Abstract
The transcription factor ETS-1 is a critical mediator of vascular inflammation and hypertrophy in hypertension. We tested the hypothesis that ETS-1 is a mediator of proinflammatory responses and neointimal hyperplasia after balloon injury of the carotid artery. For this study, we took advantage of the availability of an ETS-1 dominant-negative (DN) peptide. Sprague-Dawley rats were assigned to treatment with ETS-1 DN, a mutant peptide (ETS-1 MU), or vehicle (Veh) and subjected to balloon injury of the carotid artery. After 2, 24 hours, and 14 days, the rats were euthanized, and both carotid arteries were processed for real-time polymerase chain reaction (2 hours), immunofluorescence and immunohistochemistry (24 hours), and morphometric analysis (14 days). ETS-1 mRNA was up regulated (2.4-fold) in injured carotid arteries. By immunofluorescence, we confirmed increased nuclear expression of ETS-1 24 hours postinjury. The carotid artery mRNA expression of monocyte chemotactic protein-1, cytokine-induced neutrophil chemoattractant-2, P-selectin, E-selectin, vascular cell adhesion molecule, and intercellular adhesion molecule was increased 2 hours after injury. ETS-1 DN but not ETS-1 MU significantly reduced mRNA and protein expression for monocyte chemotactic protein-1, P-selectin, and E-selectin in injured arteries. These changes were accompanied by concomitant reductions in vascular monocyte and leukocyte infiltration. Moreover, treatment with ETS-1 DN but not ETS-1 MU resulted in a 50% reduction in neointima formation at day 14 after balloon injury. This study unveils the role of ETS-1 as a mediator of inflammation and neointima formation in a model of carotid artery balloon injury and may result in the development of novel strategies in the treatment of vascular injury.
Keywords: ETS-1 transcription factor, balloon injury, inflammation, neointima, cytokines, chemokines
Inflammation plays an important role in the pathogenesis of hypertension, atherosclerosis, restenosis, and other forms of vascular disease.1 Carotid artery balloon injury is a well-established model of vascular injury characterized by increased expression of inflammatory mediators and marked leukocyte infiltration, which initiate the process of vascular remodeling and neointima formation after endoluminal injury.1–7 In this model, adhesion molecules such as P-selectin, E-selectin, intercellular adhesion molecule (ICAM), and vascular cell adhesion molecule (VCAM), as well as chemokines that mediate neutrophil and monocyte infiltration, such as cytokine-induced neutrophil chemoattractant-2 (CINC-2) and monocyte chemotactic protein-1 (MCP-1), are expressed at high levels within 2 hours of injury. In turn, these mediators activate and stimulate the migration of large numbers of granulocytes and monocytes into the arterial wall from the periadventitial tissues, which is evident after 24 hours of injury.4,7 These early inflammatory responses correlate with the extent of subsequent neointima formation at 14 and 30 days postinjury.4,5,7
The ETS factors are a family of transcription factors that are involved in the regulation of a wide variety of biological processes, including normal development and differentiation.8 As proto-oncogenes, they have also been implicated in the pathogenesis of several different types of cancer.9,10 Over the past few years, several studies have also supported a role for several members of the ETS family in the regulation of vascular inflammation, including endothelial activation,11 the recruitment of inflammatory cells to the vessel wall, and proliferation and migration of vascular smooth muscle cells (VSMC).12 ETS-1 is induced in VSMC and endothelial cells in response to a variety of stimuli, including angiotensin II (Ang II), platelet-derived growth factor–BB, thrombin, interleukin-1β, and tumor necrosis factor-α.13–16 Moreover, as we have recently shown, Ang II increases the glomerular expression of ETS-1, and in rat mesangial cells ETS-1 mediates Ang II–stimulated fibronectin expression.17 The systemic administration of Ang II not only is associated with increases in blood pressure but also promotes the recruitment of inflammatory cells, including T cells and monocytic cells, to the vessel wall.18 The influx of inflammatory cells in response to Ang II is markedly diminished in ETS-1–deficient mice compared with littermate controls,15 suggesting an important role for ETS-1 as mediator of the proinflammatory effects of Ang II in the vasculature.
In the past few years, although emerging evidence supports a novel role for ETS-1 in regulation of vascular inflammation and remodeling, as well as its being a mediator of mesangial cell fibronectin production response to Ang II, the role of ETS-1 in other models of injury and in the absence of hypertension has not been investigated. In this study, we tested the hypothesis that ETS-1 is an early mediator of proinflammatory responses that participate in neointima formation after balloon injury of the carotid artery. For this study, we took advantage of the availability of an ETS-1 dominant-negative (DN) peptide to directly assess the role of ETS-1 in this model of injury.
Methods
Experimental Protocol
Ten-week-old male Sprague-Dawley rats were obtained from Charles River Breeding Laboratories (Wilmington, Mass). The animal protocols were approved by the institutional animal care and use committee at the University of Alabama at Birmingham and were consistent with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
ETS-1 DN Peptide
ETS-1 DN peptide and ETS-1 mutant (ETS-1 MU) peptide were synthesized (CPC Scientific Inc, San Jose, Calif) following the sequences described by Oettgen et al.18
Balloon Injury Carotid Artery and Experimental Protocols
Rats were randomly selected to receive a single injection of ETS-1 DN (10 mg · kg−1, IP), ETS-1 MU (10 mg · kg−1, IP) or vehicle (Veh) (normal saline IP). Two hours after the injection, the rats were anesthetized with ketamine (80 mg/kg, IP) and xylazine (5 mg/kg, IP) and subjected to balloon injury of the right common carotid artery as we have previously described.19 The uninjured left carotid artery served as a control.
At 2 hours postinjury (n=6 per group), the rats were euthanized with an overdose of sodium pentobarbital (100 mg · kg−1, IP), and the carotid arteries were removed and processed for measurement of mRNA levels of inflammatory mediators (MCP-1, E-selectin, P-selectin, VCAM, and ICAM) and ETS-1 by real-time reverse transcription–polymerase chain reaction (RT-PCR). At 24 hours postinjury (n=5 per group), the rats were euthanized and perfused with 10% formalin at a pressure of 120 mm Hg. Both carotid arteries were removed, fixed, and embedded in paraffin for immunohistochemical analysis.
In separate groups of rats (n=6 per group), in addition to the initial IP injection, the rats received ETS-1 DN (10 mg · kg−1 · day−1), ETS-1 MU (10 mg · kg−1 · day−1), or Veh after carotid artery injury via osmotic minipump (Alzet, Cupertino, Calif). On day 14 of treatment, the rats were euthanized and perfused with 10% formalin at a pressure of 120 mm Hg. Both carotid arteries were removed, fixed, embedded in paraffin, serially sectioned, and stained with Verhoeff elastin stain for morphometric analysis.19
To determine whether a single injection of ETS-1 DN was enough to reduce neointima formation after balloon injury, we performed additional experiments in an additional group of rats (n=8 per group), in which the rats received a single injection of ETS-1 DN (10 mg · kg−1, IP), ETS-1 MU (10 mg · kg−1, IP), or Veh (normal saline, IP) 2 hours before balloon injury. After 14 days, the rats were euthanized, and carotid arteries were collected, fixed and stained with Verhoeff elastin stain for morphometric analysis.
Real-Time Quantitative RT-PCR Analysis of mRNA Expression
Total RNA was extracted from injured or uninjured carotid arteries with TRIzol reagent (Invitrogen, Carlsbad, Calif). The RNA was reverse transcribed to cDNA, amplified by polymerase chain reaction with specific primers (Table), and quantified using SYBR Green and the 7300 Real Time PCR System (Applied Biosystems, Foster City, Calif).4 Levels of specific mRNAs were normalized using ribosomal protein S9 mRNA.20 For inflammatory mediators, mRNA results were standardized to the mean value of the Veh injured group.
Table.
Specific Sense and Antisense Primers for Quantitative Real-Time RT-PCR Analysis
| Gene | Primer Sense Sequence | Primer Antisense Sequence |
|---|---|---|
| ETS-1 | AGGCATTGTGGGTAATAACA | AAGTCCACTGTCGCTGTCTC |
| MCP-1 | ATGCAGGTCTCTGTCACGCT | GGTGCTGAAGTCCTTAGGGT |
| P-selectin | AATGAAATCGCTCACCTC | TTATTGGGCTCGTTGTCT |
| E-selectin | GCCTTACTGGCTTTAT | ACCTTTGCCTTGTGAT |
| ICAM | CAAACGGGAGATGAATGG | TGGCGGTAATAGGTGTAAAT |
| VCAM | GGGGATTCCGTTGTTCT | CAGGGCTCAGCGTCAGT |
| CINC-2 | TCAGGGACTGTTGTGG | TGACTTCTGTCTGGGTG |
| Ribosomal protein S9 |
GCTGGATGAGGGCAAGAT | CGAACAATGAAAGATGGGAT |
Immunofluorescence
Five-micrometer sections of carotid arteries were prepared from paraffin-embedded tissues. After deparaffinization, the sections were incubated with primary antibodies rabbit anti-rat ETS-1 antibody (sc-350, Santa Cruz, CA) at a 1:100 dilution at 4°C overnight. Sections were washed, incubated with the secondary antibody (Texas Red– conjugated horse anti-rabbit immunoglobulin G), and mounted with Vectashield 4′,6-diamidino-2-phenylindole mounting medium (Vector Laboratories, Burlingame, Calif). Two controls, in which we omitted the primary or secondary antibody, were included in each experiment.
Immunohistochemistry
The avidin-biotin-peroxidase immunohistochemical technique was used to detect MCP-1, E-selectin, P-selectin, monocyte infiltration, and leukocyte infiltration in paraffin-embedded sections in 24-hour-postinjury carotid arteries using a Vector Laboratories kit. In brief, after deparaffinization, MCP-1, E-selectin– and P-selectin–positive areas were immunolocalized by incubation with a rabbit polyclonal antibody against MCP-1 and E-selectin and a goat polyclonal antibody against P-selectin (Santa Cruz Biotechnology, Santa Cruz, Calif), followed by application of a biotinylated goat anti-rabbit or horse anti-goat secondary antibody, respectively (1:200), for 30 minutes. Rat monocytes were recognized by specific primary antibodies against ED1 (Serotec, Oxford, United Kingdom) and biotinylated goat anti-rabbit antibody. Rat leukocytes were recognized by specific primary antibodies against myeloperoxidase (Abcam, Cambridge, Mass) and biotinylated goat anti-rabbit antibody (Vector Laboratories). Detection of MCP-1, E-selectin, P-selectin, and monocytes was completed with Vector NovaRed (Vector Laboratories). All evaluations were performed on 3 sections from the injured arterial segment per vessel and then averaged. The expression of MCP-1 E-selectin and P-selectin was semiquantified by measuring the percentage of positive area of arterial cross section in which they were expressed (Image-Pro, MediaCybernetics, Bethesda, Md). ED1- and myeloperoxidase-positive cells were counted by an observer unaware of the experimental conditions (Image-Pro, MediaCybernetics).
Morphometric Analysis
Morphometric analysis of representative cross-sectional photomicrographs from the middle thirds of injured and contralateral control carotid arteries was performed with a computer-based Bioquant II Morphometric System (Nashville, Tenn), as we have previously described.19
Detection of Biotinylated ETS-1 DN Within the Vessel Wall
Paraffin sections of carotid artery were prepared, and the biotinylated ETS-1 DN was detected using the Vectastain ABC system (Vector Laboratories). Briefly, the tissue sections were deparaffinized, hydrated, and incubated for 30 minutes with diluted Vectastain ABC reagent. The slides were washed for 5 minutes, incubated in peroxidase substrate solution until appropriate stain intensity developed, and rinsed with tap water. The sections were then counter-stained and mounted.
Statistical Analysis
Results were expressed as means±SEM. The data were evaluated by 1-way or 2-way ANOVA. When the overall F test result of ANOVA was significant, a multiple-comparison Dunnett test was applied. The Student t test was used in 2-mean comparisons. Differences were reported as significant at P<0.05.
Results
ETS-1 Expression Is Increased After Carotid Artery Balloon Injury
To determine whether ETS-1 expression is increased after carotid artery balloon injury, we measured ETS-1 mRNA expression by real-time quantitative RT-PCR 2 hours postinjury. As shown in Figure 1, 2 hours after balloon injury, there was a 2.4-fold increase in ETS-1 mRNA expression.
Figure 1.
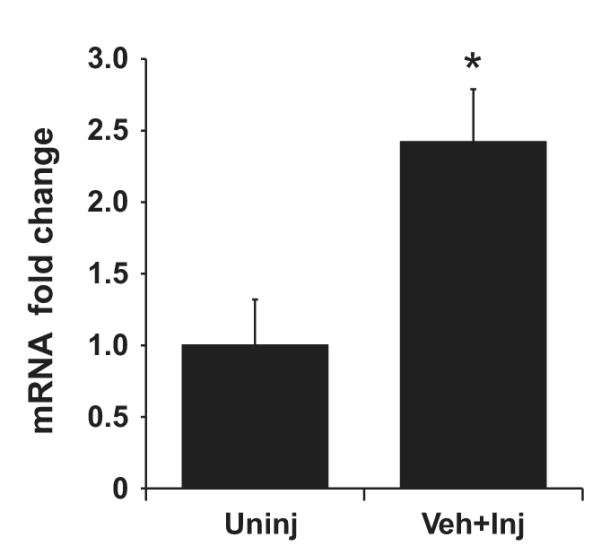
ETS-1 mRNA expression increases in injured carotid arteries. Total RNA was extracted at 2 hours postinjury (inj) and from contralateral uninjured carotid arteries (Uninj). mRNA levels measured by real-time RT-PCR were normalized using ribosomal protein S9. Results are expressed as mean±SEM. *P<0.05 vs uninjured group.
To determine whether these changes in ETS-1 mRNA after balloon injury were accompanied by changes in ETS-1 protein expression, we measured carotid artery ETS-1 expression by immunofluorescence 24 hours after balloon injury. As shown in Figure 2, in control vessels, there was normal autofluorescence limited to the elastic layer, with no detectable expression of ETS-1. In contrast, in injured vessels, there was a significant increase in ETS-1 expression, especially in the adventitia and to a lesser degree in the media. Colocalization analysis using nuclear 4′,6-diamidino-2-phenylindole stain demonstrated that ETS-1 expression in injured vessels was mostly nuclear (Figure 2).
Figure 2.
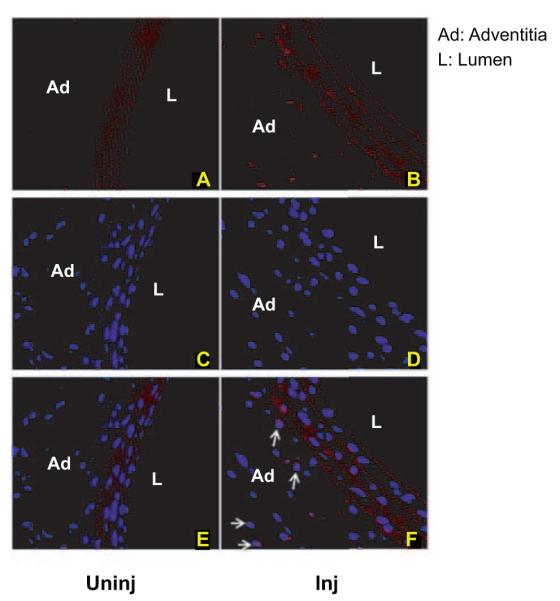
Nuclear ETS-1 expression is increased in injured carotid arteries. Shown are ETS-1 antibody immunofluorescence–stained sections at 24 hours postinjury (Inj) and uninjured carotid arteries (uninj). Perfusion-fixed and sectioned arteries were immunostained using primary antibodies against ETS-1. The secondary antibody was Texas Red conjugated (A and B). The sections were mounted with 4′,6-diamidino-2-phenylindole mounting medium (C and D). Merging of the 2 pictures with Adobe Photoshop shows nuclear colocalization of ETS-1 (E and F, arrows).
ETS-1 Mediates the Expression of Proinflammatory and Adhesion Molecules in Carotid Artery Balloon Injury
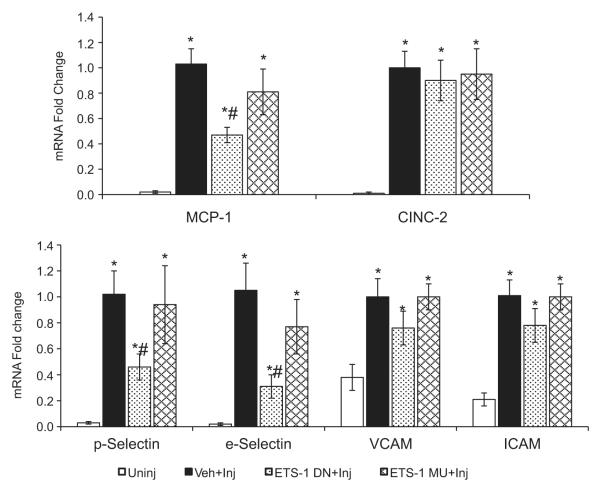
To assess the role of ETS-1 in the expression of chemokines and adhesion molecules that are upregulated in balloon injury, we measured the mRNA expression of the chemokines MCP-1 and CINC-2 and the adhesion molecules P-selectin, E-selectin, VCAM, and ICAM 2 hours after balloon injury. By RT-PCR, we determined that the mRNA expression of these molecules in control vessels was very low (Figure 3). In contrast, the mRNA expression of these molecules was markedly increased after 2 hours of balloon injury (Figure 4): MCP-1 (≈50-fold), CINC-2 (≈50-fold), P-selectin (≈30-fold), E-selectin (≈50-fold), VCAM (2.6-fold), and ICAM (4.7-fold). Treatment with ETS-1 DN resulted in significant reductions in the mRNA levels of MCP-1 (53%) and the adhesion molecules E-selectin and P-selectin (69% and 54%, respectively) (Figure 3). ETS-1 DN treatment did not significantly modify the mRNA expression of the adhesion molecules VCAM and ICAM or the neutrophil-selective chemokine CICN-2 (Figure 3). Treatment with ETS-1 MU did not modify the expression of any of the genes examined. These findings therefore demonstrate that ETS-1 regulates the transcription of several genes involved in the proinflammatory responses after carotid artery balloon injury, including MCP-1, P-selectin, and E-selectin.
Figure 3.
Effects of ETS-1 DN on mRNA expression of chemokines and adhesion molecules. The mRNA expression for the chemokines and adhesion molecules studied was measured by real-time RT-PCR. It was first normalized using ribosomal protein S9 mRNA to correct for differences in total RNA loading and then standardized to the mean value of the Veh-injured (Veh-Inj) group, which was assigned a value of 1. Results are expressed as mean±SEM. *P<0.05 vs uninjured (Uninj); #P<0.05 vs Veh group.
Figure 4.
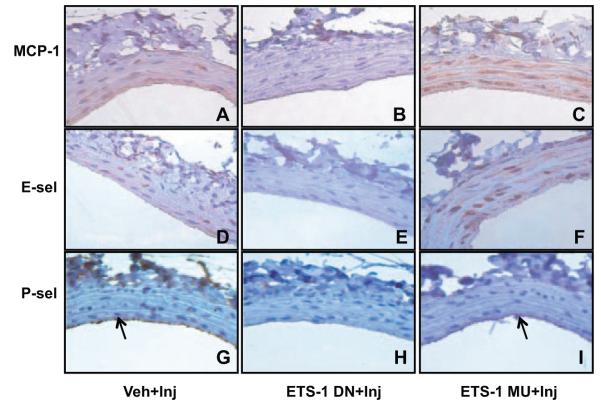
ETS-1 DN blocked MCP-1, P-selectin (P-sel), and E-selectin (E-sel) expression in injured carotid arteries. Veh-, ETS-1 DN–, and ETS-1 MU–treated rats were perfusion fixed 24 hours after balloon injury. Injured carotid arteries were sectioned and immunostained using primary antibodies for MCP-1, P-selectin, and E-selectin. MCP-1 and E-selectin were expressed mainly in the media of injured arteries (A and D), whereas P-selectin was expressed mainly in the lumen side along the injured arteries (G). Treatment with ETS-1 DN significantly reduced MCP-1, E-selectin, and P-selectin (B, E, and H), whereas treatment with ETS-1 MU had no effect (C, F, and I).
To determine whether these changes in mRNA expression were accompanied by changes in their protein expression, we performed immunohistochemical analysis for MCP-1, E-selectin, and P-selectin in the 24-hours-postinjury carotid arteries. As shown in Figure 4, injured vessels had significant expression of MCP-1 and E-selectin, mainly in the media, whereas P-selectin was increased in the media and endothelial injury areas (Figure 4).
Treatment with ETS-DN resulted in significant reductions (P<0.05 versus Veh) in the vascular expression of MCP-1 assessed as percentage of stained area: 42.0±19% (Veh) versus 18.2±11% (ETS-1 DN) (Figure 4). We observed similar changes for E selectin (19.1±7.6% [Veh] versus 9.3±4.6% [ETS-1 DN]) and P-selectin (5.8±2% [Veh] versus 1.5±0.3% [ETS-1 DN]). In contrast, treatment with ETS-1 MU did not modify the expression of MCP-1, E-selectin, or P-selectin (45±16%, 15.4±5.6%, and 4.4±1.8%, respectively).
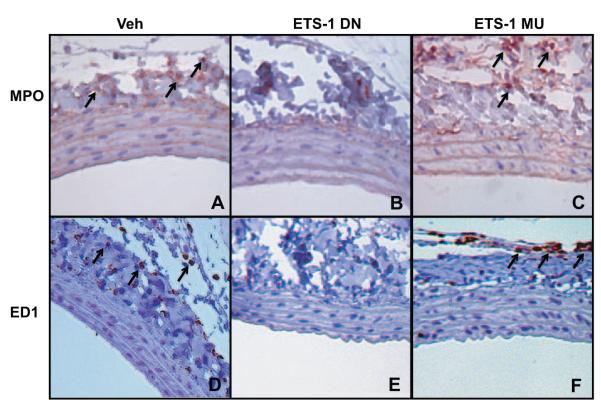
Given the important role of MCP-1 as a mediator of monocyte infiltration, we performed immunohistochemistry for ED1+ cells as a marker of monocyte infiltration in the 24-hours-postinjury carotid arteries. As shown in Figure 5, large numbers of ED1+ monocytes were present in the adventitial domains of 24-hour injured arteries of Veh-treated rats; these numbers were significantly reduced (n=3, P<0.05 versus Veh) by ETS-1 DN: 374±32/mm2 (Veh) versus 215±46/mm2 (ETS-1 DN). In contrast, treatment with ETS-1 MU had no effect (327±41/mm2). In addition, we measured leukocyte infiltration in vessels from the different groups. As shown in Figure 5, there was a significant increase in leukocyte infiltration in Veh-treated animals that was significantly reduced by ETS-1 DN (P<0.05 versus Veh): 84.8±34.9/mm2 (Veh) versus 41±2.57/mm2 (ETS-1 DN). Treatment with ETS-1 MU had no effect on leukocyte infiltration: 70.6±12.7/ mm2 (P=not significant).
Figure 5.
ETS-1 DN blocked monocyte and leukocyte infiltration in injured carotid arteries. Perfusion-fixed and sectioned arteries were immunostained using primary antibodies against leukocyte antigens (myeloperoxidase, MPO) or monocyte antigens (ED1). Infiltrating leukocyte and monocytes were localized in the adventitial and perivascular regions of 24-hour injured carotid arteries (A and D) and were significantly reduced by ETS-1 DN (B and E) but not by ETS-1 MU (C and F).
ETS-1 Mediates the Formation of Neointima After Carotid Artery Balloon Injury
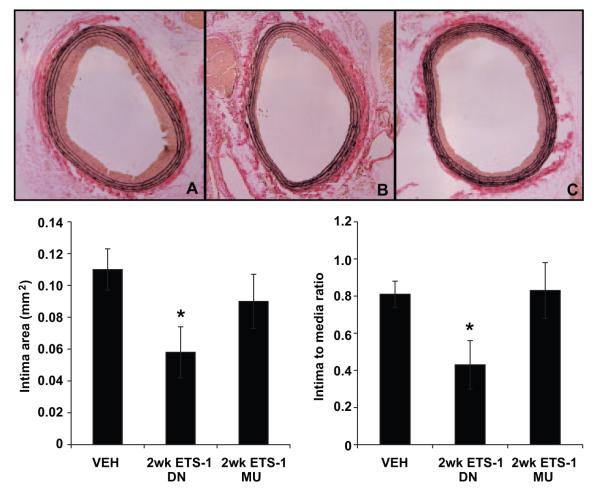
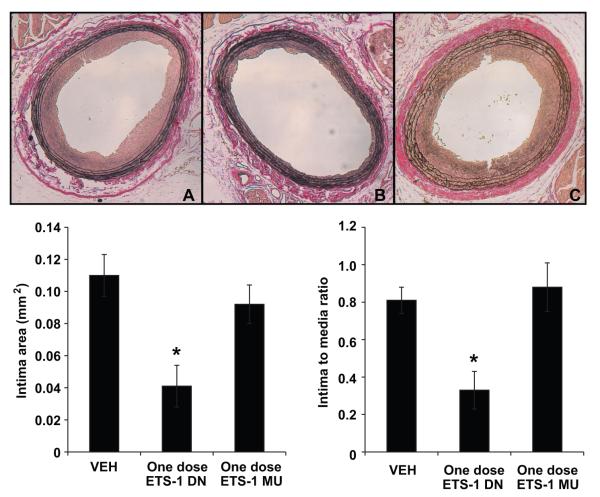
We and others have previously demonstrated that balloon injury of the carotid artery results in significant neointima formation that is evident after 14 days of injury.4,19 Morphometric analysis showed that at 14 days after balloon injury, the neointimal areas and neointima/media area ratios of injured carotid arteries were significantly reduced in vessels from rats treated with ETS-1 DN for 14 days compared with those treated with Veh (Figure 6). The intima/media ratios closely reflected the absolute intimal areas and were reduced by ≈50%. In contrast, treatment with ETS MU did not modify the total neointima area or the neointima/media ratio (Figure 6). In a separate group of rats, we determined whether a single injection of ETS-1 DN would prevent the development of neointima formation after balloon injury. Neointimal areas and neointima/media ratios of injured carotid arteries from the Veh-treated group were reduced in ETS-1 DN–treated rats but not in ETS-1 MU–treated rats (Figure 7). These findings demonstrate that blockade of ETS-1 before balloon injury is enough to prevent the development of neointima after balloon injury and highlight the important role of ETS-1 as mediator of the initial proinflammatory responses that result in the subsequent development of neointima.
Figure 6.
ETS-1 DN infusion for 14 days inhibited neointima formation in injured carotid arteries. Top, Representative light micrographs of right common carotid arteries from Veh-, ETS-1 DN–, and ETS-1 MU–treated rats at day 14 after balloon injury. Arterial sections were stained with Verhoff elastin stain. Bottom, Effects of administration of ETS-1 DN on neointima area and neointima/media ratio of injured carotid arteries in rats at 14 days after carotid artery balloon injury. Data are shown as mean±SEM. *P<0.05 vs Veh group.
Figure 7.
A single injection of ETS-1 DN inhibited neointima formation in injured carotid arteries. Top, Representative light micrographs of right common carotid arteries from Veh-, ETS-1 DN–, and ETS-1 MU–treated rats at day 14 after balloon injury. Arterial sections were stained with Verhoff elastin stain. Bottom, Effects of administration of ETS-1 DN on neointima area and neointima/media ratio of injured carotid arteries in rats at 14 days after carotid artery balloon injury. Data are shown as mean±SEM. *P<0.05 vs Veh group.
To confirm appropriate delivery of the ETS-1 DN peptide, we performed biotin staining using Vectastain (Vector Laboratories) in injured vessels from rats receiving either Veh or ETS-1 DN. We observed a weak biotin background stain in control vessels that was substantially increased in vessels from ETS-1 DN–injected animals. As shown in Figure 8, vessels from rats that received ETS-1 DN showed increased biotin stain, especially in the adventitia, confirming the presence of biotinylated peptide within the vessel wall. Injection of ETS-1 MU peptide resulted in identical peptide distribution (not shown).
Figure 8.
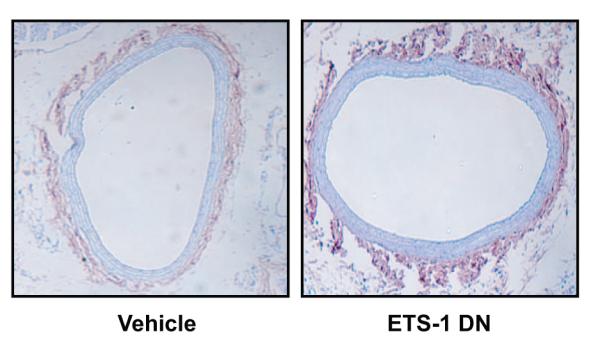
Biotin staining of biotinylated peptide in the vessel. Carotid arteries from Veh-treated rats had background biotin stain that was significantly increased in arteries from ETS-1 DN–treated rats.
Discussion
The ETS factors are a family of transcription factors that share a highly conserved DNA-binding domain (the ETS domain).21 The term ETS originates from the sequence originally described in the E26 avian erythroblastosis virus (E26 transformation-specific sequence).22 There are approximately 25 to 30 ETS family members.23 ETS-1 in particular has emerged as a critical transcription factor involved in the regulation of multiple biological processes, including normal development and differentiation, and as a proto-oncogene it has been implicated in the pathogenesis of different types of cancer.9
In addition to a highly conserved DNA binding domain, the different ETS family members have also shared distinct structural domains.21 The transcriptional activity of ETS factors can be modulated through posttranslational modifications. The activity of most ETS factors can be modulated by phosphorylation. Phosphorylation of threonine-38 by the mitogen-activated kinases extracellular signal regulated kinases 1 and 2 potentially increases the transcriptional activity of ETS-1.24 In contrast, calmodulin-dependent kinase II inhibits DNA binding via serine phosphorylation of ETS-1 inhibitory domains.25 Another important mechanism by which the function of ETS factors can be regulated is through nuclear transport. Specific regions called nuclear localization sequences within each of the ETS family members facilitate their movement from the cytoplasm into the nucleus,26 which is required for their function as transcription factors.
Several studies support a role for ETS factors in the regulation of gene expression in the endothelium. ETS-1 increases endothelial migration by enhancing the expression of matrix metalloproteinases and β3 integrin.27 ETS-1 also regulates the expression of genes involved in angiogenesis, such as the vascular endothelial growth factor receptors and angiopoietin-2.28 Indeed, DN forms of ETS-1 exhibit antiangiogenic activity in cultured endothelial cells.29 Other target genes that have been identified to be downstream of ETS-1 include the chemokine monocyte chemoattractant protein-1 (MCP-1), plasminogen activator inhibitor-1, and the adhesion molecule VCAM-1.15 Additional downstream targets of ETS-1 that are expressed by VSMC and promote cell migration include the matrix metalloproteinases stromelysin (matrix metalloproteinase-3) and type IV collagenase (matrix metalloproteinase-2).30 ETS-1 expression in VSMC also induces the expression of platelet-derived growth factor, thereby promoting VSMC proliferation.31
Recent studies have shown that ETS-1 is induced in the vasculature in response to a variety of stimuli, including the proinflammatory cytokines interleukin-116 and tumor necrosis factor,13 as well as by Ang II15 and platelet-derived growth factor.14 VSMC isolated from ETS-1-deficient mice exhibit decreased proliferative response to Ang II, and systemic administration of Ang II to ETS-1-deficient mice is associated with marked reductions in medial hypertrophy, despite similar increases in blood pressure in wild-type and ETS-1-deficient mice,15 suggesting an important role for ETS-1 in the pathogenesis of vascular injury in hypertension. In addition and as we have recently demonstrated, Ang II increases the glomerular expression of ETS-1 in vivo, and in vitro, Ang II induces the expression of ETS-1 in rat mesangial cells via increased generation of reactive oxygen species.17 Moreover, we demonstrated that ETS-1 in large part mediates the production of fibronectin stimulated by Ang II in rat mesangial cells.17
Inflammation is clearly implicated in the pathogenesis of different forms of vascular diseases, including hypertension and atherosclerosis.1 In this study, we used the balloon injury of the rat carotid artery as a model to study the role of ETS-1 as mediator of vascular injury responses. This model is characterized by an early accumulation of neutrophils followed by increased monocyte infiltration of the adventitia and subsequent neointima formation.32 As we have previously demonstrated, these inflammatory responses and development of neointima are mediated by chemokines such as MCP-1 and CINC-2 and adhesion molecules such as P-selectin, E-selectin, VCAM, and ICAM.4 Our previous studies have also demonstrated that balloon injury induces migration and proliferation of adventitial cells into the media that contribute to formation of neointima in this model of vascular injury.33
Previous studies have demonstrated strong ETS-1 protein expression after balloon injury as early as 2 hours postinjury and involving all vascular layers, suggesting that ETS-1 expression probably precedes the expression of proinflammatory cytokines that mediate the formation of neointima.12 Significant increases in ETS-1 mRNA expression suggest that these changes in ETS-1 protein expression are mediated via transcriptional mechanisms. For our study, we took advantage of the availability of an ETS-1 DN peptide to block the effects of ETS-1, as described by Oettgen et al.18 Our study demonstrates that ETS-1 is upregulated in this model of vascular injury and transcriptionally regulates the expression of the monocyte-specific chemokine MCP-1, as well as of the adhesion molecules P-selectin and E-selectin. Importantly, we also showed that ETS-1 blockade reduced monocyte infiltration, demonstrating that ETS-1 plays an important role as a mediator of monocyte infiltration in this model of vascular injury. We have also demonstrated that ETS-1 blockade also significantly reduced leukocyte infiltration, although it did not significantly change the expression of the leukocyte specific chemokine CICN-2, suggesting that other chemokines also play a role in the development of leukocyte infiltration after balloon injury.
Blockade of ETS-1 with a specific ETS-1 DN but not with a mutant peptide resulted in significant reductions in neointima formation, demonstrating a critical role for ETS-1 as a mediator of inflammation and neointima formation in this model of vascular injury. On the basis of our findings, we postulate that reductions in the expression of MCP-1, E-selectin, and P-selectin and subsequent monocyte infiltration as a result of ETS-1 blockade result in significant reductions in neointima formation. In our study, we blocked ETS-1 before balloon injury to prevent the inflammatory response and formation of neointima. In the future, we will perform interventional studies to determine whether blockade of ETS-1 ameliorates the formation of neointima when the balloon injury has already occurred.
In conclusion, the results of our study support a critical role for ETS-1 as a transcriptional mediator of endoluminal vascular injured-induced inflammation and neointima formation. We have identified several downstream effectors of the model, including MCP-1, P-selectin, and E-selectin, that are regulated by ETS-1. Future studies will be directed at identifying protein-protein interactions of ETS-1 with other transcription factors or cofactors and to additional mechanisms by which ETS-1 could play a role in neointima formation.
Perspectives
This study unveils the critical role of the transcription factor ETS-1 in the pathogenesis of vascular injury and may result in the development of novel strategies in the treatment and prevention of vascular injury associated with hypertension and atherosclerosis.
Acknowledgments
Sources of Funding This work was supported by a Merit Review Award of the Department of Veterans Affairs (E.A.J.); National Institutes of Health Research Grants ES014948 (E.A.J.), HL087980 (S.O.), and HL044195 (Y.-F.C.); and an American Heart Association Greater Southeast Affiliate Grant (D.X.).
Footnotes
Disclosures None.
References
- 1.Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Münzel T, Simoons ML, Hamm CW. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 2.Egashira K, Zhao Q, Kataoka C, Ohtani K, Usui M, Charo IF, Nishida K, Inoue S, Katoh M, Ichiki T, Takeshita A. Importance of monocyte chemoattractant protein-1 pathway in neointimal hyperplasia after periarterial injury in mice and monkeys. Circ Res. 2002;90:1167–1172. doi: 10.1161/01.res.0000020561.03244.7e. [DOI] [PubMed] [Google Scholar]
- 3.Horvath C, Welt FG, Nedelman M, Rao P, Rogers C. Targeting CCR2 or CD18 inhibits experimental in-stent restenosis in primates: inhibitory potential depends on type of injury and leukocytes targeted. Circ Res. 2002;90:488–494. doi: 10.1161/hh0402.105956. [DOI] [PubMed] [Google Scholar]
- 4.Miller AP, Feng W, Xing D, Weathington NM, Blalock JE, Chen YF, Oparil S. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation. 2004;110:1664–1669. doi: 10.1161/01.CIR.0000142050.19488.C7. [DOI] [PubMed] [Google Scholar]
- 5.Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29:289–295. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 7.Miller AP, Xing D, Feng W, Fintel M, Chen YF, Oparil S. Aged rats lose vasoprotective and anti-inflammatory actions of estrogen in injured arteries. Menopause. 2007;14:251–260. doi: 10.1097/01.gme.0000235366.39726.f6. [DOI] [PubMed] [Google Scholar]
- 8.Wasylyk B, Hahn SL, Giovane A. The Ets family of transcription factors. Eur J Biochem. 1993;211:7–18. doi: 10.1007/978-3-642-78757-7_2. [DOI] [PubMed] [Google Scholar]
- 9.Seth A, Watson DK. ETS transcription factors and their emerging roles in human cancer. Eur J Cancer. 2005;41:2462–2478. doi: 10.1016/j.ejca.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Oikawa T, Yamada T. Molecular biology of the Ets family of transcription factors. Gene. 2003;303:11–34. doi: 10.1016/s0378-1119(02)01156-3. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Brown C, Ni W, Maynard E, Rigby AC, Oettgen P. Critical role for the Ets transcription factor ELF-1 in the development of tumor angiogenesis. Blood. 2006;107:3153–3160. doi: 10.1182/blood-2005-08-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hultgårdh-Nilsson A, Cercek B, Wang JW, Naito S, Lövdahl C, Sharifi B, Forrester JS, Fagin JA. Regulated expression of the ets-1 transcription factor in vascular smooth muscle cells in vivo and in vitro. Circ Res. 1996;78:589–595. doi: 10.1161/01.res.78.4.589. [DOI] [PubMed] [Google Scholar]
- 13.Goetze S, Kintscher U, Kaneshiro K, Meehan WP, Collins A, Fleck E, Hsueh WA, Law RE. TNFalpha induces expression of transcription factors c-fos, Egr-1, and Ets-1 in vascular lesions through extracellular signal-regulated kinases 1/2. Atherosclerosis. 2001;159:93–101. doi: 10.1016/s0021-9150(01)00497-x. [DOI] [PubMed] [Google Scholar]
- 14.Naito S, Shimizu S, Maeda S, Wang J, Paul R, Fagin JA. Ets-1 is an early response gene activated by ET-1 and PDGF-BB in vascular smooth muscle cells. Am J Physiol. 1998;274:C472–C480. doi: 10.1152/ajpcell.1998.274.2.C472. [DOI] [PubMed] [Google Scholar]
- 15.Zhan Y, Brown C, Maynard E, Anshelevich A, Ni W, Ho IC, Oettgen P. Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest. 2005;115:2508–2516. doi: 10.1172/JCI24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redlich K, Kiener HP, Schett G, Tohidast-Akrad M, Selzer E, Radda I, Stummvoll GH, Steiner CW, Groger M, Bitzan P, Zenz P, Smolen JS, Steiner G. Overexpression of transcription factor Ets-1 in rheumatoid arthritis synovial membrane: regulation of expression and activation by interleukin-1 and tumor necrosis factor alpha. Arthritis Rheum. 2001;44:266–274. doi: 10.1002/1529-0131(200102)44:2<266::AID-ANR43>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 17.Pearse DD, Tian RX, Nigro J, Iorgulescu JB, Puzis L, Jaimes EA. Angiotensin II increases the expression of the transcription factor ETS-1 in mesangial cells. Am J Physiol Renal Physiol. 2008;294:F1094–F1100. doi: 10.1152/ajprenal.00458.2007. [DOI] [PubMed] [Google Scholar]
- 18.Ni W, Zhan Y, He H, Maynard E, Balschi JA, Oettgen P. Ets-1 is a critical transcriptional regulator of reactive oxygen species and p47(phox) gene expression in response to angiotensin II. Circ Res. 2007;101:985–994. doi: 10.1161/CIRCRESAHA.107.152439. [DOI] [PubMed] [Google Scholar]
- 19.Chen SJ, Li H, Durand J, Oparil S, Chen YF. Estrogen reduces myointimal proliferation after balloon injury of rat carotid artery. Circulation. 1996;93:577–584. doi: 10.1161/01.cir.93.3.577. [DOI] [PubMed] [Google Scholar]
- 20.Tai JT, Brooks EE, Liang S, Somogyi R, Rosete JD, Lawn RM, Shiffman D. Determination of temporal expression patterns for multiple genes in the rat carotid artery injury model. Arterioscler Thromb Vasc Biol. 2000;20:2184–2191. doi: 10.1161/01.atv.20.10.2184. [DOI] [PubMed] [Google Scholar]
- 21.Wasylyk C, Bradford AP, Gutierrez-Hartmann A, Wasylyk B. Conserved mechanisms of Ras regulation of evolutionary related transcription factors, Ets1 and Pointed P2. Oncogene. 1997;14:899–913. doi: 10.1038/sj.onc.1200914. [DOI] [PubMed] [Google Scholar]
- 22.Nunn MF, Seeburg PH, Moscovici C, Duesberg PH. Tripartite structure of the avian erythroblastosis virus E26 transforming gene. Nature. 1983;306:391–395. doi: 10.1038/306391a0. [DOI] [PubMed] [Google Scholar]
- 23.Oettgen P. Regulation of vascular inflammation and remodeling by ETS factors. Circ Res. 2006;99:1159–1166. doi: 10.1161/01.RES.0000251056.85990.db. [DOI] [PubMed] [Google Scholar]
- 24.Wasylyk B, Hagman J, Gutierrez-Hartmann A. Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem Sci. 1998;23:213–216. doi: 10.1016/s0968-0004(98)01211-0. [DOI] [PubMed] [Google Scholar]
- 25.Cowley DO, Graves BJ. Phosphorylation represses Ets-1 DNA binding by reinforcing autoinhibition. Genes Dev. 2000;14:366–376. [PMC free article] [PubMed] [Google Scholar]
- 26.Boulukos KE, Pognonec P, Rabault B, Begue A, Ghysdael J. Definition of an Ets1 protein domain required for nuclear localization in cells and DNA-binding activity in vitro. Mol Cell Biol. 1989;9:5718–5721. doi: 10.1128/mcb.9.12.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oda N, Abe M, Sato Y. ETS-1 converts endothelial cells to the angiogenic phenotype by inducing the expression of matrix metalloproteinases and integrin beta3. J Cell Physiol. 1999;178:121–132. doi: 10.1002/(SICI)1097-4652(199902)178:2<121::AID-JCP1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 28.Elvert G, Kappel A, Heidenreich R, Englmeier U, Lanz S, Acker T, Rauter M, Plate K, Sieweke M, Breier G, Flamme I. Cooperative interaction of hypoxia-inducible factor-2alpha (HIF-2alpha) and Ets-1 in the transcriptional activation of vascular endothelial growth factor receptor-2 (Flk-1) J Biol Chem. 2003;278:7520–7530. doi: 10.1074/jbc.M211298200. [DOI] [PubMed] [Google Scholar]
- 29.Nakano T, Abe M, Tanaka K, Shineha R, Satomi S, Sato Y. Angiogenesis inhibition by transdominant mutant Ets-1. J Cell Physiol. 2000;184:255–262. doi: 10.1002/1097-4652(200008)184:2<255::AID-JCP14>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 30.Hultgardh-Nilsson A, Lovdahl C, Blomgren K, Kallin B, Thyberg J. Expression of phenotype- and proliferation-related genes in rat aortic smooth muscle cells in primary culture. Cardiovasc Res. 1997;34:418–430. doi: 10.1016/s0008-6363(97)00030-8. [DOI] [PubMed] [Google Scholar]
- 31.Santiago FS, Khachigian LM. Ets-1 stimulates platelet-derived growth factor A-chain gene transcription and vascular smooth muscle cell growth via cooperative interactions with Sp1. Circ Res. 2004;95:479–487. doi: 10.1161/01.RES.0000141135.36279.67. [DOI] [PubMed] [Google Scholar]
- 32.Xing D, Miller A, Novak L, Rocha R, Chen YF, Oparil S. Estradiol and progestins differentially modulate leukocyte infiltration after vascular injury. Circulation. 2004;109:234–241. doi: 10.1161/01.CIR.0000105700.95607.49. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Chen SJ, Oparil S, Chen YF, Thompson JA. Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation. 2000;101:1362–1365. doi: 10.1161/01.cir.101.12.1362. [DOI] [PubMed] [Google Scholar]