Abstract
It is well accepted that a low intensity/long duration isometric contraction induces more low frequency fatigue (LFF) compared to a high-intensity/short-duration contraction. However, previous reports examined the intensity/duration of the contraction but did not control the level of fatigue when concluding fatigue is task dependent. The purpose of this study was to determine whether a long duration/low intensity fatiguing contraction would induce greater LFF than a short duration/high-intensity contraction when the quadriceps muscle was fatigued to similar levels. Eighteen healthy male subjects performed quadriceps contractions sustained at 35% and 65% of maximal voluntary contraction (MVC) on separate days, until the tasks induced a similar amount of fatigue (force generating capacity = 45% MVC). Double pulse torque to single pulse torque ratio (D/S ratio) was obtained before, immediately and 5 min after fatigue along with the electromyographic (EMG) signal from vastus medialis (VM) and rectus femoris (RF). The D/S ratio significantly (p < 0.05) increased by 8.7 ± 8.5% (mean ± SD) and 10.2 ± 9.2% after 35% and 65% tasks, respectively, and remained elevated 5 min into recovery; however, there was no significant difference in ratio between the two sessions immediately or 5 min post-fatigue (p > 0.05) even though the endurance time for the 35% fatigue task (124 ± 39.68 s) was significantly longer (p = 0.05) than that of the 65% task (63 ± 17.73 s). EMG amplitude and median power frequency (MPF) analysis also did not reveal any significant differences between these two sessions after fatigue. These findings indicate that LFF fatigue is fatigue dependent as well as task intensity/duration dependent. These findings assist us in understanding task dependency and muscle fatigue.
Keywords: Task dependency, Equivalent fatigue, Double/single ratio, Excitation–contraction coupling, Electrical stimulation
1. Introduction
Muscle fatigue is conventionally defined as “a inability to produce or sustain a desired force or power” (Gandevia, 2001). Physiological mechanisms responsible for muscle fatigue can involve all elements of the motor system from a decrease in descending drive from the supraspinal centers to activate motor neurons to a reduction in the activity of contractile proteins in skeletal muscle fibers (Bigland-Ritchie, 1981). Although multiple mechanisms usually contribute to muscle fatigue simultaneously, “task dependency of muscle fatigue” says that how a muscle is fatigued determines which of these mechanisms is activated and to what extent (Enoka and Stuart, 1992; Fuglevand et al., 1993; Enoka, 1995). Depending on how muscle is fatigued, a specific type of muscle fatigue, known as low frequency fatigue (LFF) can be induced.
LFF is a type of muscle fatigue that can last up to 24 h after exercise (Edwards et al., 1977). It is named so because of a preferential decrease in the torque elicited with electrical stimulation at a low frequency, compared to a high frequency. The mechanism for LFF is not fully understood; however, a decrease of sarcoplasmic reticulum Ca2+ release per action potential and/or impaired regulatory light chain phosphorylation (R-LC), which leads to excitation–contraction (E–C) coupling failure (Westerblad et al., 1993; Chin and Allen, 1996; Hill et al., 2001; Tubman et al., 1997), has been implicated.
Like other mechanisms of muscle fatigue, E–C coupling failure is also suggested to be task dependent. For example, when subjects sustained 20% maximal voluntary contraction (MVC) of the first dorsal interosseous muscle until they could no longer maintain that target force, a greater depression in single twitch force was observed compared to when subjects maintained 65% MVC (Fuglevand et al., 1993). The authors of the study attributed this depression in twitch force after fatigue to LFF, thus their conclusion was that LFF was also task dependent. Indeed, if a task used to fatigue muscles is a long duration with low intensity, it induces a greater extent of LFF, compared to a short-duration high-intensity task. This notion was supported by the study of Lannergren and Westerblad (1991), where they fatigued single, intact mouse muscle fibers electrically, and divided the observed tension decline into three phases. They attributed the first two phases of the force decline to the impairment in contractile elements (cross-bridge function) and the last third phase to LFF, suggesting that duration of a task was a key factor in determining to what extent LFF develops. However, LFF has also been shown not only after low intensity/long duration fatigue tasks, but also after high intensity/short-duration tasks. Skurvydas et al. (2003) found a change in the ratio of the torque elicited by electrical stimulation at 20 Hz and 50 Hz (20/50) after a maximal voluntary isometric contraction of the quadriceps sustained for 60 s. Tubman et al. (1997) found a similar change in the ratio of the torque elicited by a double pulse (166 Hz) and single pulse to support that the R-LC phosphorylation system is impaired with disuse.
These previous reports raise the question “does the duration and intensity of the contraction determine the extent of LFF or is it based on the amount of fatigue induced?” When sub maximal isometric contractions are held at two different target forces (20% and 65% for example) until they cannot sustain that target force the amount the muscle has fatigued is different (80% and 45%, respectively) (Fuglevand et al., 1993). This difference in fatigue makes the interpretation of previous results difficult.
Therefore, the purpose of the study was to determine if the intensity/duration of a fatiguing contraction influences LFF when the magnitude of the fatigue induced remains constant. We employed two tasks in which subjects held their force at different target levels (35% and 65% MVC) until the force generating capacity of the muscle had declined to the same level (45% MVC). Observing a similar amount of LFF after these two tasks would indicate that LFF is fatigue dependent, rather than duration/intensity dependent.
2. Methods
2.1. Subjects
Eighteen healthy male subjects (age = 25.7 ± 7.4 (mean ± SD) years, height = 173.1 ± 4.24 cm, and weight = 82.6 ± 15.3 kg) volunteered to participate in this study. Only male subjects were recruited in the study to control gender differences in endurance time (Hicks et al., 2001). Informed consent was obtained from all the subjects in accordance with the Human Ethics Review Committee at the University of Iowa.
2.2. Force recording
Subjects were seated upright and performed isometric contraction of the right quadriceps muscle on the Kincom isokinetic testing system (125 E Plus Chattanooga Group, Inc.). The subjects were secured with a seat belt, shoulder and thigh straps to prevent unwanted movements. The right leg was positioned with knee angle fixed at 95° (0° being full extension). We chose this angle because a previous study has demonstrated that LFF is seen more at longer muscle lengths than at shorter length (Jones et al., 1989). The knee joint axis was aligned with that of the Kincom for each subject, and sufficient space was maintained between the popliteal fossa and Kincom seat to prevent discomfort and occlusion of blood vessels. The distal pad on the movement arm of the Kincom was placed comfortably on the subjects’ shin just proximal to the malleoli. The distance from the pad to the axis of the knee joint was the lever arm, which was used for torque calculations. Visual feedback of the force generated by the subjects was available through an oscilloscope screen. This force recording set-up was used to record force elicited by electrical stimulation (see below) as well.
2.3. Electromyographic (EMG) recording
EMG activity was recorded with bipolar silver–sliver chloride surface electrodes (1 cm diameter and 2 cm inter-electrode distance, Therapeutics Unlimited, Iowa City, IA). After shaving and cleaning the skin over the vastus medialis (VM) and rectus femoris (RF) muscles using alcohol pads, electrodes were placed with conductive gel and adhesive pads. For RF, the electrode was placed at the mid-point of the line joining anterior superior iliac spine and superior patellar pole, and for VM, it was placed slightly proximal and medial to the patella. These electrodes were placed parallel to estimated muscle fibers of both muscles. A common ground electrode was placed on the distal shin of the left leg.
The signal from all the electrodes was preamplified on site with a gain of 35 and then differentially amplified (input impedance of 15MΩ at 100 Hz; frequency response 15–4000 Hz; common mode rejection ratio 87 dB at 60 Hz) with an overall gain of 200 (Therapeutics Unlimited, Iowa City, IA).
2.4. Electrical stimulation
The quadriceps was electrically stimulated to quantify the amount of LFF (by calculating a ratio of the double pulse force to the single pulse force, D/S ratio), using two gel adhesive pads (3.5 × 5 cm, Conmed Corporation Utica, NY) and a custom designed stimulator. The stimulator was under control of customized computer software, and the subjects received 18 pairs of alternating single and double pulses. Each pulse was separated by 3 s. The electrodes were applied at 50% and 85% of the distance from the right anterior superior iliac spine to the superior patellar pole.
A maximal stimulating intensity was determined by increasing the intensity until the peak force elicited with a 500 ms single pulse no longer increased. Once the maximal intensity was determined, this intensity was maintained throughout the session. The average force produced by the single pulse with the maximal intensity was approximately 60.3–80.3 N m (18.5–24.6% MVC). Double pulse force was elicited by two pulses separated by 6 ms (166 Hz), which was always preceded by the single pulse.
2.5. Experimental procedures
Each of the subjects was asked to participate in two sessions (35% MVC and 65% MVC sessions) and a third session was used if the subject did not meet the appropriate criteria for fatigue (see below). The sessions were separated by one week, and half of the subjects participated in 65% session first while the other half started with the 35% session.
The following six measurements were recorded during each session: (1) pre-fatigue D/S ratio, (2) pre-fatigue MVCs, (3) fatigue task (either 35% or 65% MVC), (4) post-fatigue D/S ratio, (5) post-5-min-fatigue MVC, and finally (6) post-5-min-fatigue D/S ratio. Strong verbal encouragement was given to the subjects during pre-, and post-fatigue MVCs, and during the fatigue task.
2.6. Pre-fatigue measurements
After subjects were seated and secured to the Kincom, the recording and stimulating electrodes were placed as described above. For the first session, all the necessary information (e.g., lever arm, electrode position) to reproduce the EMG/force recordings, as well as the stimulating set-up was recorded to keep consistency across sessions for each subject. After the maximal stimulating intensity was determined, 18 pairs of alternating single and double pulses were given to obtain pre-fatigue D/S ratio. Then the subjects were asked to produce three 3-s MVCs in knee extension with each contraction separated by 15 s.
2.7. Fatigue task
A target force (35% or 65% MVC) was calculated from the highest MVC among the three pre-fatigue MVCs, and was displayed on the screen as a horizontal line. Additional horizontal lines were placed at 45% and 55% MVC, which were used for investigators to determine the end point. For the 35% MVC task (Fig. 1a), the subject was instructed to maintain 35% MVC as long as possible. When investigators suspected that maintaining the target level was becoming very difficult for the subject due to fatigue, they asked the subject to give a maximal contraction. As soon as the investigators saw this maximal contraction was able to produce force exceeding 55% MVC, the investigators asked the subject to bring the force back to the target 35% line. If this maximal contraction failed to produce force exceeding 55% MVC, resulting in producing force fell between 55% and 45% MVC, the subject was asked to extend his knee as hard as possible until the force dropped to the 45% target line. The subject was asked to relax and the 35% MVC task was ended if the force remained under the 45% line for 3 s. In some subjects, the force generated by the maximal effort made periodically did not reach even 45% MVC line (the force falling between 35% and 45% MVC) due to too much fatigue. When this happened, the whole data for that session was completely discarded, and a third session was performed a week later. Therefore, only sessions successfully performed were used for data analysis.
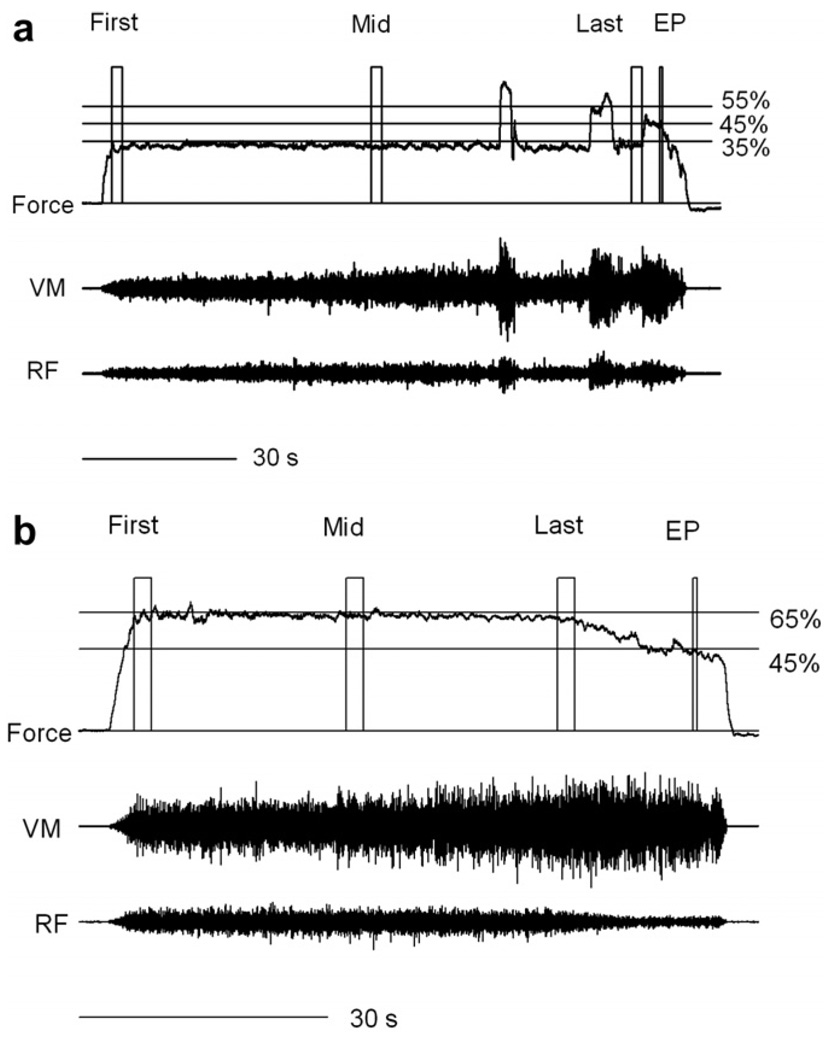
Fig. 1.
Representative example of 35% (a) and 65% (b) MVC fatigue tasks performed by one subject. From the top, voluntary force, EMG signals of vastus medialis (VM) and rectus femoris (RF) are shown. The square blocks superimposed on the force are the 2-s first, middle (Mid), last and 520-ms end point (EP) windows used to analyze the data. For the 35% task, the subject was asked to give his maximal effort three times during this specific task. The last effort did not produce force exceeding 55% MVC, so the subject was asked to give their maximal effort as long as possible until the force dropped to 45% MVC. For the 65% task, the subject was asked to sustain the target level of 65% MVC as long as possible. Even after it became impossible to sustain that target level, the subject was asked to give their maximal effort as long as possible until the force dropped to 45% MVC. Note the difference in the length of the 30-s lines between the two tasks.
For the 65% MVC task (Fig. 1b), the subject was given directions to hold the force on the 65% target line as long as possible. Even when it became impossible to hold the force on the target line of 65% MVC due to fatigue, the subject was still asked to continue giving their maximal effort as long as possible until the force dropped to the 45% line. When the force remained under 45% MVC for 3 s, the subject was asked to relax, and the 65% task was terminated.
2.8. Post-fatigue measurements
Immediately after the fatigue task, D/S ratio was recorded in the same way as pre-fatigue D/S ratio. After a rest of 5 min from the end of the fatigue task, the subject was asked to give a final 3-s MVC, and then the final D/S ratio was recorded.
2.9. Data analysis
Voluntary force and EMG signals were sampled at 1000 Hz and the double/ single twitches were sampled at 500 Hz. Force for pre- and post-fatigue MVCs was determined as the highest point in each trial. A window of 520-ms duration centered on this highest force was used to calculate EMG root mean square (RMS) amplitude and median power frequency (MPF) using fast Fourier transform (FFT) with 512 points for pre- and post-fatigue MVCs. Three windows (Fig. 1) whose duration was 2 s were selected for the first, middle and last parts of the fatigue task where the force was maintained at the target force of either 35% or 65% MVC (visual inspection). When determining the window positions for the middle and last parts of the fatigue task care was taken not to include the data recorded when the subject was asked to give his maximal effort in the 35% task. Force, EMG RMS amplitude, and MPF at the end of the fatigue task (end point) were determined from a 520-ms window centered on the point at which the force crossed 45% MVC. Since the voluntary force crossed the 45% MVC line multiple times, the very last point was chosen. Endurance time was calculated as the time between the beginning of the first window and the end point window. All the force calculated was converted to torque by multiplying by the lever arm, and torque, EMG RMS amplitude and MPF were all normalized to the highest respective values obtained before fatigue.
The peak force elicited by each of the single and double pulses was determined only for the last three pairs out of the 18 pairs in order to insure stable D/S ratios. There was no increase in the force for the last three pairs, indicating that the muscle was fully potentiated. Preliminary data indicated that the D/S ratio was very reproducible both within and between sessions (ICC’s = 0.92 and 0.96, respectively). Within and between session change in the D/S ratio was less than 2% and 4%, respectively. Thus, we had over 95% confidence that we could measure changes as low as 4% within a session. All ratios were calculated from the single pulse that immediately preceded the double pulse.
2.10. Statistics
Three-way analysis of variances (ANOVAs) for repeated measurement were used to identify the effects of fatigue (pre-fatigue, first, middle, last and end point of fatigue task, and post-fatigue), session (35% and 65%) and muscle (VM and RF) on EMG amplitude and MPF. Two-way ANOVAs for repeated measurement (session × fatigue) were used for voluntary torque and D/S ratio. Bonferroni post hoc tests were used when appropriate. A paired t-test was used to compare endurance time of the two tasks. Statistical significant differences were identified using a p-value of 0.05. All the means are reported as mean ± SD in text, whereas the error bars shown in figures are standard error.
3. Results
3.1. Voluntary torque and fatigue task
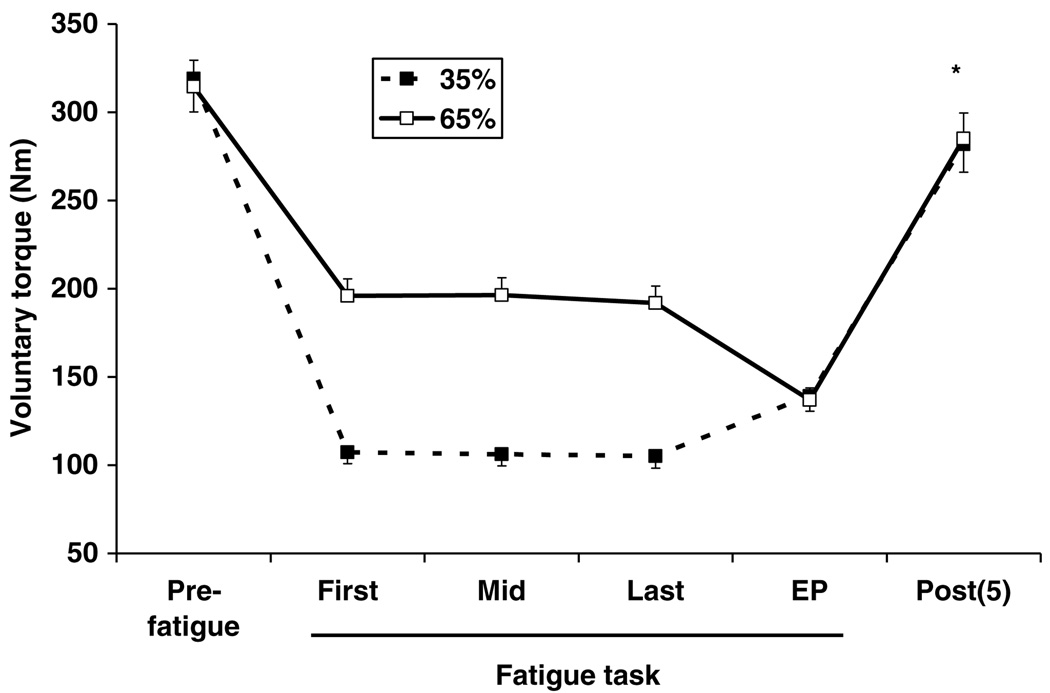
Fig. 2 shows the changes in voluntary torque in 35% and 65% sessions. When subjects were asked to maintain the target force, the actual means of the torque were 33.7 ± 2. MVC (3%), 33.3 ± 1.7% MVC and 32.8 ± 1.5% MVC during the 35% task, and 62.3 ± 1.7% MVC, 62.4 ± 1.7% MVC, and 60.9 ± 1.6% MVC during the 65% task for the first, middle and last parts of the fatigue task, respectively. At the end point, the torque obtained from both tasks was very similar (43.5 ± 1.6% MVC and 43.6 ± 2.0% MVC for 35% and 65% tasks, respectively). All of these values indicate that the subjects were able to sustain the required torque steadily and that the fatigue tasks were terminated when the force generating capacity of the muscle fell to a similar level regardless of which task was performed. The endurance time for the 35% task (124 ± 39.68 s) was significantly longer (p = 0.05) than that of the 65% task (63 ± 17.73 s).
Fig. 2.
Changes in voluntary torque during (first, middle (Mid) and last), at the end point (EP), and 5 min (post(5)) post-fatigue task in 35% and 65% sessions. The torque at post (5) was significantly lower than that of pre-fatigue (p < 0.05), which is indicated by asterisk (*) with no significant effect of session for pre- vs. post fatigue comparison.
These fatigue tasks induced significant depression in MVC torque even after 5 min from the end of the fatigue task. An ANOVA indicated that there was no effect of session, but found a significant effect of fatigue, which was determined to be present in both 35% and 65% sessions (MVC torque = 319.0 ± 79.7 N m and 281.8 ± 67.0 N m in 35% session and 314.5 ± 63.7 N m and 285.0 ± 61.8 N m in 65% session for pre-, and post-fatigue, respectively; p < 0.05 for both).
3.2. Double/single ratio
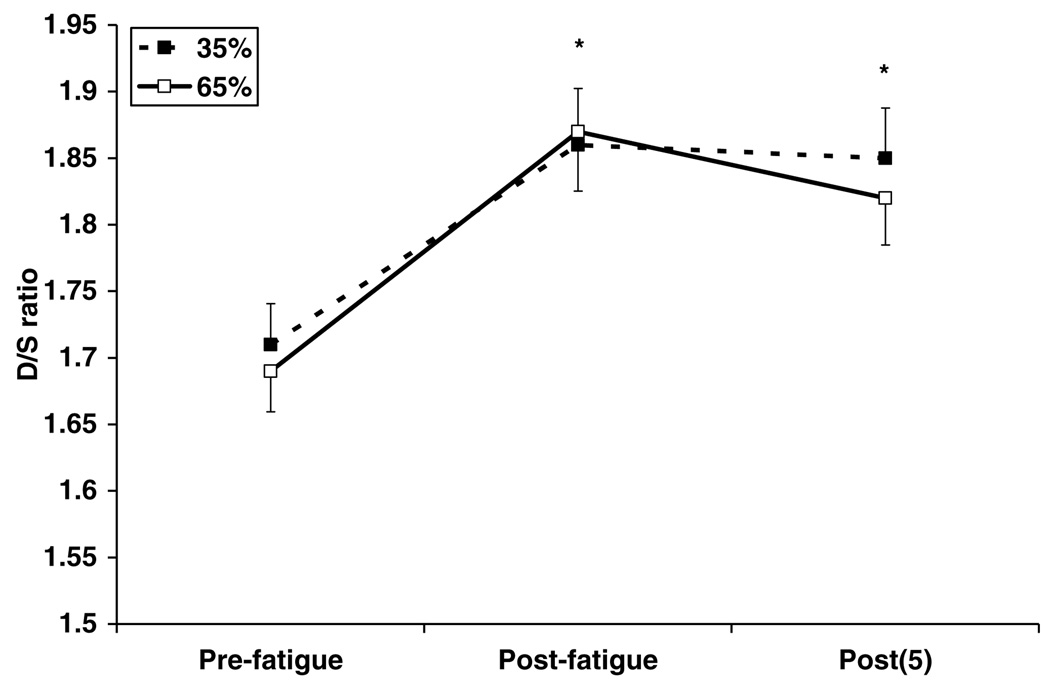
Fig. 3 shows how the ratio changed immediately and 5 min after 35% and 65% fatigue tasks. A significant effect of fatigue, but a non-significant effect of session was found on the D/S ratio. A significant increase in the ratio was found immediately after both fatigue sessions. The means of pre- and immediately post-fatigue D/S ratio were 1.71 ± 0.13 and 1.86 ± 0.18 (108.7 ± 8.5% pre-fatigue; p < 0.05) for 35% session and 1.69 ± 0.13 and 1.87 ± 0.19 (110.22 ± 9.18% pre-fatigue; p < 0.05) for 65% session. At 5 min post-fatigue, the ratio stayed significantly increased compared to the pre-fatigue ratio in both 35% and 65% sessions (1.85 ± 0.16 or 108.4 ± 7.2% pre-fatigue and 1.82 ± 0.15 or 108.1 ± 5.9% pre-fatigue after 35% and 65% tasks, respectively). These ratios, however, were not significantly different from those calculated immediately after fatigue in either of the two sessions.
Fig. 3.
Changes in D/S ratio immediately (post-fatigue) and 5 min (post (5)) after 35% and 65% fatigue tasks. Asterisk (*) indicates significant increase compared to pre-fatigue ratio (p < 0.05) with no significant difference across sessions (35% and 65%).
A secondary assessment verified the sensitivity of the D/S ratio in measuring low frequency fatigue. A sub-group of four subjects generated the 35% force condition until they could no longer maintain any force above 25%. The ratio was significantly higher when this additional fatigue was induced (1.95 ± 0.11 or 114.4 ± 6.2% pre-fatigue; p < 0.05).
3.3. EMG RMS amplitude
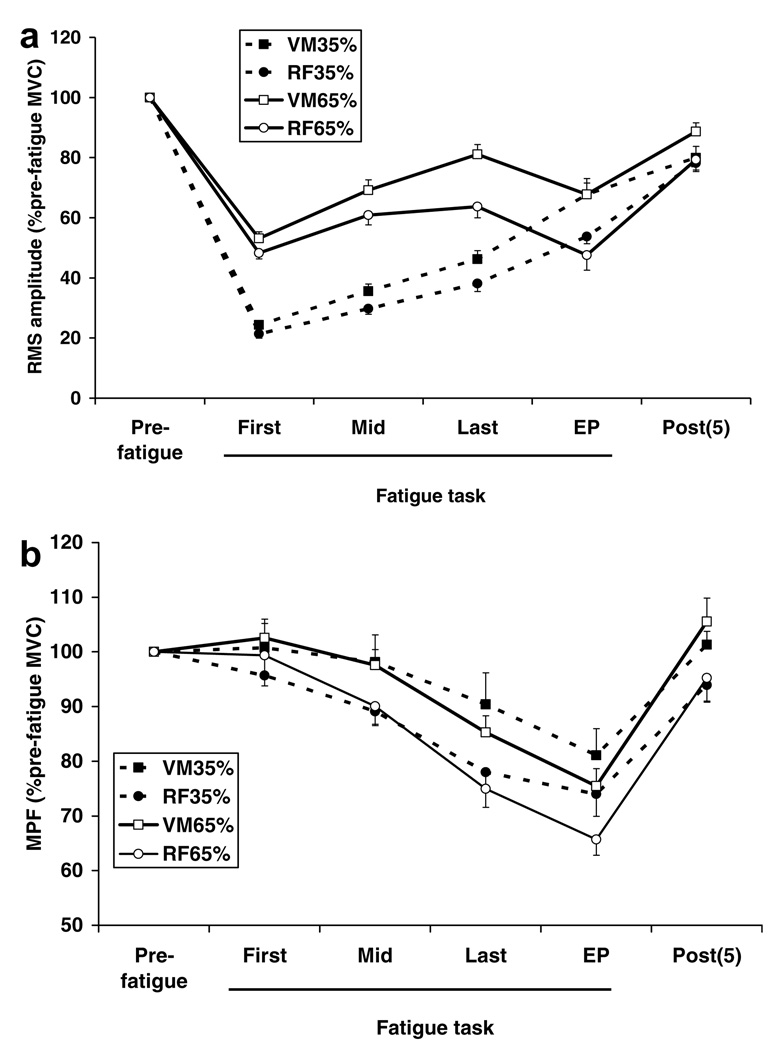
While the subjects maintained their torque at the target level, both VM and RF EMG amplitude increased in both tasks (Fig. 4a). However, the ANOVA indicated a significant interaction of muscle × fatigue in both tasks (p < 0.05 for both), indicating the rate of increase in EMG amplitude was greater for VM than RF. Non-significant differences in EMG amplitude between the two muscles at the beginning of the fatigue task (24.3 ± 6.2% MVC and 21.4 ± 5.9% MVC in 35% task, p > 0.05, and 53.1 ± 9.6% MVC and 48.3 ± 8.4% MVC in 65% task, p = 0.06, for VM and RF, respectively) became significantly different by the end of the last part of the plateau portion of the fatigue task (46.3 ± 11.7% MVC and 38.2 ± 11.3% MVC in 35% task, p < 0.01, and 81.1 ± 13.9% MVC and 63.7 ± 15.5% MVC in 65% task, p < 0.01, for VM and RF, respectively). In contrast to the significant interaction of muscle × fatigue within tasks, there was no significant interaction of session × fatigue in either VM (p = 0.08) or RF (p = 0.14), indicating that although EMG amplitude of VM and RF was always higher in the 65% task than that of 35% task, the task did not have a significant effect on the rate of increase in EMG amplitude (when the endurance time was normalized since endurance time of 35% task was significantly longer than that for 65% task).
Fig. 4.
Changes in EMG RMS amplitude (a) and median power frequency (MPF); (b) during (first, middle (Mid) and last), at the end point (EP), and 5 min (post(5)) post-fatigue task in 35% and 65% sessions.
As the force decreased with fatigue the EMG amplitude of both muscles decreased in the 65% task. In contrast, EMG amplitude of both muscles in the 35% task increased when the subjects were asked to give their maximal effort at the end of the fatigue task. These responses yielded very similar RMS amplitude for both muscles across tasks at end point. An ANOVA indicated that there was no significant effect of session, but found a significant effect of muscle, which was present in both tasks (67.7 ± 16.4% MVC and 53.8 ± 10.3% MVC in 35% task; p = 0.02, and 67.8 ± 22.3% MVC and 47.6 ± 21.3% MVC in 65% task; p < 0.01 for VM and RF, respectively). Although a recovery of RMS amplitude of both muscles was seen after 5 min from the fatigue task, there was a significant effect of fatigue compared to the pre-fatigue value, with no significant effects of session and of muscle. The effect of fatigue was present in both muscles, indicating that the recovery in EMG amplitude of both muscles was not complete even at 5 min post fatigue (VM RMS amplitude = 80.0 ± 16.1% pre-fatigue MVC and 88.7 ± 12.2% pre-fatigue MVC and RF RMS amplitude = 78.1 ± 11.9% pre-fatigue MVC and 79.3 ± 14.6% pre-fatigue MVC for 35% and 65% sessions, respectively), but the depression of EMG amplitude seen after 5 min was similar for both muscles with no difference across sessions.
3.4. Median power frequency
Fig. 4b shows how the MPF changed in 35% and 65% sessions. While the subjects maintained their target force in both tasks, MPF decreased with fatigue for both muscles; however, in contrast to RMS amplitude, an ANOVA indicated that there was no significant effect of muscle or session with no significant interaction of session × fatigue or muscle × fatigue from the start to the end point of the fatigue task. At 5 min post-fatigue, there was no significant effect of muscle, session, or fatigue when compared to pre-fatigue values. This indicates that MPF of both VM and RF obtained at 5 min post-fatigue showed complete recovery in both sessions.
4. Discussion
The primary finding of the study was that when the human quadriceps muscle was fatigued with two different tasks that differed in their sustained target level (35% MVC or 65% MVC) and their endurance time (124 ± 39.68 s and 63 ± 17.73 s for 35% task and 65% task, respectively) a similar amount of LFF, as measured by the double pulse to single pulse ratio, was induced.
4.1. Comparisons with previous studies
Our results indicate that given equivalent levels of fatigue, LFF, or impairment of E–C coupling as a result of sustained submaximal contractions, is not only dependent on the intensity and duration of the contraction, but also depends on the net fatigue level. This is the first study, to our knowledge, that was designed to answer the question as to whether LFF is task dependent or fatigue dependent. Our finding is contrary to the commonly held belief that the development of LFF is limited to low force, long-lasting contractions (Ratkevicius et al., 1995; Blangsted et al., 2005). The most likely reason why our findings differ is because we controlled for the factor, fatigue. If the quadriceps femoris had been fatigued until the subjects could no longer sustain the respective target force, as done in previous studies, the endurance time of a 20% task would have been much longer, so we might have observed LFF to a greater extent after the 20% task compared to the 65% task. However, the longer the task the greater the risk of confounding the study with central fatigue. Even with the end point criteria we employed in the present study, the endurance time for the 35% task was almost twice as long as that of the 65% task. If LFF was truly intensity/duration dependent then we would have expected greater LFF in the 35% contractions, which were twice as long. Indeed, we believe that the magnitude of fatigue induced, when controlled, negated the typical differences observed during two vastly different tasks. Interestingly, when we had four individuals hold a 35% force until they could no longer maintain a 25% force, then we obtained a D/S ratio that was ~7% greater, supporting that the ratio is sensitive. Additional support for the ratio sensitivity is borne out by recent studies from our lab showing a 25% increase in the D/S ratio following an eccentric fatigue protocol (unpublished observations).
We used the D/S ratio to quantify LFF even though others have used higher frequency trains (e.g., Skurvydas et al., 2003; Strojnik and Komi, 2000; Ratkevicius et al., 1998). Single and double pulses were employed in the present study rather than trains because they induce less co-contraction because they are more comfortable, and, therefore, allow a greater intensity of stimulation. The D/S ratio is of a short enough duration that the event is over before volitional reaction time can interfere with the force output, and they are extremely sensitive to low frequency fatigue induced in fast fatigable muscle (Shields and Shields, 1994; Shields and Chang, 1997). Moreover, our between session ratio reliability was excellent (ICC > 0.92). Therefore, we believe that the D/S ratio was highly sensitive to LFF as supported by others (Strojnik and Komi, 2000; Tubman et al., 1997).
4.2. Changes in EMG activities during the fatigue tasks
While the subjects sustained the required force at either 35% or 65% MVC, EMG RMS amplitude of both VM and RF increased progressively, but to a greater extent in VM compared to RF (Fig. 4a). This is in agreement with previous studies that also found a pronounced increase in RMS amplitude for VM compared to RF during sustained isometric contraction in knee extension (Ebenbichler et al., 1998; Mathur et al., 2005). These findings suggest that mono-articular (VM) and bi-articular (RF) muscles of the human quadriceps have different roles in fatiguing contractions even when the contraction is isometric. The lack of differences in MPF between muscles is also in line with the study of Ebenbichler et al. (1998), although, in contrast to our results, they found a significant difference in median frequency among fatigue tasks sustained at different target forces. However, Ebenbichler et al. did not control for force, further supporting that the effects on sarcolemmal action potential conduction velocity and ultimately MPF are more related to the magnitude of the fatigue rather than the isometric tasks used in this study.
Although there was a difference in the rate of increase in RMS amplitude between VM and RF, the RMS amplitude of the two muscles did not reach their respective pre-fatigue maximum values. The extent to which the amplitude can reach pre-fatigue levels is thought to depend on the initial target force: the lower the target force, the lower the EMG amplitude at the end point compared to pre-fatigue values (Fuglevand et al., 1993). However, in the present study, the EMG amplitude for both muscles was very similar (no significant effect of session) at the end point across tasks (Fig. 4a). This suggests that the end point EMG is also dependent on the end point force. Fuglevand and colleagues did not induce similar levels of fatigue across tasks, thus the lower initial force task (20% MVC) induced over 80% fatigue compared to higher force contractions (65% MVC). Mechanisms that contribute to EMG amplitude not reaching pre-fatigue levels include: increased peripheral inhibitory feedback from metabolite-sensitive muscle receptors (Bigland-Ritchie, 1981), a reduction in muscle spindle gain (Macefield et al., 1991), an increase in Golgi tendon organ (Zytnicki et al., 1990) afferent input, an increase in recurrent inhibition (Mcnabb et al., 1988), and neuromuscular propagation failure (Fuglevand et al., 1993). Although it is impossible to identify which of these processes is responsible, our findings that EMG amplitude at end point was similar across both contractions (35% and 65%) suggests that these mechanisms are also fatigue dependent, rather than intensity/duration dependent for the tasks examined in this study.
We did not assess the extent of neuromuscular transmission failure as femoral nerve activation is extremely uncomfortable. However, the lack of a difference in force at 5 min of recovery, when transmission would have recovered (Fuglevand et al., 1993; Galea, 2001), suggests that neuromuscular propagation minimally influenced the findings of this study. Other confounding factors may be fatigue-induced metabolic changes, which can impair cross-bridge function in the muscle. However, 5 min is typically thought to be ample time to wash out the fatigue-associated metabolic build-up in the muscle (Bogdanis et al., 1995). Our finding that MPF recovered fully after 5 min also supports this notion.
4.3. Voluntary torque, EMG activities and LFF after fatigue
The central nervous system is capable of using different strategies to overcome fatigue-induced E–C coupling failure when muscles are in LFF. De Ruiter et al. (2005) found an increase in motor unit discharge rates of the human vastus lateralis muscle after LFF was induced. This compensation of E–C coupling failure may have occurred in the present study when subjects performed the MVC after fatigue. However, neither the EMG amplitude of the two muscles nor the voluntary torque recovered fully to the pre-fatigue values at 5 min post-fatigue, indicating that either the increased motor unit discharge rates were not enough or LFF was so severe that it could not be compensated fully by neural strategies. Moreover, an increase in motor unit discharge rates after LFF was not detected in surface EMG amplitudes but was detected with fine wire recordings (De Ruiter et al., 2005). Therefore, observing significantly depressed surface EMG amplitude after fatigue does not necessarily mean that motor unit discharge rates did not increase in the present study. The spatial summation of motor unit action potentials may lead to extensive cancellation and or potentiation of signals reflected in the more global surface recordings.
A fatigue-induced reduction in descending drive, usually known as central fatigue may have contributed to the decreased EMG amplitude and voluntary torque at 5 min post-fatigue. However, studies suggest that 5 min is long enough for central fatigue to recover (Andersen et al., 2003; Taylor et al., 1996). Therefore, we believe that the contribution of central fatigue to the decline in EMG amplitude and MVC torque after fatigue was negligible. There was no increase in torque when one subject, who showed typical findings in this study, received supra-maximal stimulation during the post-fatigue MVC (unpublished observations). Thus, central fatigue appears to have minimally influenced the comparison between the two fatigue tasks.
5. Conclusion
In summary, our results suggest that LFF in the human quadriceps muscle is more dependent on the extent to which the muscle is fatigued and not on the duration or intensity of the contraction. This may have important implications as we strive to understand the neural control strategies used to activate muscle during fatigue. The notion that both low and high percentage MVC contractions can induce similar changes in E–C coupling may indicate that neural strategies depend on the “fatigue state” of the muscle as well as the type of task employed. These findings have implications to our understanding of mechanisms that contribute to task failure.
Biographies

Masaki Iguchi graduated from the Physical Therapy program at Tsukuba College of Technology in Tsukuba, Japan in 1995. He worked as a physical therapist in Japan before he received his BS in Health Science from Benedictine University in Lisle, IL in 2001 and his MA in Physical Therapy from The University of Iowa in 2004. He is continuing his study at The University of Iowa as a PhD student. His main research areas are muscle fatigue and motor control.

Kris Baldwin received a BS (1995) in exercise and sport science from Iowa State University and a MPT (1997) from The University of Iowa. She is currently a senior physical therapist at the University of Iowa Hospitals and Clinics in the Center for Disabilities and Development.

Charles Boeyink received his undergraduate degree from Southwest State University and a MPT (1997) from The University of Iowa. He is currently a practicing physical therapist.

Carol Engle received a BS degree in Finance from the University of Wyoming (1994) and a Masters in Physical Therapy degree from the University of Iowa Physical Therapy Graduate Program (1997). She is a physical therapist for St Vincent Healthcare in Billings, Montana where she is a team member of the Headway brain injury outpatient rehabilitation program. Carol’s special interest areas include vestibular and neurological rehabilitation.

Michael Kehoe received BA (1994) and MPT (1997) degrees from the University of Iowa in Iowa City, Iowa. He is currently a practicing physical therapist in an outpatient clinic for Iowa Health-Des Moines in Des Moines, Iowa. His interests are in orthopaedics and he is currently working towards McKenzie certification.

Anish Ganju received his B.Sc. Physical Therapy from the Institute for Physically Handicapped in New Delhi, India in 1999. He received his MA in Physical Therapy from The University of Iowa in 2003. He is continuing his study at The University of Iowa as a PhD student.

Andrew J. Messaros is Assistant Professor and Director of Anatomic Studies in the Department of Physical Therapy at The University of Toledo. He earned a PhD in Exercise Science at the University of Iowa (1998; Dr. Rich Shields) where he also served a NIH/NIA post-doctoral fellowship in the Iowa Injury Prevention Research Center (1999; Dr. Tom Cook). His research examines the effects of exercise-induced fatigue on concurrent changes to muscle mechanical properties and sensorimotor function, particularly the involuntary neural control of upper extremity and trunk posture. He is also interested in the neural control of muscle anatomic subcompartments.

Richard K. Shields received advanced degrees in Physical Therapy from the Mayo Clinic and the University of Iowa, and a PhD degree in Exercise Science from the University of Iowa. His research explores the neuromuscular and skeletal adaptations that occur in humans during natural perturbations (fatigue, disuse, trauma, disease, immobility, pathology, paralysis) and unnatural perturbations (vibration, electrical stimulation). The Christopher Reeve Foundation and the National Institutes (NIH) currently fund Dr. Shields’ research. Dr. Shields received the Neurology Section Research Excellence Award, and named a Catherine Worthingham Fellow for his advancement of science, education, and clinical practice in rehabilitation.
References
- Andersen B, Westlund B, Krarup C. Failure of activation of spinal motoneurones after muscle fatigue in healthy subjects studied by transcranial magnetic stimulation. J Physiol. 2003;551(Pt. 1):345–356. doi: 10.1113/jphysiol.2003.043562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B. EMG/force relations and fatigue of human voluntary contractions. Exercise Sport Sci Rev. 1981;9:75–117. [PubMed] [Google Scholar]
- Blangsted AK, Sjogaard G, Madeleine P, Olsen HB, Sogaard K. Voluntary low-force contraction elicits prolonged low-frequency fatigue and changes in surface electromyography and mechanomyography. J Electromyogr Kinesiol. 2005;15(2):138–148. doi: 10.1016/j.jelekin.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Bogdanis GC, Nevill ME, Boobis LH, Lakomy HK, Nevill AM. Recovery of power output and muscle metabolites following 30 s of maximal sprint cycling in man. J Physiol. 1995;482(Pt2):467–480. doi: 10.1113/jphysiol.1995.sp020533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin ER, Allen DG. The role of elevations in intracellular [Ca2+] in the development of low frequency fatigue in mouse single muscle fibres. J Physiol. 1996;491(Pt 3):813–824. doi: 10.1113/jphysiol.1996.sp021259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ruiter CJ, Elzinga MJ, Verdijk PW, Van Mechelen W, De Haan A. Changes in force, surface and motor unit EMG during post-exercise development of low frequency fatigue in vastus lateralis muscle. Eur J Appl Physiol. 2005;94:659–669. doi: 10.1007/s00421-005-1356-x. [DOI] [PubMed] [Google Scholar]
- Ebenbichler G, Kollmitzer J, Quittan M, Uhl F, Kirtley C, Fialka V. EMG fatigue patterns accompanying isometric fatiguing knee-extensions are different in mono- and bi-articular muscles. Electroencephalogr Clin Neurophysiol. 1998;109:256–262. doi: 10.1016/s0924-980x(98)00015-0. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Hill DK, Jones DA, Merton PA. Fatigue of long duration in human skeletal muscle after exercise. J Physiol. 1977;272(3):769–778. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM. Mechanism of muscle fatigue: central factors and task dependency. J Electromyogr Kinesiol. 1995;5(3):141–149. doi: 10.1016/1050-6411(95)00010-w. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Stuart DG. Neurobiology of muscle fatigue. J Appl Physiol. 1992;72(5):1631–1648. doi: 10.1152/jappl.1992.72.5.1631. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Zackowski KM, Huey KA, Enoka RM. Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J Physiol. 1993;460:549–572. doi: 10.1113/jphysiol.1993.sp019486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea V. Electrical characteristics of human ankle dorsi- and plantarflexor muscles. Comparative responses during fatiguing stimulation and recovery. Eur J Appl Physiol. 2001;85:130–140. doi: 10.1007/s004210100439. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81(4):1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Hicks AL, Kent-Braun J, Ditor DS. Sex differences in human skeletal muscle fatigue. Exercise Sport Sci Rev. 2001;29:109–112. doi: 10.1097/00003677-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Hill CA, Thompson MW, Ruell PA, Thom JM, White MJ. Sarcoplasmic reticulum function and muscle contractile character following fatiguing exercise in humans. J Physiol. 2001;531:871–878. doi: 10.1111/j.1469-7793.2001.0871h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DA, Newham DJ, Torgan C. Mechanical influences on long-lasting human muscle fatigue and delayed-onset pain. J Physiol. 1989;412:415–427. doi: 10.1113/jphysiol.1989.sp017624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannergren J, Westerblad H. Force decline due to fatigue and intracellular acidification in isolated fibres from mouse skeletal muscle. J Physiol. 1991;434:307–322. doi: 10.1113/jphysiol.1991.sp018471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield G, Hagbarth KE, Gorman R, Gandevia SC, Burke D. Decline in spindle support to alpha-motoneurones during sustained voluntary contractions. J Physiol. 1991;440:497–512. doi: 10.1113/jphysiol.1991.sp018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur S, Eng JJ, Macintyre DL. Reliability of surface EMG during sustained contractions of the quadriceps. J Electromyogr Kinesiol. 2005;15:102–110. doi: 10.1016/j.jelekin.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Mcnabb N, Frank JS, Green HJ. Recurrent inhibition during sustained contractions in humans. Society for Neuroscience. 1988 Abstract 14. [Google Scholar]
- Ratkevicius A, Skurvydas A, Lexell J. Submaximal-exercise-induced impairment of human muscle to develop and maintain force at low frequencies of electrical stimulation. Eur J Appl Physiol Occup Physiol. 1995;70(4):294–300. doi: 10.1007/BF00865025. [DOI] [PubMed] [Google Scholar]
- Ratkevicius A, Skurvydas A, Povilonis E, Quistorff B, Lexell J. Effects of contraction duration on low-frequency fatigue in voluntary and electrically induced exercise of quadriceps muscle in humans. Eur J Appl Physiol Occup Physiol. 1998;77(5):462–468. doi: 10.1007/s004210050361. [DOI] [PubMed] [Google Scholar]
- Shields RK, Chang Y-J. The effects of fatigue on the torque–frequency curve of the human paralysed soleus muscle. J Electromyogr Kinesiol. 1997;7:3–13. doi: 10.1016/s1050-6411(96)00015-6. [DOI] [PubMed] [Google Scholar]
- Shields RK, Shields KC. Effect of double pulse activation on force enhancement in the human paralyzed soleus muscles. Society for Neuroscience. 1994 Abstract 20. [Google Scholar]
- Skurvydas A, Mamkus G, Stanislovaitis A, Mickeviciene D, Bulotiene D, Masiulis N. Low frequency fatigue of quadriceps muscle after sustained maximum voluntary contractions. Medicina (Kaunas) 2003;39(11):1094–1099. [PubMed] [Google Scholar]
- Strojnik V, Komi PV. Fatigue after submaximal intensive stretch-shortening cycle exercise. Med Sci Sports Exercise. 2000;32(7):1314–1319. doi: 10.1097/00005768-200007000-00020. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Allen GM, Gandevia SC. Changes in motor cortical excitability during human muscle fatigue. J Physiol. 1996;490(Pt. 2):519–528. doi: 10.1113/jphysiol.1996.sp021163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubman LA, Rassier DE, MacIntosh BR. Attenuation of myosin light chain phosphorylation and posttetanic potentiation in atrophied skeletal muscle. Pflugers Archiv – Eur J Physiol. 1997;434:848–851. doi: 10.1007/s004240050474. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Duty S, Allen DG. Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol. 1993;75:382–388. doi: 10.1152/jappl.1993.75.1.382. [DOI] [PubMed] [Google Scholar]
- Zytnicki D, Lafleur J, Horcholle-Bossavit G, Lamy F, Jami L. Reduction of Ib autogenetic inhibition in motoneurons during contractions of an ankle extensor muscle in the cat. J Neurophysiol. 1990;64:1380–1389. doi: 10.1152/jn.1990.64.5.1380. [DOI] [PubMed] [Google Scholar]