Abstract
Introduction
Despite modern targeted therapy, metastatic renal cell carcinoma (RCC) remains a deadly disease. Interferon alpha (IFNα) is currently used to treat this condition, mainly in combination with the targeted anti-VEGF antibody, bevacizumab. Apo2 ligand/Tumor necrosis factor related apoptosis inducing ligand (TRAIL) is a novel anti-neoplastic agent now in early phase clinical trials. IFNα and TRAIL can act synergistically to kill cancer cells, but this has never been tested in the context of RCC. We hypothesized that TRAIL and IFNα can synergistically induce apoptosis in RCC cells.
Methods
RCC cell lines were treated with recombinant TRAIL and/or IFNα. Viability and apoptosis were assessed by MTS assay, flow cytometery, and western blot. Synergy was confirmed by isobologram analysis. IFNα induced changes in signaling by RCC cells were assessed by western blot, flow cytometry, and ELISA.
Results
TRAIL and IFNα act synergistically to increase apoptotic cell death in RCC cells. Treatment with IFNα alters these cells ability to activate ERK and inhibiting ERK with UO126 abrogates the apoptotic synergy between TRAIL and IFNα. IFNα does not induce changes in TRAIL or death receptor expression, nor does it change other known mediators of the intrinsic and extrinsic apoptotic cascade in RCC cells.
Conclusion
TRAIL plus IFNα synergistically induces apoptosis in RCC cells. The mechanism is due at least in part to IFNα mediated changes in ERK activation. Combination therapy with TRAIL and IFNα may be a novel approach to systemically treat advanced RCC and warrants further testing in vivo.
Keywords: Apo2 ligand/Tumor necrosis factor related apoptosis inducing ligand (TRAIL), kidney cancer, immunotherapy, apoptosis, ERK
Introduction
Metastatic RCC is notoriously resistant to traditional cytotoxic chemotherapy and is treated with various forms of immunotherapy and targeted therapies. Immunotherapy includes both interferon α (IFNα) and interleukin 2 (IL-2) which induce a response in 10–20% of cases, only a third of which are durable complete responses.1 Targeted therapies include small molecule tyrosine kinase inhibitors (TKI), monoclonal antibodies to VEGF (bevacizumab), and inhibitors of mTOR.2 Although these targeted therapies have improved the outlook for patients with advanced RCC, cures remain very rare and novel forms of therapy for advanced RCC are being actively investigated.
Although IFNα (a type I interferon) can directly induce apoptosis in a number of cancer cell lines including melanoma, multiple myeloma, and ovarian cancer,3, 4 its mechanism of action in the context of RCC has never been fully elucidated and is thought to be mediated by modulating the immune system. IFNα was one of the first therapies to demonstrate a survival advantage in RCC and is less toxic than the alternative immunotherapy, high dose IL-2.1 As a consequence, it was extensively used prior to the advent of targeted therapies. Currently IFNα use has significantly declined, though it is still used in combination with bevacizumab.
Apo2 ligand/Tumor necrosis factor related apoptosis inducing ligand (TRAIL) is a member of the TNF receptor superfamily that includes CD95 ligand (FasL) and tumor necrosis factor alpha (TNFα). The utility of TRAIL as a therapeutic anti-cancer agent has been extensively investigated since all the members of the TNF superfamily can induce apoptosis via the extrinsic pathway5, 6, however only TRAIL, but not FasL and TNFα, is relatively well tolerated when given systemically 7, 8 In xenograft models tumor regression has been reported in response to systemic TRAIL and TRAIL based therapeutics are currently in early phase clinical trials.9, 10
TRAIL initiates apoptosis by binding one of its cognate death domain (DD) containing receptors, DR4 or DR5.11 These receptors then interact with the Fas associating death domain containing protein (FADD) adapter protein12, 13, which aggregate to allow the death effecter domain (DED) of FADD to interact with the DED of initiator caspases 8 and 10. This leads to a proteolytic cascade ultimately resulting in apoptosis and cell death. TRAIL can also bind to two receptors (DcR1 and DcR2) that function as decoy receptors that bind ligand but do not induce apoptosis because they lack a functional DD.
Synergy between TRAIL mediated killing and more traditional chemotherapeutic agents and/or radiation therapy has been demonstrated both in vitro and in vivo in a wide variety of malignancies including RCC.7, 14, 15 In fact some tumor cells that are resistant to either therapy alone will undergo apoptosis in response to combination therapy.14, 15 IFNα can up-regulate TRAIL expression in a variety of cell types3, 16 and IFNβ, another Type I interferon, can sensitize cells to TRAIL induced apoptosis.17 Thus in this study we tested the hypothesis that TRAIL and IFNα would act synergistically to induce cell death in RCC.
Materials and Methods
Cell lines/Reagents
RCC cell lines A-498, ACHN, and 786-O were purchased from ATCC (Manassas, VA). Antibodies: Caspase 8, caspase 3, PARP, Bcl-2, ERK, p-ERK, Bcl-XL, XIAP, cIAP-1, Survivin, horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology, Beverly, MA), c-FLIP (R&D Systems, Minneapolis, MN), actin (Sigma, St. Louis, MO), Smac/DIABLO (Oncogene Research Products, San Diego, CA), DR4, DR5, DcR1, and DcR2 (Alexis Biochemicals, San Diego, CA) and phycoerythrin (PE)-F′ secondary antibody (Jackson ImmunoResearch Lab, West Grove, PA). TRAIL and IFNα from CALBIOCHEM (La Jolla, CA). Reagents: zVAD.fmk (Alexis Biochemicals, San Diego, CA), Annexin V-FITC Apoptosis detection kit (BioVision, Mountain View, CA), and DAPI (Sigma).
Cell Viability
Cell viability was determined using the MTS method (Promega, Madison, WI) using the manufacturers protocol. In brief, cells were seeded in 96 well culture plate, grown overnight, and treated as indicated. MTS/PMS solution was added for one hour and absorbance at 490 nm measured. All experiments were done in triplicate and the results taken as the mean plus/minus the standard error.
Isobologram analysis for synergy
To determine if combination treatment was additive or synergistic, cells were exposed to varying doses of IFNα (250 IU/ml to 4,000 IU/ml) for 72 hours, TRAIL (62.5 ng/ml to 1000 ng/ml) for 24 hours, or the combination. Cell viability was determined utilizing the MTS method. An additive or synergistic response was determined by isobologram analysis. The analysis is based on the median-effect principal. The software (CalcuSyn, Biosoft, Ferguson, MO) allows one to calculate the Combination Index (CI), for the drug combination compared with each agent alone.18 If the CI=1, this indicates an additive effect, CI>1 indicates antagonism and CI<1 indicates synergy. CI of less than 0.5 is considered strong synergy. A scoring system (+++++ to −−−−− indicating very strong synergy to very strong antagonism) is used as recommended by Chou & Talalay.18 All experiments were done in triplicate and representative results are shown.
Western Blot
Cells were lysed in lysate buffer (50 mM Tris pH 8.0, 150 mM NaCl, 5 mM EDTA, 0.5% NP-40) with protease inhibitor solution (Roche, Mannheim, Germany). Protein concentration was determined using Bio-Rad protein assay (Hercules, CA). Equal protein was loaded onto 10–12% SDS-PAGE gel, transferred to nitrocellulose or PVDF membrane, blocked with 5% dry milk in TBST, incubated with primary antibody, washed, then incubated with secondary HRP labeled antibody, washed, and the bands visualized using Lumiglo Reagent and Peroxide (Cell Signaling Technology, Beverly, MA).
Flow Cytometry
Cells were allowed to grow overnight, treated as indicated, trypsinized, then resuspended in media with 10% FBS and washed twice with cold phosphate buffered saline (PBS). Determination of DR expression level: cells were blocked with 1% bovine serum albumin in PBS, incubated with primary antibody, washed, then resuspended in 1% BSA in PBS with secondary antibody. Cells were washed again, resuspended in PBS, and counts read utilizing a BD FACsCALIBUR flow cytometer. Cell cycle analysis: after washing cells were fixed in cold 70% EtOH, stained with 1 μg/ml DAPI/0.1%Triton X-100 and analyzed on the flow cytometer. Apoptotic versus non-apoptotic cell death: after washing cells were incubated with Annexin V then PI following the manufacturers recommendations, and analyzed on the flow cytometer. All flow cytometry analysis was performed using commercially available software (FlowJo).
ELISA
ELISA assays measuring soluble TRAIL in the conditioned media from cells exposed to IFNα for up to 72 hours were done using the Human TRAIL ELISA construction kit (RHF760CK) from Antigenix (Huntington Station, NY). Results were plotted against a TRAIL standard processed in parallel on the same ELISA plate. All experiments were done in triplicate.
Tritiated Thymidine Incorporation
RCC cells were seeded onto 35mm dishes and treated as indicated. Tritiated thymidine was added and the cells incubated for two hours. The media was removed and the cells incubated with 10% TCA solution, washed twice and incubated with 0.2 N NaOH. Aliquots were combined with scintillation fluid and counted on a scintillation counter. All experiments were done in triplicate and representative results are shown.
Results
TRAIL and IFNα act synergistically to induce RCC cell death
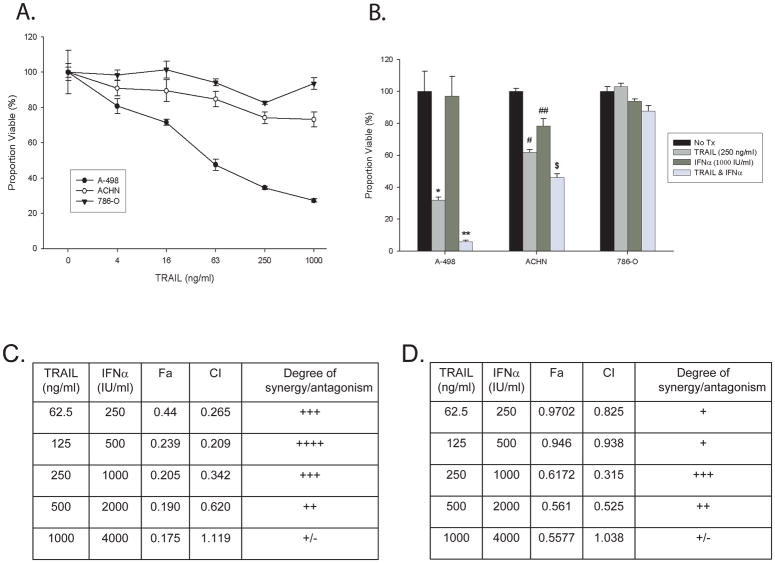
To test whether IFNα would alter RCC cells response to TRAIL we screened a panel of commercially available cell lines for their sensitivity to TRAIL mediated cell death and selected three cell lines that demonstrated differing responses to TRAIL (Figure 1a). A-498 cells had dramatic decreases in cell viability with small doses of TRAIL over 24 hours, 786-O cells were resistant to TRAIL at high doses and ACHN cells were of intermediate sensitivity and are known to be sensitive to IFNα. The maximal response to IFNα alone for all three cell lines was at a dose of 1000 IU/ml for 72 hours (data not shown).
Figure 1. RCC cell lines undergo synergistic cell death in response to TRAIL plus IFNα.
A. MTS assay of RCC cell lines treated with TRAIL at the indicated dose for 24 hours. B. MTS assay of RCC cell lines treated with recombinant TRAIL (250 ng/ml) for 24 hours, IFNα (1000 IU/ml) for 72 hours, or a combination of IFNα for 72 hours with TRAIL added for the last 24 hours. (* p=0.002, ** p<0.001 vs. control, p=0.01 vs. TRAIL alone, # p=0.009, ## p=0.06, $ p<0.001 vs. control or TRAIL alone, student t-test) C–F: A-498 (C, E) or ACHN (D, F) cells were exposed to varying doses of IFNα (250 IU/ml to 4,000 IU/ml) for 72 hours, TRAIL (62.5 ng/ml to 1000 ng/ml) for 24 hours, or the combination of the two agents. Cell viability was determined utilizing the MTS method as described above. Synergy was then determined using the CalcuSyn software as described in the methods and shown in tabular format (C&D) and graphically (E&F) at ED50 (red line) and ED75 (green line).
To determine if TRAIL plus IFNα was more effective at inducing cell death than either drug alone, the three RCC cell lines were treated with TRAIL, IFNα, or the combination and cell viability was measured by the MTS assay (Figure 1b). For A-498 and ACHN cells, TRAIL plus interferon induced more cell death than either treatment alone. To determine whether this was an additive or synergistic effect, isobologram analysis was undertaken with A-498 and ACHN cells treated with increasing doses of TRAIL, IFNα, or both (see methods section). Strong synergy was present for A-498 cells (Figure 1c & 1e), while with ACHN cells it was present but not as strong (Figure 1d &1f).
TRAIL and IFNα act by different mechanisms
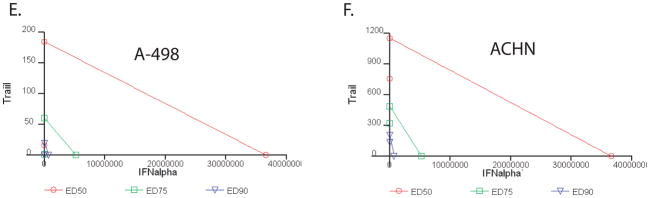
We confirmed that decreased viability of all the cell lines mediated by TRAIL was due to caspase dependent, apoptotic cell death by treating with TRAIL and measuring the degree of caspase 8, caspase 3, and PARP cleavage by Western blot (Figure 2a). As expected, in A-498 and ACHN cell lines (the two sensitive RCC’s) there was clear processing of caspase 8, caspase 3, and PARP cleavage after treatment with TRAIL, with very little cleavage in the resistant 786-O cell line. Although there was still initial processing of caspase 8, downstream cleavage of caspase 3 and PARP was inhibited with the addition of the pan-caspase inhibitor zVAD.fmk, confirming that the apoptotic cell death was caspase dependent. We tested whether IFNα on its own would result in processing of caspases/PARP or increase TRAIL induced processing (Figure 2b). As expected, IFNα on its own did not induce caspase or PARP cleavage. The combination of IFNα plus TRAIL resulted in a modest increase in Caspase 8 and 3 cleavage, but no obvious increase in PARP cleavage relative to TRAIL alone.
Figure 2. TRAIL mediated cell death of RCC cells is consistent with caspase dependent apoptosis. IFNα does not induce apoptosis on its own.
A) Western blot of caspase 8, caspase 3, and PARP cleavage in A-498, ACHN, and 786-O cells after treatment with TRAIL for 4 hours in the presence or absence of zVAD.fmk (100 μM/ml). B) Western blot of caspase 8, caspase 3, PARP cleavage in A-498 cells treated with IFNα, recombinant TRAIL, or a combination of both.
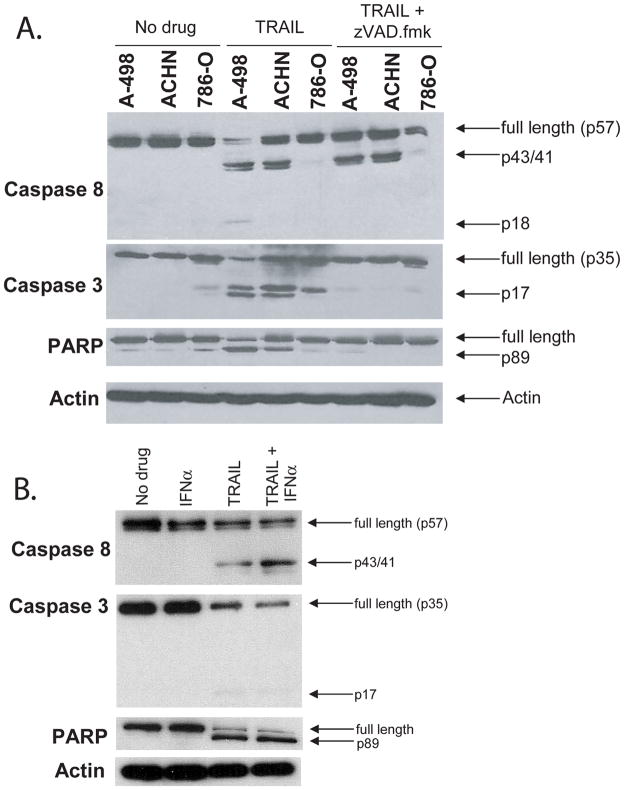
Since IFNα alone did not alter cell viability on the MTS assay (Figure 1b) and did not result in caspase or PARP processing (Figure 2b), we postulated that IFNα was decreasing RCC growth kinetics. To test this hypothesis, we measured cell growth by measuring tritiated thymidine incorporation of A-498 cells after exposure to IFNα. There was a significant decrease in tritiated thymidine incorporation following IFNα treatment in both a dose and time dependent fashion (Figures 3a and 3b), suggesting that exposure of A-498 cells to IFNα does not cause cell death, but could induce cell cycle arrest. This was confirmed by flow cytometry of A-498 cells stained with PI after exposure to IFNα for increasing time intervals (Figure 3c). By 72 hours the proportion of cells in S phase had increased from 24% to 33%, while those in G2 decreased from 11.2% to 5.8%, consistent with cell cycle arrest at the S-G2 interface. It should be emphasized; however, that this correlation does not prove that arrest at this interface in and of itself increases susceptibility to subsequent TRAIL mediated apoptosis. Note the absence of any significant increase in the proportion of cells in the sub-G1 fraction, confirming that A-498 cells were not undergoing cell death in response to IFNα.
Figure 3. IFNα induces a modest change in cell proliferation consistent with cell cycle arrest at S-G2 interface.
A and B. Thymidine incorporation of A-498 cells (A) was measured after treatment with increasing doses of IFNα as described in the methods. (* p<0.001, ** p<0.001, # p<0.001) B. Tritiated thymidine incorporation was measured in A-498 cells and ACHN cells treated with IFNα (1,000 I.U./mL.) for the time indicated. Tritiated thymidine incorporation was then measured as outlined in methods. (A-498: *p=0.015, ** p<0.001, # p<0.001; ACHN: * p=0.051, ** p=0.002, # p=0.001 student t-test) C. A-498 cells were left untreated or treated with IFNα at 1000 IU/mL for 6, 18, or 72 hours, stained with DAPI, and analyzed by flow cytometry.
Synergy of TRAIL plus IFNα is primarily due to increased apoptotic cell death
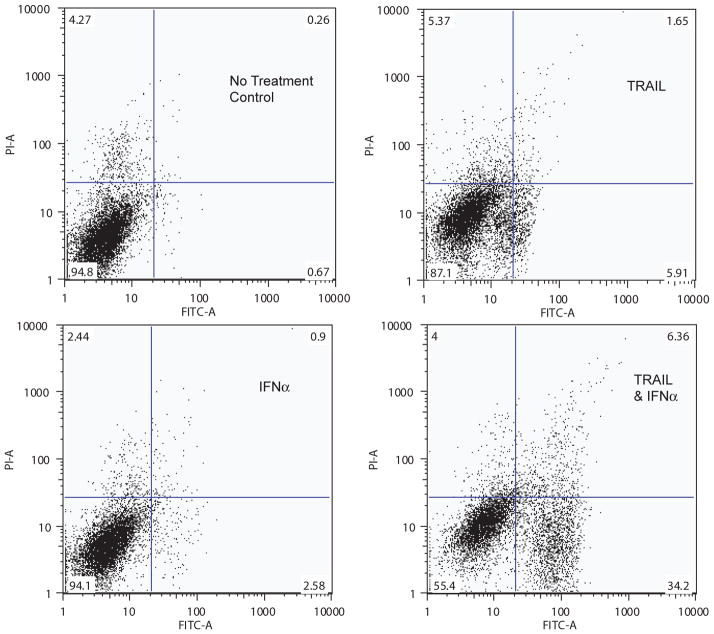
Since TRAIL induced apoptotic cell death, we postulated that the enhanced, synergistic cell death induced by the addition of IFNα was largely due to an increase in apoptosis rather than necrosis. We tested this hypothesis by treating A-498 cells with TRAIL, IFNα, or both and measured the amount and type of cell death (apoptosis versus necrosis versus mixed) by flow cytometry after staining the cells with Annexin V and PI (Figure 4). After exposure to TRAIL, the proportion of viable cells (Annexin V −/PI −) was reduced with the majority of dead/dying cells undergoing apoptotic cell death (Annexin V +/PI −). The combination of both TRAIL and IFNα resulted in a dramatic shift of cells that stained positive for Annexin V and negative for PI, suggesting that the synergism between TRAIL and interferon was largely due to an increase in apoptosis. As anticipated, IFNα induced little change in the proportion of dead or dying cells on its own.
Figure 4. RCC response to treatment with combination of TRAIL plus IFNα is predominantly due to an increase in the proportion of cells undergo apoptosis (annexin V positive, PI negative).
A-498 cells were left untreated or treated with IFNα at 72 hours and/or with TRAIL 100 ng/mL as indicated for the last 24 hours. Cells were incubated first with Annexin V then PI and analyzed by flow cytometry as outlined in the methods.
IFNα induced modulation of ERK activation contributes to synergy of TRAIL and IFNα
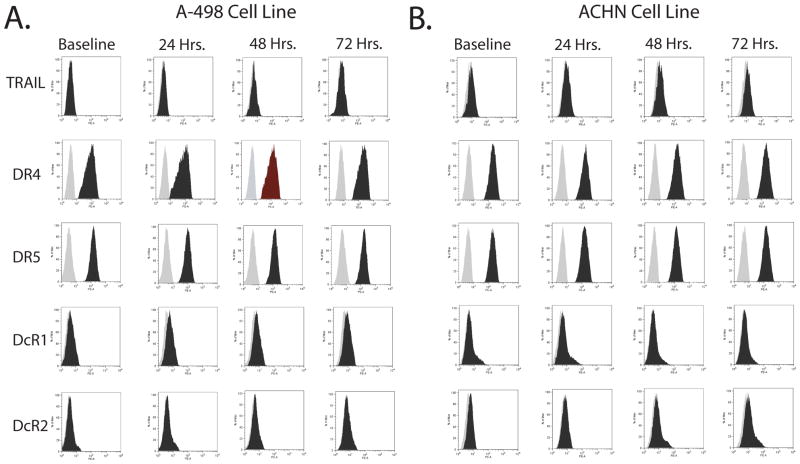
We hypothesized that IFNα is acting to increase RCC cells susceptibility to TRAIL mediated apoptosis by changing the expression of one or more genes known to regulate TRAIL mediated apoptosis. We undertook a candidate gene approach to define this target by sequentially testing known mediators/regulators of TRAIL mediated apoptosis via the extrinsic or intrinsic pathways. To test whether the synergy was due to alterations in the surface expression of TRAIL itself or it’s cognate death and decoy receptors A-498 and ACHN cells were treated with IFNα for up to 72 hours and death receptor surface expression determined by flow cytometry (Figure 5). No change in TRAIL or cell surface death receptor expression was seen.
Figure 5. Synergy between TRAIL and IFNα in A-498 and ACHN RCC cells is not due to IFNα induced changes in cell surface expression of TRAIL or its cognate death receptors.
Flow cytometry data measuring surface expression of TRAIL and its cognate receptors in A-498 (A) or ACHN (B) cells treated for 0, 24, 48, and 72 hours with 1,000 IU/ml IFNα. The lighter plot represents the negative control, the darker plot the surface expression of the specific death receptor.
TRAIL is a type 2 cell surface molecule that can be proteolytically cleaved and secreted in soluble form into the supernatant. We therefore tested whether TRAIL is secreted into the supernatant of A-498 or ACHN cells grown in culture and whether this is increased after exposure to IFNα by performing ELISA for TRAIL on the supernatant of these cell lines after exposure to INFα 1,000 IU/ml. for 0, 24, 48, or 72 hours. The lower limit for detection was 1.5 ng/ml of TRAIL based on our standard curves. No soluble TRAIL could be detected by ELISA at baseline or after exposure to IFNα for up to 72 hours for either A-498 or ACHN (data not shown).
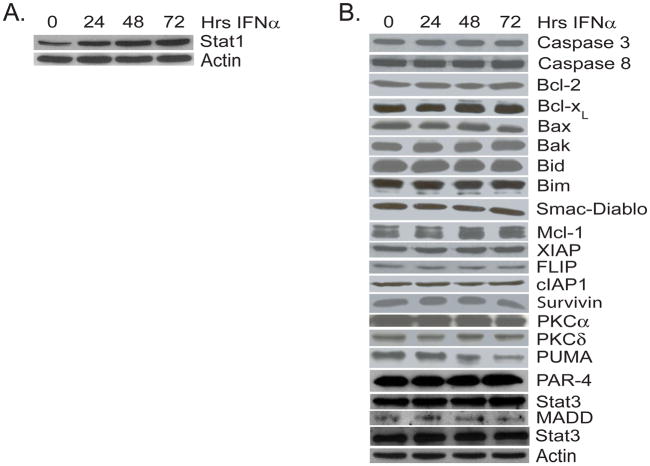
Since IFNα did not induce changes in TRAIL or death receptor expression we tested whether it was altering the levels of one or more known mediators of apoptosis. First we confirmed that A-498 cells responded to IFNα by verifying that there was an increase in the protein level of its known downstream signaling target STAT1 72 hours after treatment (Figure 6a). We then looked for changes in other known mediators of the apoptotic cascade, however there was no significant change in the protein expression level of any of the tested proteins (XIAP, FLIP, survivin, caspase 8, caspase 3, Bcl-2, Bcl-XL, Bid, Bax, Bak, Mcl, Bim, cIAP, PKCα, PKCδ, PUMA, Noxa, or SMAC/Diablo, Figure 6b) that could explain the observed synergistic response.
Figure 6. Synergy between TRAIL and IFNα in A-498 cells is not due to INFα induced changes in the expression of mediators of apoptosis.
Western blot analysis of cell lysates from A-498 cells treated for 0, 24, 48, and 72 hours with INFα (1,000 IU/ml) with antibodies against STAT1 (A) or known mediators of apoptosis including XIAP, FLIP, Bcl-2, Bcl-xL, Smac/DIABLO, Survivin, Caspase 8, and Caspase 3. For each of these experiments, staining for actin was done as a loading control (representative blots shown).
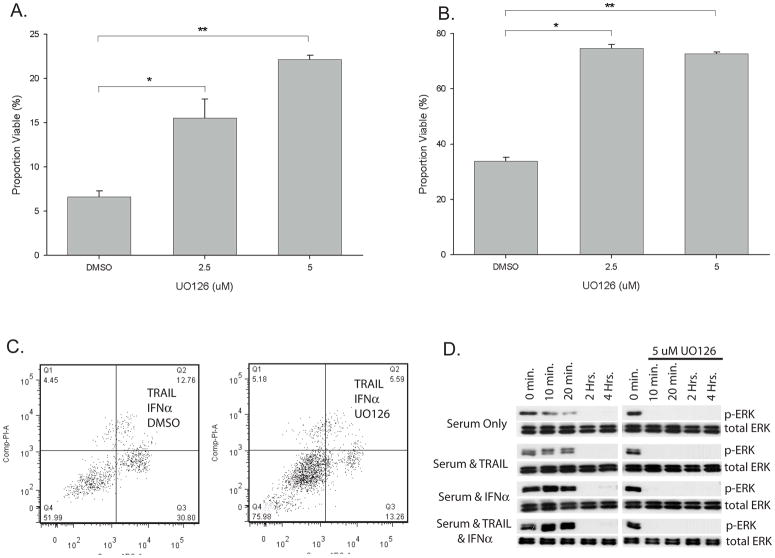
Since IFNα failed to alter the levels of TRAIL, death receptors, or protein mediators of apoptosis we looked for other signaling pathways that could be altered with IFNα treatment. TRAIL is known to activate signaling pathways including PI3 Kinase-AKT, ERK, p38 MAPK, and mTOR. To test whether inhibition of any of these pathways impacts the synergy of TRAIL and IFNα, A-498 cells were treated with IFNα and TRAIL in the presence or absence of increasing doses of inhibitors of these pathways (data not shown). Only inhibition of ERK with UO126 had a dose dependent impact on the synergy with TRAIL plus INFα (Figure 7a). Similar results were seen with ACHN cells (Figure 7b). To test if this was altering the proportion of cells undergoing apoptosis, flow cytometry and Annexin V/PI staining was performed on A-498 cells after treatment with IFNα plus TRAIL, in the presence or absence of UO126 (Figure 7c). UO126 decreased the proportion of cells undergoing apoptotic cell death relative to control, suggesting that IFNα may be inducing its synergistic effects with TRAIL by altering ERK signaling. To test this possibility we serum starved A-498 cells for 24 hours and then measured ERK activation by western blot after serum stimulation for up to four hours with or without pre-treatment with IFNα (Figure 7d). Note that RCC cells respond to the stress of serum starvation by increased phospho-ERK which decreases with time after the addition of serum. The baseline level of phospho-ERK as well as at later time points is increased with the addition of IFNα. This suggests that exposure of RCC cell lines to IFNα alters ERK signaling and makes them more susceptible to cell death when treated in combination with TRAIL.
Figure 7. Synergistic cell death from the combination of TRAIL plus IFNα is at least in part dependent on ERK activation.
A & B. MTS assay of A-498 (A) or ACHN (B) RCC cell lines treated with a combination of recombinant TRAIL (50 ng/mL A-498, 200 ng/mL ACHN) for 24 hours, IFNα (1000 IU/mL) for 72 hours, with the ERK inhibitor UO126 at the dose (μM) indicated or DMSO control for 72 hours. (A: * p=0.004, **p<0.001; B: * p<0.001, ** p<0.001 student t-test) C. A-498 cells treated with the combination of IFNα at 72 hours plus TRAIL for the last 24 hours in the presence of UO126 (5 μM) or DMSO control, stained with Annexin V/PI and analyzed by flow cytometry. D. Western blot of A-498 cells treated with or without IFNα (1,000 IU/mL) for 72 hours, serum starved for 24 hours, then treated with serum (10% FBS) for the time indicated. At the time serum was added, cells were additionally treated as indicated with or without TRAIL (50 ng/ML) in the presence of either the ERK inhibitor UO126 (5 μM) or DMSO control.
Discussion
Our data demonstrate that TRAIL and IFNα act synergistically to induce cell death in RCC cells in vitro. We also demonstrate that this increased cell death is predominantly due to increased apoptosis in response to exogenous TRAIL in cells treated with IFNα. While prior studies have explored the use of TRAIL in RCC cells either alone or in combination with chemotherapy, this is the first study to look at TRAIL in combination with IFNα. 19, 20 Although IFNα is no longer commonly utilized as monotherapy in the management of advanced RCC, it remains part of a frequently used doublet with the anti-VEGF antibody bevacizumab. Our study forms the rationale to explore the combination of IFNα, an established therapy for advanced RCC, with an actively investigated biologic therapy, TRAIL or DR directed monoclonal antibodies. We currently plan on directly testing if IFNα also synergizes with the DR directed monoclonal antibodies that are currently in phase I clinical trials. Future work will also test this combination utilizing in vivo animal models. Options would include testing RCC cell line xenografts in nude mice or testing the combination utilizing the RENCA model system in immunocompetent BALBc mice.
Regulation of TRAIL mediated apoptosis can occur at several different levels, including up or down regulation of TRAIL itself, altering the surface expression of effector death receptors (DR4 and DR5) or decoy receptors (DcR1 and DcR2), or changes in regulatory molecules that can affect the extrinsic or intrinsic apoptotic cascade such as cellular FLICE-inhibitory protein (c-FLIP), Bcl-2, Bcl-XL, XIAP, cIAP, Bid, Bax, Bak, Mcl, Bim, cIAP, PKCα, PKCδ, PUMA, Smac/DIABLO, survivin, and Noxa.21, 22 Surprisingly, IFNα treatment of RCC cells did not change the levels of any of these molecules involved in apoptosis regulation.
It has been well established that TRAIL can induce ERK activation in a variety of different cell types.23, 24 These data are consistent with our results where TRAIL, IFNα and the combination of both increase ERK activation. However in contrast to most studies where inhibition of TRAIL-induced ERK activation results in increased apoptosis25, in our study ERK inhibition decrease cell death and increases cell viability. This finding is consistent with at least two other studies, including one which looked at TRAIL mediated apoptosis in prostate cancer cells.26, 27 ERK inhibition can protect cells from stress induced cell death In a number of contexts including prostate cancer28, glioma29, and pancreatic cancer cells.30 Our data now suggests that, INFα alters ERK activation and inhibition of ERK can abrogate the RCC cell death induced by the combination of TRAIL and IFNα.
Conclusions
TRAIL combined with IFNα acts synergistically to kill RCC cells in vitro. The mechanism is at least partly dependent on changes in ERK signaling. These studies suggest that the combination of IFNα and TRAIL based therapies may be a therapeutic strategy for advanced RCC. They form the rationale to test the combination of these agents further in the in vivo setting and in the clinical setting in the future.
Acknowledgments
The project described was supported in part by Award Number K08 CA113452 (P.E.C.) from the National Institutes of Health. RZ is supported by DK065123; DK075594, DK65123; an AHA established investigator award; a Merit award from the Department of Veterans Affairs and the George O’Brien Center Grant (RCH, AP, DEK and RZ)
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Bukowski RM. Cytokine therapy for metastatic renal cell carcinoma. Semin Urol Oncol. 2001;19:148. [PubMed] [Google Scholar]
- 2.Rini BI. Metastatic renal cell carcinoma: many treatment options, one patient. J Clin Oncol. 2009;27:3225. doi: 10.1200/JCO.2008.19.9836. [DOI] [PubMed] [Google Scholar]
- 3.Chen Q, Gong B, Mahmoud-Ahmed AS, et al. Apo2L/TRAIL and Bcl-2-related proteins regulate type I interferon-induced apoptosis in multiple myeloma. Blood. 2001;98:2183. doi: 10.1182/blood.v98.7.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chawla-Sarkar M, Leaman DW, Borden EC. Preferential induction of apoptosis by interferon (IFN)-beta compared with IFN-alpha2: correlation with TRAIL/Apo2L induction in melanoma cell lines. Clin Cancer Res. 2001;7:1821. [PubMed] [Google Scholar]
- 5.Pitti RM, Marsters SA, Ruppert S, et al. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 6.Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 7.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 9.Leong S, Cohen RB, Gustafson DL, et al. Mapatumumab, an antibody targeting TRAIL-R1, in combination with paclitaxel and carboplatin in patients with advanced solid malignancies: results of a phase I and pharmacokinetic study. J Clin Oncol. 2009;27:4413. doi: 10.1200/JCO.2008.21.7422. [DOI] [PubMed] [Google Scholar]
- 10.Plummer R, Attard G, Pacey S, et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- 11.Mongkolsapaya J, Grimes JM, Chen N, et al. Structure of the TRAIL-DR5 complex reveals mechanisms conferring specificity in apoptotic initiation. Nat Struct Biol. 1999;6:1048. doi: 10.1038/14935. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22:8628. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 13.LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10:66. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- 14.Asakuma J, Sumitomo M, Asano T, et al. Selective Akt inactivation and tumor necrosis actor-related apoptosis-inducing ligand sensitization of renal cancer cells by low concentrations of paclitaxel. Cancer Res. 2003;63:1365. [PubMed] [Google Scholar]
- 15.Bouralexis S, Findlay DM, Atkins GJ, et al. Progressive resistance of BTK-143 osteosarcoma cells to Apo2L/TRAIL-induced apoptosis is mediated by acquisition of DcR2/TRAIL-R4 expression: resensitisation with chemotherapy. Br J Cancer. 2003;89:206. doi: 10.1038/sj.bjc.6601021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong B, Almasan A. Genomic organization and transcriptional regulation of human Apo2/TRAIL gene. Biochem Biophys Res Commun. 2000;278:747. doi: 10.1006/bbrc.2000.3872. [DOI] [PubMed] [Google Scholar]
- 17.Chawla-Sarkar M, Leaman DW, Jacobs BS, et al. IFN-beta pretreatment sensitizes human melanoma cells to TRAIL/Apo2 ligand-induced apoptosis. J Immunol. 2002;169:847. doi: 10.4049/jimmunol.169.2.847. [DOI] [PubMed] [Google Scholar]
- 18.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Zhang X, Barrisford GW, et al. Lexatumumab (TRAIL-receptor 2 mAb) induces expression of DR5 and promotes apoptosis in primary and metastatic renal cell carcinoma in a mouse orthotopic model. Cancer Lett. 2006 doi: 10.1016/j.canlet.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Griffith TS, Fialkov JM, Scott DL, et al. Induction and regulation of tumor necrosis factor-related apoptosis-inducing ligand/Apo-2 ligand-mediated apoptosis in renal cell carcinoma. Cancer Res. 2002;62:3093. [PubMed] [Google Scholar]
- 21.Griffith TS, Stokes B, Kucaba TA, et al. TRAIL gene therapy: from preclinical development to clinical application. Curr Gene Ther. 2009;9:9. doi: 10.2174/156652309787354612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 23.Secchiero P, Melloni E, Heikinheimo M, et al. TRAIL regulates normal erythroid maturation through an ERK-dependent pathway. Blood. 2004;103:517. doi: 10.1182/blood-2003-06-2137. [DOI] [PubMed] [Google Scholar]
- 24.Song JJ, Lee YJ. Differential cleavage of Mst1 by caspase-7/-3 is responsible for TRAIL-induced activation of the MAPK superfamily. Cell Signal. 2008;20:892. doi: 10.1016/j.cellsig.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran SE, Holmstrom TH, Ahonen M, et al. MAPK/ERK overrides the apoptotic signaling from Fas, TNF, and TRAIL receptors. J Biol Chem. 2001;276:16484. doi: 10.1074/jbc.M010384200. [DOI] [PubMed] [Google Scholar]
- 26.Li P, Wang X, Li N, et al. Anti-apoptotic hPEBP4 silencing promotes TRAIL-induced apoptosis of human ovarian cancer cells by activating ERK and JNK pathways. Int J Mol Med. 2006;18:505. [PubMed] [Google Scholar]
- 27.Kim YH, Lee DH, Jeong JH, et al. Quercetin augments TRAIL-induced apoptotic death: involvement of the ERK signal transduction pathway. Biochem Pharmacol. 2008;75:1946. doi: 10.1016/j.bcp.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao D, Singh SV. Phenethyl isothiocyanate-induced apoptosis in p53-deficient PC-3 human prostate cancer cell line is mediated by extracellular signal-regulated kinases. Cancer Res. 2002;62:3615. [PubMed] [Google Scholar]
- 29.Lee WC, Choi CH, Cha SH, et al. Role of ERK in hydrogen peroxide-induced cell death of human glioma cells. Neurochem Res. 2005;30:263. doi: 10.1007/s11064-005-2449-y. [DOI] [PubMed] [Google Scholar]
- 30.Sahu RP, Zhang R, Batra S, et al. Benzyl isothiocyanate mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of MAPK in human pancreatic cancer cells. Carcinogenesis. 2009 doi: 10.1093/carcin/bgp157. [DOI] [PMC free article] [PubMed] [Google Scholar]