Abstract
Patients with Parkinson's disease (PD) often have cognitive deficits from the time of diagnosis. Except in patients with dementia, the impact of cognitive symptoms on daily function is not well documented. This study had two objectives: (1) to determine the functional significance of cognitive deficits in nondemented patients with PD and (2) to assess the sensitivity of two measures of global cognitive abilities to identify individuals with impaired ADL function. One hundred eleven subjects with PD and a range of cognitive abilities were included. Of these, 20 were diagnosed with PDD. All subjects were assessed with the Mattis Dementia Rating Scale to two (DRS-2) and the Mini-Mental State Examination (MMSE). ADL function was reported by an informant using the Alzheimer's Disease Cooperative Study Activities of Daily Living Inventory (ADCS-ADL). The ability of the DRS-2 and MMSE to capture the impact of cognitive impairment on ADL function was assessed in the entire cohort and in subsets of nondemented individuals. After adjustment for covariates, cognition as measured by the DRS-2 was strongly related to ADL function in the entire cohort (partial correlation coefficient = 0.55, P < 0.001). The association remained strong when only nondemented subjects were included (r = 0.42, P < 0.001). The DRS-2 was significantly more accurate than the MMSE, particularly for detecting milder degrees of ADL impairment (ROC area = 0.87 vs. 0.75, P = 0.0008). Cognition is associated with impairment in ADL function, even in nondemented patients with PD. However, sensitive cognitive assessment measures may be needed to identify these functionally relevant impairments.
Keywords: Parkinson's disease, cognitive impairment, ADL
Cognitive impairment is common in Parkinson's disease (PD), with dementia affecting up to 80% of patients with PD over the course of their illness.1 When dementia is present, it greatly impairs one's ability to complete activities of daily living (ADLs).2,3 More subtle cognitive impairment may be present in the majority of patients with PD.4,5 The impact of milder cognitive deficits on ADL function in patients with PD has not been extensively evaluated.6 Although treatment for PDD is available,7 less severe cognitive impairment is typically not treated, partly due to the moderate effectiveness of therapy and also due to the belief that cognitive impairment short of dementia does not materially affect daily life.
We investigated the association between cognitive impairment and ADL function in a cohort of patients with PD. The primary purpose of this study was to determine whether cognitive impairment has a clinically meaningful impact on ADL function in nondemented as well as demented patients. A secondary purpose of this study was to compare the usefulness of the Mattis Dementia Rating Scale-2 (DRS-2)8 and the Mini-Mental State Examination (MMSE),9 to correctly classify patients with functionally significant cognitive deficits.
METHODS
Subjects
Patients aged 60 or older with a diagnosis of PD on the basis of British Brain Bank criteria10 and a range of cognitive status were recruited from the University of Pennsylvania's Parkinson's Disease and Movement Disorders Center. Subjects were required to have a caregiver available to respond to questions about their ADL function. The study was approved by the University of Pennsylvania Institutional Review Board. Informed consent was obtained from all subjects before administration of any study instruments.
Assessments
The neuropsychological evaluation was administered by trained research staff. If a knowledgeable informant was present at the visit, he or she completed the Alzheimer's Disease Cooperative Study-Activities of Daily Living Inventory (ADCS-ADL).11 If the knowledgable informant was not present at the time of the visit, he or she was contacted at home to complete the questionnaire either by telephone or to self-complete and return by mail.
Neuropsychological Tests and Classification of Cognitive Impairment
Cognitive status was assessed using the MMSE and DRS-2. The MMSE is a 30-point test of general cognitive ability used commonly in clinical practice.9 The DRS-2 is a more detailed measure of general cognitive ability. It has been validated in patients with PD,12 and a cut-off score of ≤123 has been shown to accurately identify patients with PDD.13 Severity of depressive symptoms was assessed using the 15-item Geriatric Depression Scale (GDS-15).14
Diagnosis of Dementia
The diagnosis of dementia was made by the treating physician for each subject. Treating physicians applied criteria from the Diagnostic and Statistical Manual of Mental Disorders IV-TR (DSM IV-TR)15 to make the diagnosis of dementia.
Motor Examination
Clinical examinations, including Unified Parkinson's Disease Rating Scale (UPDRS)16 and Hoehn and Yahr (HY)17 were conducted by the patients' treating doctors who are all movement disorders specialists. Motor examinations were conducted while patients were receiving their regular schedule of PD medications. The motor exam was performed within 2 months of the date of cognitive testing.
Activities of Daily Living
The Alzheimer's Disease Cooperative Study Activities of Daily Living Inventory (ADCS-ADL)11 is one of the most widely used ADL scales for Alzheimer's disease and was used as an outcome measure in the only large-scale treatment trial for PDD.7 It is completed by a knowledgeable informant for each participant during the research visit. The ADCS-ADL asks the informant to report whether or not the patient has performed an activity within the past 4 weeks and to what degree of complexity or independence he or she was able to perform it. It is made up of a total of 23 items. Six items pertain to Basic ADL (BADL) function (max score = 22), and 17 pertain to Instrumental ADL (IADL) function (max score = 56). The overall maximum score is 78.
Analysis
Demographic and clinical variables were tabulated, and descriptive statistics, including means, percentages, and standard deviations, were calculated. Scatter plots were constructed to show the relationship between raw DRS and MMSE scores and ADL function. Three disability cut-offs were established on the ADCS-ADL scale: 45, 55, and 65 points. The cut-points of 45 and 55 are based on scores obtained by patients with PDD7 and mild-to-moderate AD18 in clinical trials, respectively. The cut point of 65 is derived from this cohort. We identified subjects with the no more than mild-to-moderate parkinsonism (HY ≤ 2) and minimal cognitive impairment based on a DRS-2 score >133. A DRS-2 score of 133 was the mean score achieved by nondemented subjects in a prior study.13 The mean ADCS-ADL score for this group (n = 45) was 73.8 (±4.2). An ADCS-ADL score of 65 is ~2 standard deviations below the mean for this minimally impaired group (73.8 − 8.4= 65.4).
Analysis of Effect Size
To examine the impact of cognitive impairment on daily function, partial correlation coefficients were calculated for the association between the ADCS-ADL total score and the DRS-2 and MMSE. Correlation coefficients were calculated first without adjustment for covariates and again adjusting for factors that were significant in univariate analysis (age, GDS score, and HY stage). Effect sizes were calculated for subsets of subjects without dementia: (1) those without a clinical diagnosis of dementia and DRS-2 scores above 123, and (2) those without a dementia diagnosis and MMSE score > 25. These scores represent the best DRS-2 cut-off for dementia (123) in a prior study of PD and PDD,13 and the MMSE cut-off recommended by the MDS task force on PDD.19 We also analyzed the subset of subjects with MMSE scores > 27, representing a common benchmark for “intact” cognition.
Discriminant Analysis
To compare the discriminant validity of the MMSE with the DRS-2 for identifying significant disability, receiver operating characteristic (ROC) analysis was used. Analyses were on the basis of cut-points of 45, 55, and 65 on the ADCS-ADL scale. ROC analysis was performed using the DRS or MMSE alone and incorporating each of these measures into a logistic discriminant model that included age, gender, education level, and HY stage. We also calculated the odds of being below each disability cut-point depending on DRS-2 and MMSE scores.
All analyses were conducted at an alpha = 0.05 significance level, without adjustment for multiple comparisons. Analyses were carried out using Stata, version 10, College Station, TX.
RESULTS
Subject Characteristics and Cognitive Status
A total of 111 participants completed all study assessments. The mean (SD) age of the participants was 72.8 (±7.1) years and 72% were male. Mean disease duration was 8.9 years (range 1–27). The mean HY score was 2.3 (HY 1 = 8; HY 2 = 57, HY 2.5 = 22, HY 3 = 17, and HY 4 = 7). 83% had completed at least some college, 14% had a high school diploma or GED, and 5% had not completed high school.
The mean MMSE score was 26 (±3.8), the mean DRS-2 score was 131.2 (±12.9), and the mean ADCSADL score was 66.1 (±14.2). 37 subjects (33%) scored below 65 on the ADCS-ADL; 20 (18%) scored below 55; and 12 (11%) scored below 45. 20 (18%) were clinically diagnosed with dementia; 20 (18%) had DRS scores ≤ 123; 40 (36%) were either diagnosed with dementia or had a MMSE score ≤ 25. 23 (21%) with either diagnosed with dementia or had DRS-2 score ≤ 123. Clinical diagnosis of dementia agreed with diagnosis on the basis of a DRS score of ≤123 in 89% and a MMSE score of ≤25 in 77% of subjects. A large number of subjects had relatively preserved cognition. 60 (54%) had MMSE scores of 28 or higher.
In univariate analysis, age (Pearson's r = 0.35, P = 0.0002), GDS score (r = 0.43, P < 0.0001), and HY stage (r = 0.53, P < 0.0001) were strongly associated with ADL function in the entire cohort. Gender and education were not significantly associated with ADL function and were not included as covariates in subsequent multivariable models. In subsequent models, only HY score was independently associated with ADL function (partial correlation = 0.33, P < 0.001, for the whole cohort and 0.52, P < 0.001 in the nondemented subset with MMSE > 25).
Effect Sizes for the Association Between Cognition and ADL Function
Table 1 shows partial correlation coefficients for the association between cognitive measures and ADL function. Based on DRS-2 scores, there is a strong relationship between cognition and ADL function, even when demented subjects are excluded. A trend persists in the subset of patients that would be considered cognitively normal by common clinical criteria. The MMSE and DRS-2 show similar partial correlation coefficients when all subjects, including those with more severe cognitive impairment, are considered. However, the DRS-2 performs substantially better than the MMSE in less impaired subgroups. The relationship between cognition as rated by the MMSE and ADL function is also more affected by adjustment for covariates than the same relationship rated by the DRS to 2.
TABLE 1.
The correlation between cognitive impairment and ADL function in with PD with and without dementia
| Mattis DRS-2 |
MMSE |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Unadjusted | P-value | Adjusted | P-value | Unadjusted | P-value | Adjusted | P-value | |
| All subjects | n = 111 | 0.66 | <0.001 | 0.55 | <0.001 | 0.57 | <0.001 | 0.46 | <0.001 |
| Non demented and DRS>123 | n = 91 | 0.52 | <0.001 | 0.42 | <0.001 | 0.36 | 0.001 | 0.37 | 0.001 |
| Non demented and MMSE>25 | n = 71 | 0.45 | <0.001 | 0.32 | 0.008 | 0.20 | 0.097 | 0.21 | 0.096 |
| Non demented and MMSE>27 | n = 56 | 0.48 | <0.001 | 0.27 | 0.052 | 0.32 | 0.018 | 0.15 | 0.269 |
Partial correlation coefficients are shown for the entire cohort including demented and nondemented individuals, and then for subgroups with decreasing severity of of cognitive impairment. The association is smaller in magnitude, but still present in all but the least affected subgroup of patients. Cognition is measured either by the DRS-2 or by the MMSE. ADL function is measured by the ADCS-ADL scale. Partial correlation coefficients are adjusted for age, GDS score, and HY stage.
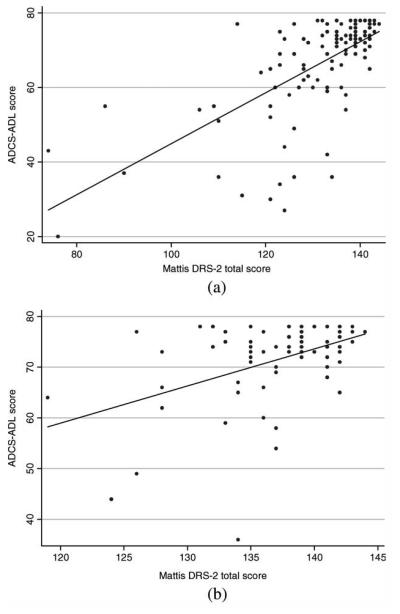
Figure 1 shows scatter plots for the relationship between ADL function and DRS-2 scores. The relationship between cognitive function and ADLs does not appear to plateau until a DRS-2 score of 140 is reached. The regression coefficient for the effect of the DRS-2 score on the ADCS-ADL score, adjusted for age, gender, education, and motor impairment is 0.51 (95%CI 0.36, 0.67), which may be interpreted as a drop of 0.51 points on the ADCS-ADL for each 1 point drop in DRS-2 total score. The effect is slightly smaller when the analysis is confined to nondemented subjects (coefficient = 0.41, 95% CI 0.10, 0.71).
FIG. 1.
Scatter plots of the relationship between DRS-2 scores and ADCS-ADL scores for the entire study cohort including demented and nondemented subjects (panel A), and only those subjects not diagnosed with dementia, and MMSE scores> 25 (panel B). The slope of the regression line is steeper when demented and nondemented individuals are included (beta = 0.51 vs. 0.41). However, in both cases, there is a significant association between cognition and ADL function. Only at DRS-2 scores above 140 does, there appear to be a loss of effect of cognition on ADL function.
The effect of cognitive impairment on ADL function was due to limitations in instrumental ADLs (IADLs) to a greater extent than basic ADLs (BALDs). After adjustment for covariates, there was a strong association between DRS-2 scores the IADLs for the entire cohort (partial correlation coefficient = 0.55, P < 0.001), for clinically nondemented subjects with DRS-2 scores > 123 (pcorr = 0.42, P = <0.001), nondemented subjects with MMSE scores >25 (pcorr = 0.34, P = 0.004), and for nondemented individuals with MMSE> 27 (pcorr = 0.31, P = 0.022). There was a significant association between DRS-2 scores and BADL function in the entire cohort (pcorr = 0.34, P < 0.001) and for nondemented subjects with DRS-2 scores> 123 (pcorr = 0.24, P = 0.028) but not for nondemented subjects with MMSE scores > 25.
Discriminant Analysis
ROC areas for the DRS and MMSE to detect mild, moderate, and severe disability are shown in Table 2. The DRS was significantly more accurate than the MMSE in identifying mildly disabled subjects (P = 0.001). This increased accuracy is reflected in both greater sensitivity and specificity of the DRS for mild disability. For the cut-off of 65 on the ADCS-ADL scale, the DRS score that best balanced sensitivity and specificity was ≥134 (sens = 76 spec = 81) and the optimal MMSE score was ≥28 (sens = 64 spec = 65). The superiority of the DRS-2 is also reflected by the fact that adding covariates to the logistic discriminant model contributes little additional accuracy in the case of the DRS-2, but does contribute additional accuracy to the MMSE. The DRS-2 was somewhat more accurate than the MMSE in identifying subjects belonging to the moderate and severe disability levels. To identify moderate disability (ADCS-ADL = 55), the optimal DRS score was ≥128 (sens = 86 spec = 85); and the optimal MMSE score was ≥26 (sens = 79 spec =75); for severe disability (ADCS-ADL = 45), the optimal DRS score was ≥127 (sens = 81 spec = 83); and the optimal MMSE score was ≥26 (sens = 75 spec = 75).
TABLE 2.
The ability of the MMSE and DRS-2 to identify mild, moderate, and severe disability
| MMSE |
MMSE Model |
DRS-2 |
DRS-2 Model |
||||||
|---|---|---|---|---|---|---|---|---|---|
| ADCS-ADL cut-off | disabled (%) | ROC area | s.e. | ROC area | s.e. | ROC area | s.e. | ROC area | s.e. |
| All subjects (n = 111) | |||||||||
| 65 | 37 (33%) | 0.75 | 0.05 | 0.86 | 0.04 | 0.87 | 0.04 | 0.90 | 0.03 |
| 55 | 20 (18%) | 0.85 | 0.04 | 0.94 | 0.02 | 0.91 | 0.03 | 0.95 | 0.03 |
| 45 | 12 (11%) | 0.83 | 0.06 | 0.92 | 0.03 | 0.88 | 0.04 | 0.91 | 0.05 |
| Non-demented only (n = 71) | |||||||||
| 65 | 11 (15%) | 0.58 | 0.08 | 0.86 | 0.08 | 0.80 | 0.08 | 0.88 | 0.08 |
| 55 | 4(6%) | 0.71 | 0.11 | 0.98 | 0.02 | 0.85 | 0.08 | 0.98 | 0.02 |
ROC areas are given both for the cognitive scale as a single test and as part of a discriminant model that also includes age, GDS score, and HY stage. The nondemented subgroup is composed of subjects who were not diagnosed with dementia and had MMSE scores>25. There are only 2 nondemented subjects with ADCS-ADL scores below 45, and this level was not included for the nondemented group. ROC areas are significantly higher for the DRS-2 alone compared with the MMSE alone at the 65 cut-off for the entire cohort (P = 0.0008) and for the nonde-mented subgroup (P = 0.021). The DRS-2 containing model is also significantly more accurate than the MMSE containing model at the 65 cut off for the entire cohort only (P = 0.02). The MMSE was less accurate than the model containing the MMSE for the 65 and 55 cut-offs for the entire cohort and for the nondemented subgroup (P < 0.05 for all). The DRS-2 containing model was only more accurate than the DRS-2 alone for the 65 cut-off in the nondemented subgroup (P = 0.04).
Greater degrees of cognitive impairment were also associated with increased odds of disability. Among nondemented subjects with DRS-2 scores > 123, each one point drop in DRS-2 score was associated with a 19% increased odds of being below 65 on the ADCSADL (OR = 1.19, 95% CI 1.03, 1.36, P = 0.012) after adjustment for covariates. Among nondemented subjects with a MMSE score > 25, there was a trend for increased odds of being below 65 on the ADCSADL (OR = 1.19, 95% CI 0.99, 1.43, P 5 0.06).
DISCUSSION
The results of this study confirm that there is a strong association between cognitive impairment and ADL difficulties in patients with PD and show that this association persists among nondemented patients with PD. It is also present to some extent in patients with nominally intact cognition, even after adjustment for covariates including mood and motor performance.
A secondary purpose of this study was to compare the usefulness of the MMSE and the DRS-2 in identifying clinically meaningful cognitive impairment. Not surprisingly, the DRS-2 was substantially more accurate than the MMSE, particularly in the case of more mildly impaired subjects. This finding is consistent with the frequent observation that the MMSE is not sensitive to mild cognitive deficits.20-22 In this study, the MMSE was both less sensitive and less specific than the DRS-2. Although the DRS-2 requires substantially more time to complete than the MMSE, relying on the MMSE alone opens the possibility of missing instances of clinically relevant cognitive impairment.
Our data show that the association between cognition and ADL function depends substantially on IADLS. This finding is consistent with a prior study that showed that cognitive deficits were associated with impairment in IADL but not BADL function in PD patients.2 Our study extends these findings by demonstrating that the same associations are present in patients with PD not diagnosed with dementia. IADL function was associated with cognition across all subgroups in our study. In cognitively intact patients, the association between overall ADL function and cognition was not statistically significant, but the association between cognition and IADL function remained. Other studies that have investigated the relationship between cognitive impairment and ADL function in nondemented patients with PD have not found as strong a relationship.6 One explanation for this discrepancy is the way that ADL function was assessed. The ADCSADL scale emphasizes IADLs that depend highly on cognitive processes such as memory and organization. Instruments used in prior studies have not focused on IADLs. For example, an analysis using the AMC linear disability scale did not find an association between cognition and ADL function in nondemented patients with PD.6 The AMC scale emphasizes tasks dependent on motor rather than cognitive function and may not be as sensitive to the impact of cognitive impairment on ADL function as the ADCS-ADL scale.23,24
The Movement Disorder Society (MDS) task force on PDD has recently published a recommended definition of PDD,25 and a detailed procedure for implementing the definition.19 The MDS definition of PDD states that there must be a decline in cognitive function from a premorbid level and that decline must involve at least two cognitive domains. It also includes a criterion that cognitive symptoms must be severe enough to affect daily life independent of the effects of motor or autonomic symptoms of PD. Although we used DSM criteria rather than the MDS criteria to define dementia, this study is relevant to the MDS definition in several ways. First, we demonstrate an independent effect of cognition on ADL function in the setting of coexisting motor impairment. Second, our analyses supports the recommended cut-off of >25 on the MMSE, which was optimal for categorizing both moderate and severe disability. However, the sensitivity and specificity of the MMSE for mild disability is modest, and ADL function is affected in individuals with MMSE scores above 25. Thus, our data leaves open the possibility that some individuals who are not “demented” based on MDS criteria, may nonetheless have significant disability on the basis of cognitive dysfunction. This situation is probably more common when applying the published DRS-2 cut-off of ≤123.13 Even though the overall accuracy of the DRS-2 is superior to the MMSE, this benchmark proved to be less specific for disability than a MMSE score of >25.
Our study has several limitations. All patients had motor ratings obtained while taking regularly scheduled dopaminergic medications. Ratings in the medication “on” state may not accurately capture motor disability. To mitigate this problem, we used Hoehn and Yahr stage rather than UPDRS scores to adjust for motor impairment. HY stage is largely driven by axial symptoms, which are less influenced by dopaminergic medication and highly related to disability.6,26 However, HY stage is also correlated with cognitive dys-function. Thus, adjusting for motor performance with the HY may underestimate the impact of cognitive impairment on daily function. Another limitation is that the study uses a convenience sample recruited from a specialty clinic. As a result, our subjects may not be representative of the population of community-dwelling patients. In particular, our subjects were notably well-educated and nondepressed, and neither of these factors was associated with impaired ADL function in our sample. Additionally, the ADCS-ADL is completed by caregivers, which may mitigate the impact of depressive symptoms experienced by the patients. While the frequency of cognitive impairment may be different in a population-based sample, it is less likely that selection bias would result in a different relationship between cognition and ADL function. Finally, the ADCS-ADL cut-offs we chose to define disability have not been validated. To our knowledge, there is no data on appropriate cut-offs on this scale. We chose scores that had been obtained in well-defined clinical trial populations for the moderate and severe cut-off. The mild cut-off was defined as a level of disability significantly worse than would be seen in patients with PD with mild to moderate motor signs and relatively preserved cognition. This cut-off may greater disability than a cut-off that was obtained from normal individuals. Additional studies would be needed to validate these cut-offs against a criterion measure of disability.
In summary, this study demonstrates that cognitive impairment significantly affects daily function in non-demented patients with PD as well as those with dementia and has some impact on ADL function even in very mildly impaired individuals. It may be reasonable for clinicians to begin to consider medical and non-medical therapies for cognitive impairment before definitive signs of dementia are present. Our data also show that the DRS-2 is a more accurate test than the MMSE for detecting functionally significant cognitive changes. It is unlikely that physicians will choose to administer this test in a clinical setting because of its length. Rather, a brief and accurate screen for functionally significant cognitive impairment is needed. The Montreal Cognitive Assessment (MoCA), a 10-min cognitive screening tool, has demonstrated high sensitivity and specificity for detecting MCI27 and has been shown in PD to be more sensitive than the MMSE for detecting mild cognitive deficits.20,28 Further analysis of the relationship between the MoCA and ADL dys-function in PD may demonstrate the means to feasibly detect mild, but functionally significant cognitive decline in patients with PD.
Footnotes
Potential conflict of interest: Nothing to report.
Financial Disclosures: The authors disclose no financial relationships that are relevant to this manuscript.
Author Roles: Emily Rosenthal—conception, organization, and execution of research project; review and critique of analysis; writing of first draft of manuscript. Laura Brennan—organization and execution of research project; review and critique of manuscript. Sharon X. Xie—conception of research project; review and critique of analysis; review and critique of manuscript. Joshua Milber—-execution of research project; review and critique of manuscript. Howard Hurtig, MD—conception and organization of research project; review and critique of manuscript. Daniel Weintraub, MD—review and critique of analysis; review and critique of manuscript. Jason Karlawish, MD—review and critique of analysis; review and critique of manuscript. Andrew Siderowf, MD, MSCE—conception and organization of research project; design and executionof analysis; review and critique of manuscript.
REFERENCES
- 1.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease—an 8-year prospective study. Arch Neurol. 2003;60:387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 2.Cahn DA, Sullivan EV, Shear PK, Pfefferbaum A, Heit G, Silverberg G. Differential contributions of cognitive and motor component processes to physical and instrumental activities of daily living in Parkinson's Disease. Arch Clin Neuropsychol. 1998;13:575–583. [PubMed] [Google Scholar]
- 3.Bronnick K, Ehrt U, Emre M, et al. Attentional deficits affect activities of daily living in dementia-associated with Parkinson's disease. J Neurol Neurosurg Psychiatry. 2006;77:1136–1142. doi: 10.1136/jnnp.2006.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 5.Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson's patients in the UK. The CamPaIGN Study. Brain. 2004;127(Part 3):550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- 6.Muslimovic D, Post B, Speelman JD, Schmand B, de Haan RJ, For the CARPA Study Group Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology. 2008;70:2241–2247. doi: 10.1212/01.wnl.0000313835.33830.80. [DOI] [PubMed] [Google Scholar]
- 7.Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med. 2004;351:2509–2518. doi: 10.1056/NEJMoa041470. [DOI] [PubMed] [Google Scholar]
- 8.Psychological Assessment Resources . Dementia Rating Scale. Psychological Assessment Resources; Odessa, FL: 1973. [Google Scholar]
- 9.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of subjects for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Hughes AJ, Daniel SE, Lees AJ. The clinical features of Parkinson's disease in 100 histologically proven cases. Adv Neurol. 1993;60:595–599. [PubMed] [Google Scholar]
- 11.Galasko D, Bennett DA, Sano K, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. Alzheimer Dis Assoc Disord Suppl. 1997;2:33–39. [PubMed] [Google Scholar]
- 12.Brown GG, Rahill AA, Gorell JM, et al. Validity of the Dementia Rating Scale in assessing cognitive function in Parkinson's disease. J Geriatr Psychiatry Neurol. 1999;12:180–188. doi: 10.1177/089198879901200403. [DOI] [PubMed] [Google Scholar]
- 13.Llebaria G, Pagonabarraga J, Kulisevsky J, et al. Cut-off score of the Mattis Dementia Rating Scale for screening dementia in Parkinson's disease. Mov Disord. 2009;23:1546–1550. doi: 10.1002/mds.22173. [DOI] [PubMed] [Google Scholar]
- 14.Weintraub D, Oehlberg KA, Katz IR, Stern MB. Test characteristics of the 15-Item Geriatric Depression Scale and Hamilton Depression Rating Scale in Parkinson disease. Am J Geriatr Psychiatry. 2006;14:169–175. doi: 10.1097/01.JGP.0000192488.66049.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Task Force on DSM-IV . Diagnostic and statistical manual of mental disorders DSM-IV-TR (Text Revision) Fourth ed. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 16.Fahn S, Elton RL. Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne D, Goldstein M, editors. Recent developments in Parkinson's disease. Macmillan Health Care Information; Florham Park, NJ: 1987. pp. 153–164. [Google Scholar]
- 17.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 18.Brodaty H, Corey-Bloom J, Potocnik FCV, Truyen L, Gold M, Damaraju CRV. Galantamine prolonged-release formulation in the treatment of mild to moderate Alzheimer's disease. Dement Geriatr Cogn Disord. 2005;20:120–132. doi: 10.1159/000086613. [DOI] [PubMed] [Google Scholar]
- 19.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 20.Nazem S, Siderowf AD, Duda JE, et al. Montreal cognitive assessment performance in patients with Parkinson's disease with “normal” global cognition according to mini-mental state examination score. J Am Geriatr Soc. 2009;57:304–308. doi: 10.1111/j.1532-5415.2008.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwamm LH, Van Dyke C, Kiernan RJ, Merrin EL, Mueller J. The Neurobehavioral Cognitive Status Examination: comparison with the Cognitive Capacity Screening Examination and the Mini-Mental State Examination in a neurosurgical population. Ann Intern Med. 1987;107:486–491. doi: 10.7326/0003-4819-107-4-486. [DOI] [PubMed] [Google Scholar]
- 22.Faustman WO, Moses JA, Jr, Csernansky JG. Limitations of the Mini-Mental State Examination in predicting neuropsychological functioning in a psychiatric sample. Acta Psychiatr Scand. 1990;81:126–131. doi: 10.1111/j.1600-0447.1990.tb06464.x. [DOI] [PubMed] [Google Scholar]
- 23.Weisscher N, Post B, de Haan RJ, Glas CAW, Speelman JD, Vermeulen M. The AMC Linear Disability Score in patients with newly diagnosed Parkinson disease. Neurology. 2007;69:2155–2161. doi: 10.1212/01.wnl.0000295666.30948.9d. [DOI] [PubMed] [Google Scholar]
- 24.Holman R, Weisscher N, Glas C, et al. The Academic Medical Center Linear Disability Score (ALDS) item bank: item response theory analysis in a mixed patient population. Health Qual Life Outcomes. 2005;3:83. doi: 10.1186/1477-7525-3-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 26.Levy G, Tang MX, Cote LJ, et al. Motor impairment in PD: relationship to incident dementia and age. Neurology. 2000;55:539–544. doi: 10.1212/wnl.55.4.539. [DOI] [PubMed] [Google Scholar]
- 27.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 28.Gill DJ, Freshman A, Blender JA, Ravina B. The montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson's disease. Mov Disord. 2008;23:1043–1046. doi: 10.1002/mds.22017. [DOI] [PubMed] [Google Scholar]