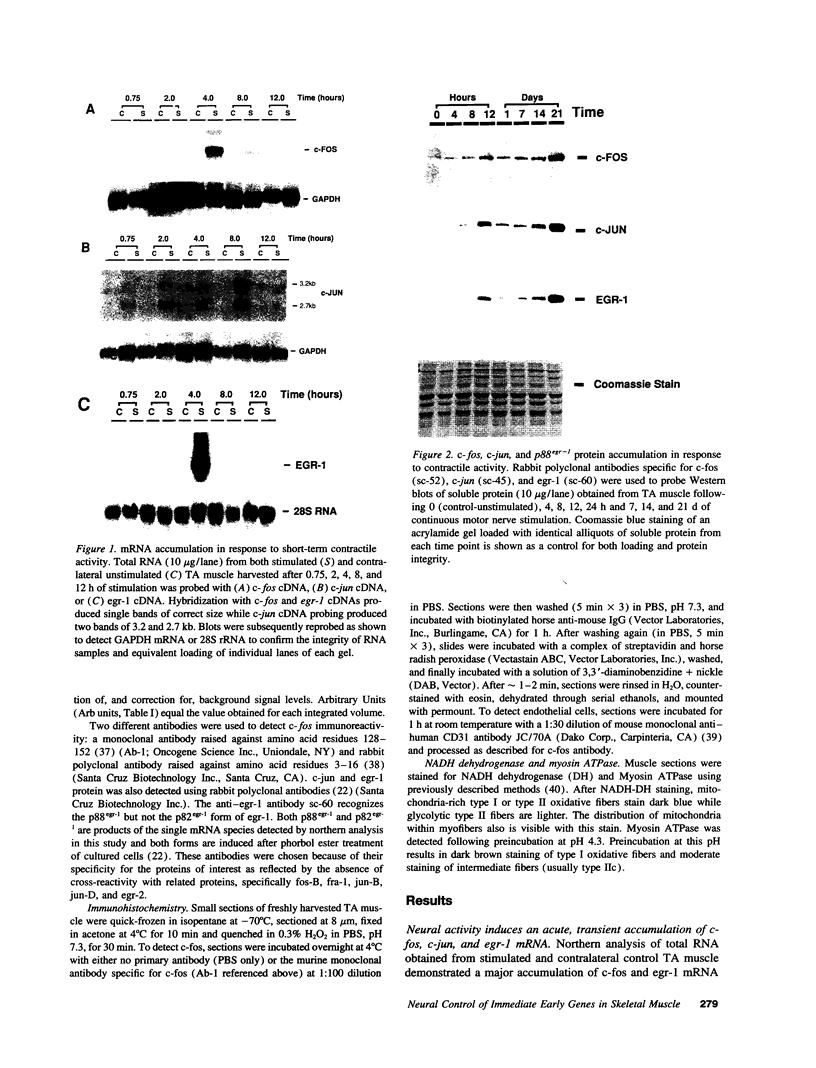
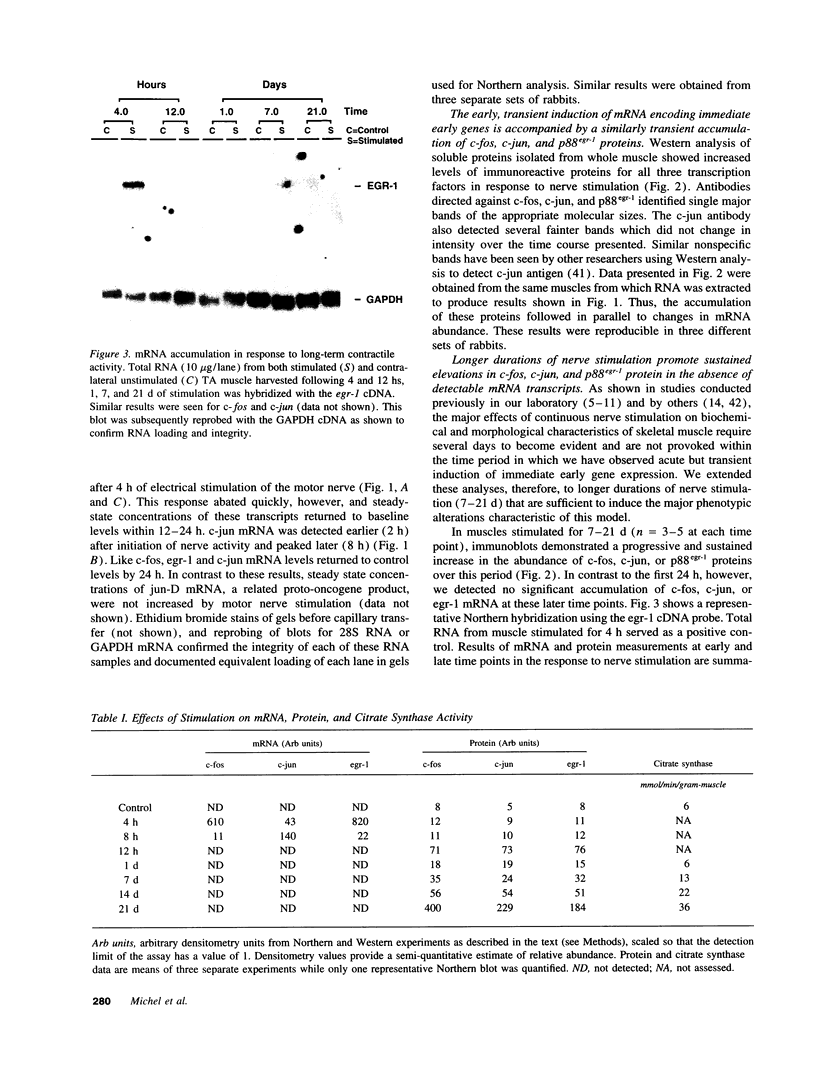
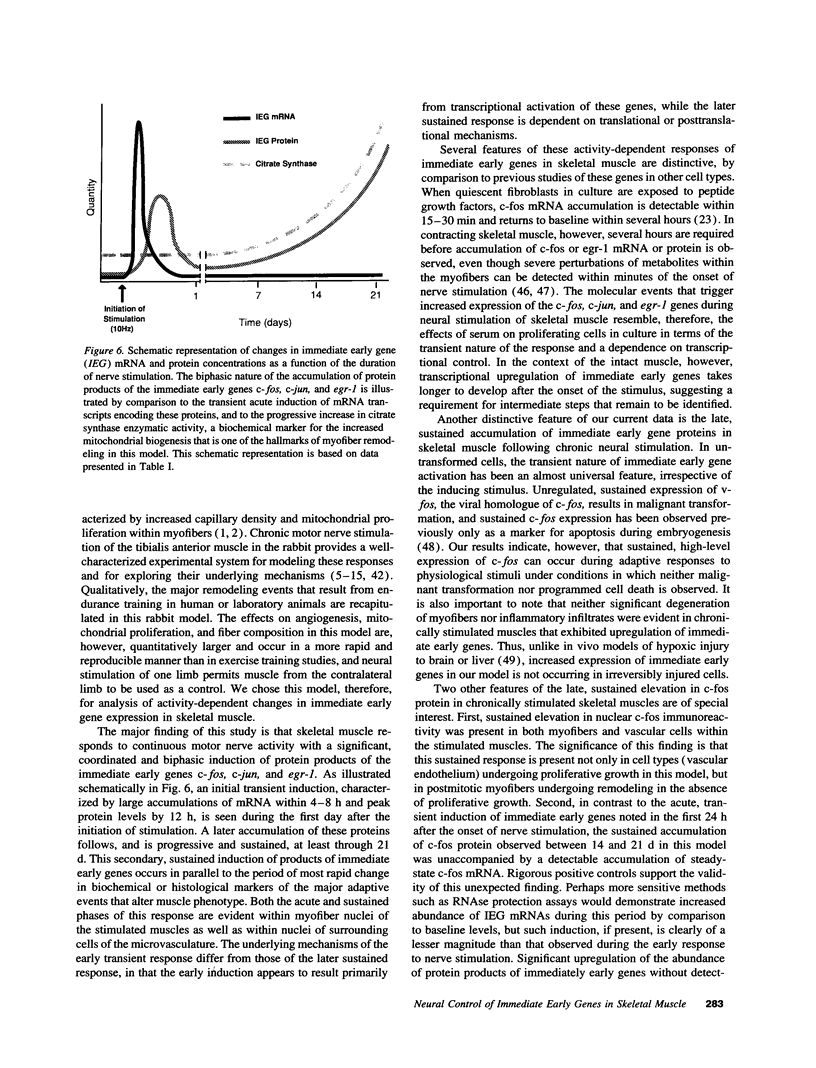
Sustained contractile activity of skeletal muscle promotes angiogenesis, as well as transformation of contractile protein isoforms and mitochondrial proliferation within myofibers. Since the products of immediate early genes such as c-fos, c-jun, and egr-1 function in many signaling pathways governing cellular responses to external stimuli, we sought to determine whether sustained contractile activity induces their expression in skeletal muscle. Low voltage electrical stimulation was applied to the motor nerve innervating rabbit tibialis anterior muscles for periods ranging from 45 min to 21 d. Northern and Western analysis demonstrated marked but transient inductions of c-fos, c-jun, and egr-1 mRNA and protein within the first 24 h. Longer durations of stimulation were associated with a secondary and sustained rise in the abundance of c-fos, c-jun, and p88egr-1 protein that, surprisingly, was not accompanied by detectable changes in mRNA. Immunohistochemistry demonstrated c-fos immunoreactivity within myofiber and vascular cell nuclei during both early and late phases of this response. These findings reveal a complex pattern of c-fos, c-jun, and egr-1 expression in response to nerve stimulation and suggest that these proteins could function in regulatory pathways that modify muscle phenotype.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annex B. H., Kraus W. E., Dohm G. L., Williams R. S. Mitochondrial biogenesis in striated muscles: rapid induction of citrate synthase mRNA by nerve stimulation. Am J Physiol. 1991 Feb;260(2 Pt 1):C266–C270. doi: 10.1152/ajpcell.1991.260.2.C266. [DOI] [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Brown W. E., Salmons S., Whalen R. G. The sequential replacement of myosin subunit isoforms during muscle type transformation induced by long term electrical stimulation. J Biol Chem. 1983 Dec 10;258(23):14686–14692. [PubMed] [Google Scholar]
- Cao X. M., Koski R. A., Gashler A., McKiernan M., Morris C. F., Gaffney R., Hay R. V., Sukhatme V. P. Identification and characterization of the Egr-1 gene product, a DNA-binding zinc finger protein induced by differentiation and growth signals. Mol Cell Biol. 1990 May;10(5):1931–1939. doi: 10.1128/mcb.10.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine K. G., Baracchini E., Goldman D. Coupling muscle electrical activity to gene expression via a cAMP-dependent second messenger system. J Biol Chem. 1993 Feb 5;268(4):2893–2898. [PubMed] [Google Scholar]
- Christy B., Nathans D. DNA binding site of the growth factor-inducible protein Zif268. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8737–8741. doi: 10.1073/pnas.86.22.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M., Young D., Hughes P., MacGibbon G., Lawlor P., Singleton K., Sirimanne E., Beilharz E., Gluckman P. Is c-Jun involved in nerve cell death following status epilepticus and hypoxic-ischaemic brain injury? Brain Res Mol Brain Res. 1993 Jun;18(4):347–352. doi: 10.1016/0169-328x(93)90101-t. [DOI] [PubMed] [Google Scholar]
- Eisenberg B. R., Salmons S. The reorganization of subcellular structure in muscle undergoing fast-to-slow type transformation. A stereological study. Cell Tissue Res. 1981;220(3):449–471. doi: 10.1007/BF00216750. [DOI] [PubMed] [Google Scholar]
- Giardina S. L., Evans S. W., Gandino L., Robey F. A., Bonvini E., Longo D. L., Varesio L. Generation of a murine monoclonal antibody that detects the fos oncogene product. Anal Biochem. 1987 Feb 15;161(1):109–116. doi: 10.1016/0003-2697(87)90659-2. [DOI] [PubMed] [Google Scholar]
- Green H. J., Cadefau J., Pette D. Altered glucose 1,6-bisphosphate and fructose 2,6-biphosphate levels in low-frequency stimulated rabbit fast-twitch muscle. FEBS Lett. 1991 Apr 22;282(1):107–109. doi: 10.1016/0014-5793(91)80455-c. [DOI] [PubMed] [Google Scholar]
- Henriksson J., Chi M. M., Hintz C. S., Young D. A., Kaiser K. K., Salmons S., Lowry O. H. Chronic stimulation of mammalian muscle: changes in enzymes of six metabolic pathways. Am J Physiol. 1986 Oct;251(4 Pt 1):C614–C632. doi: 10.1152/ajpcell.1986.251.4.C614. [DOI] [PubMed] [Google Scholar]
- Holloszy J. O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967 May 10;242(9):2278–2282. [PubMed] [Google Scholar]
- Hudlicka O., Tyler K. R. The effect of long-term high-frequency stimulation on capillary density and fibre types in rabbit fast muscles. J Physiol. 1984 Aug;353:435–445. doi: 10.1113/jphysiol.1984.sp015345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isfort R. J., Cody D. B., Asquith T. N., Ridder G. M., Stuard S. B., LeBoeuf R. A. Induction of protein phosphorylation, protein synthesis, immediate-early-gene expression and cellular proliferation by intracellular pH modulation. Implications for the role of hydrogen ions in signal transduction. Eur J Biochem. 1993 Apr 1;213(1):349–357. doi: 10.1111/j.1432-1033.1993.tb17768.x. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988 Jan;85(2):339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L. D., Inoue J., Verma I. M. Signal transduction: the nuclear target. Curr Opin Cell Biol. 1992 Jun;4(3):496–501. doi: 10.1016/0955-0674(92)90017-7. [DOI] [PubMed] [Google Scholar]
- Kraus W. E., Bernard T. S., Williams R. S. Interactions between sustained contractile activity and beta-adrenergic receptors in regulation of gene expression in skeletal muscles. Am J Physiol. 1989 Mar;256(3 Pt 1):C506–C514. doi: 10.1152/ajpcell.1989.256.3.C506. [DOI] [PubMed] [Google Scholar]
- Lemaire P., Vesque C., Schmitt J., Stunnenberg H., Frank R., Charnay P. The serum-inducible mouse gene Krox-24 encodes a sequence-specific transcriptional activator. Mol Cell Biol. 1990 Jul;10(7):3456–3467. doi: 10.1128/mcb.10.7.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987 Nov 6;238(4828):797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Minotti J. R., Johnson E. C., Hudson T. L., Zuroske G., Murata G., Fukushima E., Cagle T. G., Chick T. W., Massie B. M., Icenogle M. V. Skeletal muscle response to exercise training in congestive heart failure. J Clin Invest. 1990 Sep;86(3):751–758. doi: 10.1172/JCI114771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow N. G., Kraus W. E., Moore J. W., Williams R. S., Swain J. L. Increased expression of fibroblast growth factors in a rabbit skeletal muscle model of exercise conditioning. J Clin Invest. 1990 Jun;85(6):1816–1820. doi: 10.1172/JCI114640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyses L., Nouskas J., Vetter H. Inhibition of endothelin-1 induced myocardial protein synthesis by an antisense oligonucleotide against the early growth response gene-1. Biochem Biophys Res Commun. 1991 Nov 27;181(1):22–27. doi: 10.1016/s0006-291x(05)81376-2. [DOI] [PubMed] [Google Scholar]
- Nguyen H. Q., Hoffman-Liebermann B., Liebermann D. A. The zinc finger transcription factor Egr-1 is essential for and restricts differentiation along the macrophage lineage. Cell. 1993 Jan 29;72(2):197–209. doi: 10.1016/0092-8674(93)90660-i. [DOI] [PubMed] [Google Scholar]
- Ordway G. A., Li K., Hand G. A., Williams R. S. RNA subunit of mitochondrial RNA-processing enzyme is induced by contractile activity in striated muscle. Am J Physiol. 1993 Dec;265(6 Pt 1):C1511–C1516. doi: 10.1152/ajpcell.1993.265.6.C1511. [DOI] [PubMed] [Google Scholar]
- Paffenbarger R. S., Jr, Hyde R. T., Wing A. L., Lee I. M., Jung D. L., Kampert J. B. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993 Feb 25;328(8):538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- Parums D. V., Cordell J. L., Micklem K., Heryet A. R., Gatter K. C., Mason D. Y. JC70: a new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J Clin Pathol. 1990 Sep;43(9):752–757. doi: 10.1136/jcp.43.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette D., Vrbová G. Adaptation of mammalian skeletal muscle fibers to chronic electrical stimulation. Rev Physiol Biochem Pharmacol. 1992;120:115–202. doi: 10.1007/BFb0036123. [DOI] [PubMed] [Google Scholar]
- Rabin R., Gordon S. L., Lymn R. W., Todd P. W., Frey M. A., Sulzman F. M. Effects of spaceflight on the musculoskeletal system: NIH and NASA future directions. FASEB J. 1993 Mar;7(5):396–398. doi: 10.1096/fasebj.7.5.8462780. [DOI] [PubMed] [Google Scholar]
- Ransone L. J., Verma I. M. Nuclear proto-oncogenes fos and jun. Annu Rev Cell Biol. 1990;6:539–557. doi: 10.1146/annurev.cb.06.110190.002543. [DOI] [PubMed] [Google Scholar]
- Rao G. N., Berk B. C. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res. 1992 Mar;70(3):593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- Ryder K., Nathans D. Induction of protooncogene c-jun by serum growth factors. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8464–8467. doi: 10.1073/pnas.85.22.8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J., Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J. 1993 Apr;12(4):1681–1692. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmons S., Vrbová G. The influence of activity on some contractile characteristics of mammalian fast and slow muscles. J Physiol. 1969 May;201(3):535–549. doi: 10.1113/jphysiol.1969.sp008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambucetti L. C., Curran T. The Fos protein complex is associated with DNA in isolated nuclei and binds to DNA cellulose. Science. 1986 Dec 12;234(4782):1417–1419. doi: 10.1126/science.3491427. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Lamph W. W., Verma I. M. Regulation of proto-oncogene fos: a paradigm for early response genes. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):749–760. doi: 10.1101/sqb.1988.053.01.085. [DOI] [PubMed] [Google Scholar]
- Smeyne R. J., Vendrell M., Hayward M., Baker S. J., Miao G. G., Schilling K., Robertson L. M., Curran T., Morgan J. I. Continuous c-fos expression precedes programmed cell death in vivo. Nature. 1993 May 13;363(6425):166–169. doi: 10.1038/363166a0. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sukhatme V. P., Cao X. M., Chang L. C., Tsai-Morris C. H., Stamenkovich D., Ferreira P. C., Cohen D. R., Edwards S. A., Shows T. B., Curran T. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988 Apr 8;53(1):37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Suva L. J., Ernst M., Rodan G. A. Retinoic acid increases zif268 early gene expression in rat preosteoblastic cells. Mol Cell Biol. 1991 May;11(5):2503–2510. doi: 10.1128/mcb.11.5.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinakaran G., Ojala J., Bag J. Expression of c-jun/AP-1 during myogenic differentiation in mouse C2C12 myoblasts. FEBS Lett. 1993 Mar 22;319(3):271–276. doi: 10.1016/0014-5793(93)80561-8. [DOI] [PubMed] [Google Scholar]
- Uemura Y., Kowall N. W., Moskowitz M. A. Focal ischemia in rats causes time-dependent expression of c-fos protein immunoreactivity in widespread regions of ipsilateral cortex. Brain Res. 1991 Jun 21;552(1):99–105. doi: 10.1016/0006-8993(91)90665-i. [DOI] [PubMed] [Google Scholar]
- Williams R. S., Garcia-Moll M., Mellor J., Salmons S., Harlan W. Adaptation of skeletal muscle to increased contractile activity. Expression nuclear genes encoding mitochondrial proteins. J Biol Chem. 1987 Feb 25;262(6):2764–2767. [PubMed] [Google Scholar]
- Williams R. S. Mitochondrial gene expression in mammalian striated muscle. Evidence that variation in gene dosage is the major regulatory event. J Biol Chem. 1986 Sep 15;261(26):12390–12394. [PubMed] [Google Scholar]
- Williams R. S., Salmons S., Newsholme E. A., Kaufman R. E., Mellor J. Regulation of nuclear and mitochondrial gene expression by contractile activity in skeletal muscle. J Biol Chem. 1986 Jan 5;261(1):376–380. [PubMed] [Google Scholar]