Abstract
Background
Direct to consumer (DTC) BRCA testing may expand access to genetic testing and enhance cancer prevention efforts. However, it is not know if current DTC websites provide adequate risk information for informed medical decision-making.
Methods
284 women with a personal or family history of breast/ovarian cancer were randomly assigned to view a “mock” DTC commercial website (control condition: CC, n=93) or the same “mock” website that included information on the potential risks of obtaining genetic testing online. Risk information was framed two ways: risk information attributed to expert sources (ES, n=98) and unattributed risk information (URI, n=93). Participants completed an online survey. Endpoints were intentions to get BRCA testing, testing site preference and beliefs about DTC BRCA testing.
Results
Sample characteristics: mean age 39 (range 18–70), 82% white, mean education 3 yrs. college. Women exposed to risk information had lower intentions to get BRCA testing than women in the CC (adjusted odds ratio (OR) 0.48; 95% confidence interval (CI) 0.26–0.87, p=0.016), less positive beliefs about online BRCA testing (adjusted OR 0.48; 95% 0.27–0.86, p=0.014). Women in the ES condition were more likely to prefer clinic based testing than women in the CC (adjusted OR 2.05; 95% CI 1.07–3.90, p=0.030).
Conclusion
Exposing women to information on the potential risks of online BRCA testing altered their intentions, beliefs and preferences for BRCA testing. Policy makers may want to consider content and framing of risk information on DTC websites as they formulate regulation for this rapidly growing industry.
Keywords: communication, direct to consumer, BRCA, informed consent, cancer
Introduction
The landscape of cancer care is being significantly altered by two major trends in medicine: the explosion in publicly available cancer information and the shift in medicine towards patient autonomy and health consumerism(1–5). Commercial entities have been quick to capitalize on these changes through the use of direct to consumer (DTC) advertising and sales. While the ethics of DTC advertising have been widely debated, the upsurge in DTC sales of health products is potentially much more concerning. “Lifestyle drugs”, such as Viagra, are sold to consumers directly over the internet without a physician visit and high technology screening tests are available for patients on a self referral basis (6, 7). One of the most controversial developments in DTC sales is the sale of genetic tests. Over 30 internet-based companies now sell a variety of DTC genetic tests including cancer susceptibility testing, pharmacogenetic testing, and single nucleotide polymorphism evaluation (8).
Potential disadvantages of DTC genetic testing include early market entry before the clinical validity of a test had been established, over utilization in low risk individuals, concern over the quality of tests, inadequate counseling, misinterpretation of the test results and poor guidance in post-test decision making (9–16). On the other hand, DTC genetic testing may have advantages such as greater patient autonomy, enhanced patient privacy, expanded access to genetic testing and general consumer education (10–15, 17).
In the United States there is currently little mandatory oversight of laboratories that provide genetic tests or of the claims that companies can make regarding genetic testing services (18, 19). The American College of Medical Genetics has issued a policy statement concluding that genetic testing should be provided to the public only through qualified health professionals and that self-ordering of genetic tests is potentially harmful (16). Because of the concerns over DTC genetic testing, including evidence from a U.S. Government Accountability Office report which found that DTC nutrigenetic testing significantly misled consumers, new measures have been taken to regulate the industry (9, 15, 20). Recently, California and New York have moved to limit DTC genetic testing for state residents (21). Additionally, groups have called for the government to establish guidelines dictating the type of information that must be included in promotional material in ensure adequate informed consent. Specifically, the American Society for Human Genetics argued that all companies should provide information on all of the risks associated with testing in order to increase the potential for informed decision making (15).
The purpose of our study was to evaluate whether exposure to information on the potential risks of DTC BRCA testing in different formats would alter women’s beliefs about online BRCA testing and intentions to get BRCA testing. We hypothesized that women exposed to risk and benefit information for DTC genetic testing would have more negative beliefs about DTC genetic testing and have lower intentions to purchase genetic testing online, and that this effect would be greater for risk information presented by experts than unattributed risk information. Additional outcome variables relate to participant’s attitudes about DTC genetic testing. The theoretical basis of our study is the Integrated Model of Behavioral Prediction which has previously shown that behavioral beliefs, attitudes and intentions can be important predictors of health related behavior (22, 23). We chose to study BRCA testing because it is currently available DTC and because it is a complex test that presents many potential risks when delivered DTC. Importantly, we focused on the potential risks of getting BRCA testing online, not the general risks of getting a test for a BRCA mutation. We also decided to manipulate source credibility in order to determine if we could increase the persuasive impact of the risk message. Prior work has shown that when information is delivered by a highly credible source (one that is perceived as trustworthy and expert), it is often more persuasive in changing attitudes and increasing behavioral compliance than a low-credibility source (24).
Materials and Methods
Study Design
This study is a randomized experiment to evaluate the effects of exposure to risk information for DTC BRCA testing on women’s beliefs about online BRCA testing and intentions to get BRCA testing. The study was conducted from October 2006- October 2007. Participants completed a baseline telephone interview and then were randomized into three equal groups before viewing one of three online “mock” websites: the “mock” website alone (control condition, CC) or the “mock” website with the addition of either unattributed risk information (URI) or expert source risk information (ES). After viewing the online stimulus material the participants’ completed an online survey. We obtained institutional board approval from the University of Pennsylvania.
Study Population
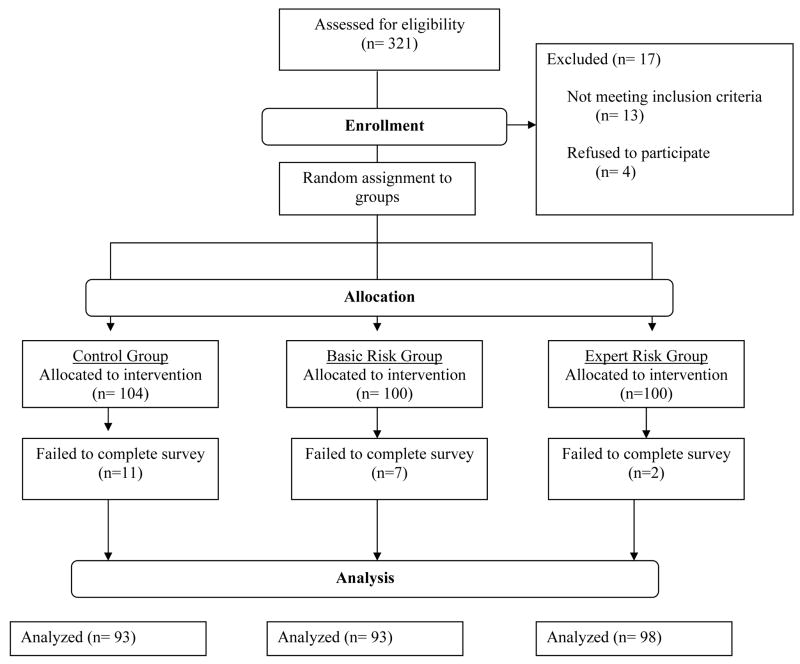
Women were invited to participate in the study if they were 18–80 years old and if they had a first or second degree relative with breast or ovarian cancer or if they had been diagnosed with breast or ovarian cancer before age fifty. Participants were excluded from the study if they had prior BRCA mutation testing, genetic counseling for hereditary breast and ovarian cancer (HBOC) syndrome, if they were unable to provide informed consent or if they were non-English speaking. Our inclusion criteria intended to recruit women who had an increased risk of having a BRCA gene mutation over the risk for women in the general population and for whom referral for genetic counseling might be considered. While there are no standardized referral criteria for BRCA mutation testing(25), we estimated the risk of carrying a BRCA gene mutation for all participants using the model on the Myriad Genetics website developed by Frank et al. (26, 27) Patients were recruited through print and online advertisements and flyers. Figure 1 provides a flow diagram of enrollment and attrition.
Figure 1.
Consort Flow Diagram
Data Collection
Baseline data on potential covariates (awareness of BRCA testing, perceptions of having a BRCA mutation, interest in BRCA testing, personal and family history of cancer, Ashkenazi Jewish heritage, cancer worry and genetic determinism) were collected via a 20 minute telephone. At the end of the telephone interview, participants were given the link to the stimulus material which they could view at any time. Participants who had not viewed the site within 24 hours received a reminder telephone call. After participants viewed the stimulus material, they were automatically directed to an online survey.
Intervention
Our stimulus material consisted of a password protected “mock” website that was hosted on the Annenberg School for Communication web server. The stimulus material contained modified visual and text images from a commercial website that sells online BRCA testing.(28) The names, phone numbers and other possible direct identifiers were changed or eliminated on the “mock” website. The stimulus material contained 47 control pages that had general information on BRCA testing, testing procedures, the pros and cons of testing and endorsements for online testing. Participants in the risk conditions were exposed to the same information as control participants but, in addition, viewed one page that outlined the possible risks of online genetic testing. In line with behavioral models such as Cognitive-Social Theory, we selected risk information that we felt would be relevant to our population and that may offer a new framing of the potential risks and benefits of DTC BRCA testing(29). We identified four key concerns about DTC BRCA testing through a literature review(11–15, 18, 19, 30–32), expert consultation and pre-testing with women who had a personal or family history of breast or ovarian cancer. The four key concerns (privacy, poor comprehension of test results, inadequate emotional support, and inadequate support for subsequent medical decision making) were presented to participants in the risk conditions (Table 1). Information in the URI and the ES conditions were similar in content however, the ES condition also included information on the source of the risk information along with a citation. Participants could access the “mock” website from any computer connected to the internet and were not directly observed during mock website navigation. Once connected, participants were able to freely navigate the site for up to 30 minutes. Following 30 minutes or when the participants chose to exit (by selecting an exit icon), the mock website displayed 6 core web pages to ensure participant exposure to key concepts related to online BRCA testing (major indications for BRCA testing, possible decisions affected by BRCA status, some pros and cons of BRCA testing, logistics of online testing, services associated with online testing and advantages of online testing). We included a mandatory viewing of the core web pages in order to lower the chance that variation in our outcomes would be attributed to variations in exposure to key logistical or factual BRCA information. Participants in the intervention groups also viewed the assigned risk page to ensure risk information exposure.
Table 1.
Risk Information
| Domain | Example unattributed risk | Example expert risk |
|---|---|---|
| Emotional support | You might not have the emotional support from experienced doctors and counselors who have helped many people cope with testing for hereditary breast and ovarian cancer. With internet testing, you don’t have the traditional doctor-patient relationship or meaningful, face- to- face genetic counseling. | You might not have the emotional support from experienced doctors and counselors who have helped many people cope with testing for hereditary breast and ovarian cancer if you have testing online. Patricia Roche, J.D. and George Annas, J.D., M.P.H. of the Boston University School of Public Health have expressed concern that using the internet for genetic testing sidesteps the doctor-patient relationship and eliminates meaningful, face- to- face genetic counseling. |
| Privacy | You might not have the same privacy protections if you have testing online. Online companies are not regulated by the Health Insurance Portability and Accountability Act (HIPAA). | You may not have the same privacy protections if you obtain genetic testing online. Adam Wolfberg, MD. M.P.H., of Tufts University, points out that, unlike physicians and hospitals, companies that offer online testing are not subject to the privacy restrictions of the Health Insurance Portability and Accountability Act (HIPAA). |
| Comprehension of test results | You might not understand the test results as completely if you have testing online. The results of genetic tests are not always “black and white”. That makes interpretations and explanations of the meaning of tests difficult. | You might not understand the test results as completely if you have testing online. “Genetic test results can be difficult to interpret… and genetic counseling or physician consultation might help people better appreciate the meaning of their test results” explain Sarah Gollust, B.A., Benjamin Wilfond, M.D. and Sara Chandros Hull, Ph.D., a genetics team at the National Institutes of Health |
| Subsequent medical decision-making | You might not get information that can help you make important medical and lifestyle decisions. Genetic test results are complex and you don’t want to make any decisions based on incomplete, inaccurate or misunderstood information. | You might not get information that can help you make important medical and lifestyle decisions if you have testing online. “Genetic test results can be complex and serious. You don’t want to make any decisions based on incomplete, inaccurate or misunderstood information,” cautions The Food and Drug Administration, the Centers for Disease Control and Prevention (CDC) and the Federal Trade Commission (FTC) in a recent statement about online and “at-home” genetic tests. |
Survey Instrument
Participants were automatically directed to a password protected online survey after viewing the stimulus material. We developed the baseline interview and survey based on literature review and expert consultation and used existing, validated measures when possible(33–43). The beginning of the survey included a short introduction that described BRCA gene mutation testing and defined internet companies and cancer genetic clinics.
Measures
Sociodemographic and Health Characteristics
We obtained self-reported information about age, race/ethnicity, education, marital status and health status through survey questions.
Outcome measures
Outcome measures are listed in Table 2. Beliefs about online BRCA testing were measured by 5 randomly ordered questions. After reverse coding the discrimination item, we created a belief index by summing the 5 belief items together (Cronbach’s alpha 0.69, inter-item covariance 0.88). Due to the non-normal and skewed distribution of our outcome variables and a desire to identify participants with strong intentions, beliefs, and preferences, we dichotomized our outcome measures. Intentions, testing preference site, trust and perceptions of enough information were dichotomized using the following schema: 1–4 (extreme left value to neutral) were coded as the reference group (zero) and 5–7 (right of neutral to extreme right) were coded as one. The belief item was dichotomized with the following schema 5–27 were coded as the reference group and 28–35 were coded as one (corresponding to an approximate 75% and 25% split).
Table 2.
Outcome Measures
| Outcome | Question | Scale |
|---|---|---|
| Intend to get BRCA Testing | “How likely is it that you will get a BRCA test in the next 12 months?” | 1–7 point rating scale from very unlikely to very likely |
| Positive Beliefs about Internet Testing | “Please tell me how much you agree or disagree with each statement about internet testing for a breast cancer gene mutation. Using an internet company to get a BRCA test would…”
|
1–7 point rating scale from very strongly disagree to strongly agree |
| Preference for Clinic Testing | “If you were to get a BRCA test, are you more likely to get a test from an internet company or by going to a cancer genetics clinic?” | 1–7 point scale from definitely internet company to definitely cancer genetics clinic |
| Trust in Internet Testing | “I would trust the results more if I was tested for a BRCA mutation through…” | 1–7 point scale from a cancer genetics clinic to an internet company |
| Believe Internet Testing is Wise | “Getting a BRCA test from an internet company in the next 12 months would be….” | 1–7 point scale from a foolish to wise |
| Site Provides Enough Risk Information | “The information that you viewed on the simulated website provided enough information about the possible risks that people might face if they decided to purchase BRCA testing from an internet company” | 1–7 point scale from strongly disagree to strongly agree |
Statistical Analysis
We used simple frequencies to determine participant characteristics and percent of participants in each outcome response category. Our study was powered to detect moderate to large effect sizes between groups. We estimated our probability of detecting a moderate effect (delta of 0.5) between any two groups to be between 86%–94% and our probability of detecting a large effect (delta of 0.75) between any two groups to be over 99% (assuming a two sided alpha of 0.05). Differences between groups were evaluated with Pearson’s chi square and ANOVA testing. We compared the combined risk group (URI and ES risk groups combined) with the control group and the individual risk groups (URI and ES risk groups separately) with the control group. We used multiple logistic regression to adjust the associations between information exposure by group and our outcomes for potential confounding variables that were different by group at baseline. All hypothesis tests were two-tailed and used a significance level of p=0.05. All statistical analysis was conducted in STATA 10 (Stata Corp. College Station, Tex).
Results
The characteristics of the participants by condition are reported in Table 3. Overall, the mean age of participants was 39 years (range 18–70 years). Eighty-two percent of participants were white, 58% married, and they had a mean education of 3 years of college. The calculated risk of having a gene mutation in our study population ranged from 2.8% to 30%, with an average risk of 6.4%, which is well above the estimated population prevalence of 0.06–0.69(44–46)%. Participant characteristics were balanced between groups except Hispanic ethnicity and interest in BRCA testing at baseline. Variables balanced at baseline but not listed in Table 3 include awareness of BRCA testing, perceptions of having a BRCA mutation (absolute and comparative), cancer worry (frequency and extent) and perceptions of genetic determinism. Participants in the unattributed risk condition had a lower reported interest in BRCA testing at baseline and participants in the expert risk condition were less likely to be Hispanic.
Table 3.
Participant Characteristics by Group
| Characteristic | Control Mean (n=93) |
Unattributed Risk Mean (n=93) |
Expert Risk Mean (n=98) |
χ2-value (or f-value) |
p-value |
|---|---|---|---|---|---|
| Age, mean (SD) | 39.0 (12.3) | 39.7 (13.2) | 38.2 (12.7) | 0.53 | 0.77 |
| Education, mean (SD) | 15.46 (2.52) | 15.63 (2.44) | 14.91 (2.49) | 0.57*** | 0.84 |
| Sex (% Women) | 100 | 100 | 100 | --- | --- |
| Ethnicity (%) | --- | --- | --- | 6.35 | 0.61 |
| White | 86.67 | 80.65 | 78.35 | --- | --- |
| Black | 8.89 | 12.9 | 10.31 | --- | --- |
| Asian | 2.22 | 2.15 | 7.22 | --- | --- |
| Other | 2.22 | 4.3 | 4.12 | --- | --- |
| Hispanic (%) | 5.56 | 3.23 | 0 | 5.26 | 0.07 |
| Employed Full-Time (%) | 56.67 | 63.44 | 50 | 3.47 | 0.176 |
| Married (%) | 55.56 | 63.44 | 55.21 | 1.66 | 0.44 |
| Health Insurance (%) | 87.78 | 84.95 | 86.6 | 0.32 | 0.85 |
| Works in Health Care (%) | 27.78 | 18.68 | 20.62 | 2.4 | 0.3 |
| History of Cancer (%) | --- | --- | --- | --- | --- |
| Breast | 8.6 | 12.9 | 7.14 | 1.97 | 0.38 |
| Ovarian | 0 | 1.1 | 0 | 2.06 | 0.35 |
| Frank Risk, mean (SD) | 0.069 (0.04) | 0.059 (0.4) | 0.065 (0.04) | 1.6 | 0.45 |
| Interested in Testing at Baseline (%) | --- | --- | --- | 10.98 | 0.03* |
| No | 5.38 | 16.13 | 8.16 | --- | --- |
| Yes | 66.67 | 47.31 | 64.29 | --- | --- |
| Don’t Know | 27.96 | 36.56 | 27.55 |
p < 0.05
f-value, not χ2
Unadjusted main effects are shown for the control group and the combined risk group in Table 4 and for the control group and the individual risk groups in Table 5.
Table 4.
Frequency of Outcomes: Combined Risk Group
| Outcome Measure | No Risk (%) |
Combined Risk Group (%) |
χ2-value | p value |
|---|---|---|---|---|
| Intend to get BRCA Testing | 30 | 17 | 5.9 | 0.015 |
| Positive Beliefs about Internet Testing | 33 | 19 | 6.2 | 0.012 |
| Preference for Clinic Testing | 62 | 72 | 2.8 | 0.095 |
| Trust in Internet Testing | 64 | 65 | 0.03 | 0.870 |
| Believe Internet Testing is Wise | 43 | 36 | 1.6 | 0.213 |
| Site Provides Enough Risk Information | 63 | 68 | 0.83 | 0.364 |
Table 5.
Frequency of Outcomes: Individual Risk Groups
| Outcome Measure | No Risk (%) |
Unattributed Risk (%) |
Expert Risk (%) |
χ2-value | p value |
|---|---|---|---|---|---|
| Intend to get BRCA Testing | 30 | 15 | 19 | 6.4 | 0.040 |
| Positive Beliefs about Internet Testing | 33 | 21 | 17 | 6.7 | 0.036 |
| Preference for Clinic Testing | 62 | 67 | 77 | 5.3 | 0.071 |
| Trust in Internet Testing | 64 | 59 | 71 | 3.2 | 0.202 |
| Believe Internet Testing is Wise | 43 | 34 | 37 | 1.7 | 0.436 |
| Site Provides Enough Risk Information | 63 | 70 | 67 | 1.0 | 0.606 |
We modeled the relationships between the different conditions and our outcomes controlling for the variables that were not balanced at baseline (Table 6). Participants who were exposed to risk information had lower intentions to get BRCA testing (adjusted odds ratio (OR) 0.48; 95% confidence interval (CI) 0.26–0.87, p=0.016) and less positive beliefs about online BRCA testing (adjusted OR 0.48; 95% 0.27–0.86, p=0.014) than controls. Participants in the URI group had lower intentions to get BRCA testing than participants in the CC (OR 0.42; 95% CI 0.20–0.88, p=0.022). Women in the ES group had higher reported preference for clinic testing (adjusted OR 2.05; 95% CI 1.07–3.90, p=0.030) and more negative beliefs about internet testing (OR 0.41; 95% CI 0.20–0.83, p=0.013) than women in the CC. While it appears that there might be differing effects for the two risk conditions on intentions to get tested, testing preference site and beliefs about internet testing, it is important to note that for each outcome the confidence intervals of the expert risk and unattributed risk group overlap.
Table 6.
Multivariate Models* for Individual Risk Groups Compared to the Control Group
| Outcome Measure | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Intend to get BRCA Testing | ||
| Combined Risk | 0.48 (0.26–.87) | 0.016 |
| Unattributed risk | 0.42 (0.20–0.88) | 0.022 |
| Expert Risk | 0.53 (0.27–1.05) | 0.072 |
| Positive Beliefs about Internet Testing | ||
| Combined Risk | 0.48 (0.27–0.86) | 0.014 |
| Unattributed risk | 0.56 (0.28–1.10) | 0.092 |
| Expert Risk | 0.41 (0.20–0.83) | 0.013 |
| Preference for Clinic Testing | ||
| Combined Risk | 1.53 (.90–2.62) | 0.117 |
| Unattributed risk | 1.18 (0.64–2.18) | 0.590 |
| Expert Risk | 2.05 (1.07–3.90) | 0.030 |
| Trust in Internet Testing | ||
| Combined Risk | 1.06 (0.63–1.82) | 0.813 |
| Unattributed risk | 0.78 (0.43–1.44) | 0.431 |
| Expert Risk | 1.46 (0.78–2.73) | 0.233 |
| Believe Internet Testing is Wise | ||
| Combined Risk | 0.73 (0.44–1.22) | 0.234 |
| Unattributed risk | 0.70 (0.38–1.27) | 0.240 |
| Expert Risk | 0.76 (0.42–1.38) | 0.374 |
| Site Provides Enough Risk Information | ||
| Combined Risk | 1.27 (0.75–2.17) | 0.371 |
| Unattributed risk | 1.37 (0.74–2.55) | 0.317 |
| Expert Risk | 1.19 (0.65–2.18) | 0.581 |
Controlling for baseline interest in testing and Hispanic ethnicity
The first line below the outcome variable reports the multivariate model for the combined risk group and covariates.
The third and fourth lines below the outcome variable report the multivariate model for the individual risk groups and covariates.
Discussion
To our knowledge, this is the first study to investigate the impact of exposure to risk information on women’s beliefs about and intentions to purchase DTC BRCA testing. This study demonstrates that women’s beliefs about DTC genetic testing, intentions to get BRCA testing and preference for where they get tested are altered by exposure to information on the possible risks of online BRCA testing.
Consistent with our hypothesis, we found that women who are exposed to information on some of the possible risks of DTC BRCA testing have more negative beliefs about DTC BRCA testing and have lower intentions to get BRCA testing than women in the control condition. These findings, while in line with our expectations, raise a number of important issues worth considering. First, if calls for increased risk information on DTC websites aim to optimize informed medical decision making our findings suggest that the inclusion of a limited amount of risk information in a commercial website will influence women’s beliefs and possibly their behavior. Our purpose in including information about the possible risks of DTC BRCA testing was to expose women to more “balanced” information than that which is often seen on commercial websites. Fair and balanced information provision is a key concept in the regulation of DTC advertising (18). Many of the commercial websites promote products but have little to no information on the risks of testing or on the risks of testing through the internet (9). The fact that the women in our experiment who were exposed to risk information had more negative beliefs about DTC testing and were more likely to prefer clinic based testing may mean that they had a greater appreciation for some of the possible risks of internet based testing and were perhaps more informed. However, the true impact of risk information exposure on informed consent is difficult to determine in a study of this nature and further research in this area is needed.
Second, the inclusion of information on the possible risks of internet BRCA testing on commercial websites may actually lower women’s intention to get any type of BRCA testing. One possible explanation for this finding is that the item assessing overall intentions for testing was interpreted as assessing intentions for DTC BRCA testing given the setting in which the questionnaire was completed. In order to investigate this issue further, we examined the association between exposure to risk information on testing preference site and on beliefs about DTC BRCA testing for women who did not intend to get BRCA testing (analysis not shown). We found that women in the risk conditions who did not intend to get testing reported a higher preference for clinic based BRCA testing (75% vs. 63%, p=0.08) and had more negative beliefs about DTC BRCA testing (85% vs. 68%, p=0.006) than women in the control condition. While we speculate that these findings suggest that the women in our experiment may in fact be reporting lower intentions to get DTC BRCA testing rather than any type of BRCA testing, we cannot reach firm conclusions based our data. The alternative and potentially more concerning explanation (when considering women with an elevated risk for HBOC) is that the inclusion of risk information on commercial websites actually decreases overall intentions to get BRCA testing. Prior work has shown that educational interventions can decrease interest in genetic testing (47–49). In addition, there is ample literature suggesting that genetic testing is underutilized despite the fact that mutation carriers and other high-risk women have good options for cancer risk reduction (35, 50–59). Possible reasons for low rates of utilization include lack of access to testing facilities, lack of awareness of genetic testing, psychological and family factors, perceptions of cancer survivability, cost, concerns over discrimination and lack of physician recommendation (52, 60–65). If in trying to optimize informed consent in the area of DTC BRCA testing we inadvertently discourage at risk women from obtaining a BRCA test we may be doing more harm than good. These findings stress the need to conduct careful studies investigating the potential impact of regulation on behavior in order to reduce the potential for adverse consequences.
The third important finding of our study is that by including risk information on commercial websites we may shift demand for BRCA testing from the internet to the clinic. The current standard of care for BRCA testing is testing within the context of a multi-disciplinary cancer risk evaluation program. While this method of care delivery may be optimal, experts are concerned that there may not be enough genetic practitioners to meet the increasing demand for genetics services (66–68). Multidisciplinary risk evaluation clinics are not available in all states and physicians often lack the training or knowledge to provide genetic counseling (67, 69–73). As genetic testing becomes more commonplace, health educators and providers must prioritize continuing genetic medical education in order to keep up with consumer demand.
Our second hypothesis, that risk information presented by experts would be more persuasive than unattributed risk information, was not well supported by our data. While the data suggest that there may be some differences for risk frames, particularly that the ES frame may be more influential in shaping preferences for testing site than the URI frame, our small sample size and the resulting wide and overlapping confidence intervals make distinctions between risk frames impossible. In addition, while we tried to match the content of the ES and the URI conditions as closely as possible, the language for the privacy and comprehension categories was not completely equivalent and could have confounded the small differences seen. Our inability to find differences between the two risk conditions leads us to believe either that the expert source condition was not more persuasive than the unattributed risk condition or that we have failed to detect a difference between our groups when one really exists. At this time, the estimated population level uptake of DTC BRCA testing is relatively unknown and we have assumed that moderate to large effect sizes would be of clinical importance. If, however, DTC BRCA testing becomes widely used, future work evaluating manipulations that detect small effects will be important. Future research on source credibility framing, as well as other types of framing, should be conducted in order to determine the most effective way to present risk information to consumers in the context of DTC genetic testing.
Our study has several limitations. The first limitation is that our relatively small sample size provided little power to detect differences in outcomes by risk frame. The second limitation is that our groups showed two imbalances at baseline: Hispanic ethnicity and interest in BRCA testing. However, we when we added these covariates into our models, the effect of exposure on outcomes was preserved. The third limitation is that our sample was predominately white, relatively well educated and all participants had access to the internet. While internet users are the likely target audience for DTC genetic testing, we acknowledge that the findings from this sample might not generalize to other ethnic or socioeconomic groups. Additionally, commercial websites often change over time and current websites for DTC BRCA testing may provide different information to consumers than they provided when our “mock” website was developed. Furthermore, we studied only a limited number of potential risks of online BRCA testing in women who had a family or personal history of breast or ovarian cancer. The findings of this study may not generalize to other types of risk information, other types of DTC genetic testing or to average-risk populations.
With the rise in DTC access to cancer-related technologies policymakers will need to determine if consumers should have access to online genetic testing and if the information and claims on company websites should be regulated. This study demonstrates that exposure to information on the potential risks of online BRCA testing alters women’s beliefs about, preferences for and intentions related to BRCA testing. Because there might be positive and negative consequences of regulating commercial DTC information, we must ensure that we understand the potential impact of the proposed regulation on patients’ beliefs and intentions so that we can create policy that truly optimizes cancer prevention strategies.
Acknowledgments
Funding/Support: NCI 5P50CA095856-05., Robert Wood Johnson Foundation and the American Society for Clinical Oncology
References
- 1.Balint J, Shelton W. Regaining the initiative. Forging a new model of the patient-physician relationship. Jama. 1996;275:887–91. doi: 10.1001/jama.275.11.887. [DOI] [PubMed] [Google Scholar]
- 2.Laine C, Davidoff F. Patient-centered medicine. A professional evolution Jama. 1996;275:152–6. [PubMed] [Google Scholar]
- 3.Rimal RN, Ratzan SC, Arnston P, Freimuth VS. Reconceptualizing the ‘Patient’: Health Care Promotion as Increasing Citizens’ Decision-Making Competencies. Health Communication. 1997;9:61–74. [Google Scholar]
- 4.Johnson JD. Cancer-related information seeking. Cresskill, NJ: Hampton Press, Inc; 1997. [Google Scholar]
- 5.Kaplan RM, Frosch DL. Decision making in medicine and health care. Annual Review of Clinical Psychology. 2005;1:525–56. doi: 10.1146/annurev.clinpsy.1.102803.144118. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong K, Schwartz JS, Asch DA. Direct sale of sildenafil (Viagra) to consumers over the Internet. N Engl J Med. 1999;341:1389–92. doi: 10.1056/NEJM199910283411810. [DOI] [PubMed] [Google Scholar]
- 7.Illes J, Fan E, Koenig BA, Raffin TA, Kann D, Atlas SW. Self-referred whole-body CT imaging: current implications for health care consumers. Radiology. 2003;228:346–51. doi: 10.1148/radiol.2282021083. [DOI] [PubMed] [Google Scholar]
- 8.Genetics and Public Policy Center [homepage on the Internet] Washington, DC: Genetics and Public Policy Center; c2002–08 [updated 2008 Nov 3; cited 2008 Nov 20]. Direct-to-Consumer Genetic Testing Companies; [about 1 page]. Available from: http://www.dnapolicy.org/resources/DTCcompanieslist.pdf. [Google Scholar]
- 9.Gollust SE, Wilfond BS, Hull SC. Direct-to-consumer sales of genetic services on the Internet. Genet Med. 2003;5:332–7. doi: 10.1097/01.GIM.0000076972.83711.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levitt DM. Let the consumer decide? The regulation of commercial genetic testing. J Med Ethics. 2001;27:398–403. doi: 10.1136/jme.27.6.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfberg AJ. Genes on the Web--direct-to-consumer marketing of genetic testing. N Engl J Med. 2006;355:543–5. doi: 10.1056/NEJMp068079. [DOI] [PubMed] [Google Scholar]
- 12.Williams-Jones B. Where there’s a web, there’s a way: commercial genetic testing and the Internet. Community Genet. 2003;6:46–57. doi: 10.1159/000069538. [DOI] [PubMed] [Google Scholar]
- 13.McCabe LL, McCabe ER. Direct-to-consumer genetic testing: access and marketing. Genet Med. 2004;6:58–9. doi: 10.1097/01.gim.0000105753.01536.be. [DOI] [PubMed] [Google Scholar]
- 14.Mykitiuk R. Caveat emptor: direct-to-consumer supply and advertising of genetic testing. Clin Invest Med. 2004;27:23–32. [PubMed] [Google Scholar]
- 15.Hudson K, Javitt G, Burke W, Byers P. ASHG Statement on direct-to-consumer genetic testing in the United States. Obstet Gynecol. 2007;110:1392–5. doi: 10.1097/01.AOG.0000292086.98514.8b. [DOI] [PubMed] [Google Scholar]
- 16.ACMG statement on direct-to-consumer genetic testing. Genet Med. 2004;6:60. doi: 10.1097/01.GIM.0000106164.59722.CE. [DOI] [PubMed] [Google Scholar]
- 17.Siegel R, Palca J. DNA: The Machinery Behind Human Beings - Part 1. National Public Radio, All things Considered. 2005 Oct 10; Available from: http://www.npr.org/templates/story/story.php?storyId=4953053. In.
- 18.Javitt GH, Stanley E, Hudson K. Direct-to-consumer genetic tests, government oversight, and the First Amendment: what the government can (and can’t) do to protect the public’s health. Oklahoma Law Rev. 2004;57:251–302. [PubMed] [Google Scholar]
- 19.Javitt GH. Policy implications of genetic testing: not just for geneticists anymore. Adv Chronic Kidney Dis. 2006;13:178–82. doi: 10.1053/j.ackd.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Nutrigenetic Testing: Tests purchased from four web sites mislead consumers. United States Government Accountability Office: GAO-06-977T: Testimony before the Special Committee on Aging, U.S. Senate. 109th Cong., 2nd Sess. (July 27, 2006). Available from: http://www.gao.gov/new.items/d06977t.pdf. In.
- 21.Pollack A. Gene Testing Questioned by Regulators. The New York Times. 2008 June 26; [Google Scholar]
- 22.Fishbein M, Ajzen I. Belief, attitude, intention, and behavior: an introduction to theory and research. Reading, MA: Addison-Wesley; 1975. [Google Scholar]
- 23.Fishbein M, Yzer M. Communication Theory. Vol. 13. 2003. Using Theory to Design Effective Health Behavior Interventions; pp. 164–83. [Google Scholar]
- 24.Pornpitakpan C. The Persuasiveness of Source Credibility: A Critical Review of Five Decades’ Evidence. Journal of Applied Social Psychology. 2004;34:243–81. [Google Scholar]
- 25.Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: recommendation statement. Ann Intern Med. 2005;143:355–61. doi: 10.7326/0003-4819-143-5-200509060-00011. [DOI] [PubMed] [Google Scholar]
- 26.Myriad Genetics [homepage on the Internet] Salt Lake City: Myriad Genetics; c1991–2008 [updated 2006 Spring; cited 2008 Nov 20]. BRCA Risk Calculator and Mutation Prevalence Tables; [about 2 pages]. Available from http://www.myriadtests.com/provider/brca-mutation-prevalence.htm. In. [Google Scholar]
- 27.Frank TS, Deffenbaugh AM, Reid JE, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20:1480–90. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 28.DNAdirect [homepage on the Internet] San Francisco: DNAdirect; c2003–08 [updated 2008; cited 2005 Oct 03]: Available from: http://www.dnadirect.com/ [Google Scholar]
- 29.Miller SM, Shoda Y, Hurley K. Applying cognitive-social theory to health-protective behavior: breast self-examination in cancer screening. Psychol Bull. 1996;119:70–94. doi: 10.1037/0033-2909.119.1.70. [DOI] [PubMed] [Google Scholar]
- 30.Roche PA, Annas GJ. DNA testing, banking, and genetic privacy. N Engl J Med. 2006;355:545–6. doi: 10.1056/NEJMp068136. [DOI] [PubMed] [Google Scholar]
- 31.Gollust SE, Hull SC, Wilfond BS. Limitations of direct-to-consumer advertising for clinical genetic testing. Jama. 2002;288:1762–7. doi: 10.1001/jama.288.14.1762. [DOI] [PubMed] [Google Scholar]
- 32.Federal Trade Commission, Bureau of Consumer Protection, Division of Consumer and Business Education [homepage on the Internet]. Washington DC: Federal Trade Commission; c1914–2008 [updated 2008 Mar 29; cited 2006 Jul]. FTC Facts for Consumers, At Home Genetic Tests: A Healthy Dose of Skepticism May Be the Best Prescription. Available from : http://www.ftc.gov/bcp/edu/pubs/consumer/health/hea02.htm. In.
- 33.Lerman C, Trock B, Rimer BK, Jepson C, Brody D, Boyce A. Psychological side effects of breast cancer screening. Health Psychol. 1991;10:259–67. doi: 10.1037//0278-6133.10.4.259. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong K, Weber B, Ubel PA, Guerra C, Schwartz JS. Interest in BRCA1/2 testing in a primary care population. Prev Med. 2002;34:590–5. doi: 10.1006/pmed.2002.1022. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. Jama. 2005;293:1729–36. doi: 10.1001/jama.293.14.1729. [DOI] [PubMed] [Google Scholar]
- 36.Llewellyn-Thomas HA, McGreal MJ, Thiel EC, Fine S, Erlichman C. Patients’ willingness to enter clinical trials: measuring the association with perceived benefit and preference for decision participation. Soc Sci Med. 1991;32:35–42. doi: 10.1016/0277-9536(91)90124-u. [DOI] [PubMed] [Google Scholar]
- 37.Shea JA, Micco E, Dean LT, McMurphy S, Schwartz JS, Armstrong K. Development of a revised Health Care System Distrust scale. J Gen Intern Med. 2008;23:727–32. doi: 10.1007/s11606-008-0575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jallinoja P, Hakonen A, Aro AR, et al. Attitudes towards genetic testing: analysis of contradictions. Soc Sci Med. 1998;46:1367–74. doi: 10.1016/s0277-9536(98)00017-3. [DOI] [PubMed] [Google Scholar]
- 39.Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33:AS264–79. [PubMed] [Google Scholar]
- 40.Mossey JM, Shapiro E. Self-rated health: a predictor of mortality among the elderly. Am J Public Health. 1982;72:800–8. doi: 10.2105/ajph.72.8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Idler EL, Angel RJ. Self-rated health and mortality in the NHANES-I Epidemiologic Follow-up Study. Am J Public Health. 1990;80:446–52. doi: 10.2105/ajph.80.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Connelly JE, Philbrick JT, Smith GR, Jr, Kaiser DL, Wymer A. Health perceptions of primary care patients and the influence on health care utilization. Med Care. 1989;27:S99–109. doi: 10.1097/00005650-198903001-00009. [DOI] [PubMed] [Google Scholar]
- 43.Human Genetic Commission [homepage on the Internet] London: Human Genetics Commission; c1999–2008 [updated 2008 Nov; cited 2008 Nov 20]. YouGov: Public attitudes to genetic testing, A quantitative study for the Human Genetics Commission, 27th December 2002 to 5th January 2003. Available from: http://www.hgc.gov.uk/UploadDocs/DocPub/Document/evidence_yougov.pdf. In. [Google Scholar]
- 44.Ford D, Easton DF, Peto J. Estimates of the gene frequency of BRCA1 and its contribution to breast and ovarian cancer incidence. Am J Hum Genet. 1995;57:1457–62. [PMC free article] [PubMed] [Google Scholar]
- 45.Risch HA, McLaughlin JR, Cole DE, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98:1694–706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 46.Rahman N, Stratton MR. The genetics of breast cancer susceptibility. Annu Rev Genet. 1998;32:95–121. doi: 10.1146/annurev.genet.32.1.95. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz MD, Benkendorf J, Lerman C, Isaacs C, Ryan-Robertson A, Johnson L. Impact of educational print materials on knowledge, attitudes, and interest in BRCA1/BRCA2: testing among Ashkenazi Jewish women. Cancer. 2001;92:932–40. doi: 10.1002/1097-0142(20010815)92:4<932::aid-cncr1403>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 48.Green MJ, Biesecker BB, McInerney AM, Mauger D, Fost N. An interactive computer program can effectively educate patients about genetic testing for breast cancer susceptibility. Am J Med Genet. 2001;103:16–23. doi: 10.1002/ajmg.1500. [DOI] [PubMed] [Google Scholar]
- 49.Green MJ, Peterson SK, Baker MW, et al. Effect of a computer-based decision aid on knowledge, perceptions, and intentions about genetic testing for breast cancer susceptibility: a randomized controlled trial. Jama. 2004;292:442–52. doi: 10.1001/jama.292.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lerman C, Narod S, Schulman K, et al. BRCA1 testing in families with hereditary breast-ovarian cancer. A prospective study of patient decision making and outcomes. Jama. 1996;275:1885–92. [PubMed] [Google Scholar]
- 51.Botkin JR, Smith KR, Croyle RT, et al. Genetic testing for a BRCA1 mutation: prophylactic surgery and screening behavior in women 2 years post testing. Am J Med Genet A. 2003;118A:201–9. doi: 10.1002/ajmg.a.10102. [DOI] [PubMed] [Google Scholar]
- 52.Biesecker BB, Ishibe N, Hadley DW, et al. Psychosocial factors predicting BRCA1/BRCA2 testing decisions in members of hereditary breast and ovarian cancer families. Am J Med Genet. 2000;93:257–63. doi: 10.1002/1096-8628(20000814)93:4<257::aid-ajmg1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 53.Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations [comment] New England Journal of Medicine. 2002;346:1616–22. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 54.Rebbeck TR, Levin AM, Eisen A, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst. 1999;91:1475–9. doi: 10.1093/jnci/91.17.1475. [DOI] [PubMed] [Google Scholar]
- 55.Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation [comment] New England Journal of Medicine. 2002;346:1609–15. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- 56.Shih HA, Couch FJ, Nathanson KL, et al. BRCA1 and BRCA2 mutation frequency in women evaluated in a breast cancer risk evaluation clinic. J Clin Oncol. 2002;20:994–9. doi: 10.1200/JCO.2002.20.4.994. [DOI] [PubMed] [Google Scholar]
- 57.Meijers-Heijboer H, van Geel B, van Putten WL, et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation [comment] New England Journal of Medicine. 2001;345:159–64. doi: 10.1056/NEJM200107193450301. [DOI] [PubMed] [Google Scholar]
- 58.Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer [comment] New England Journal of Medicine. 1999;340:77–84. doi: 10.1056/NEJM199901143400201. [DOI] [PubMed] [Google Scholar]
- 59.Eisen A, Lubinski J, Klijn J, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case-control study. J Clin Oncol. 2005;23:7491–6. doi: 10.1200/JCO.2004.00.7138. [DOI] [PubMed] [Google Scholar]
- 60.Hall MJ, Olopade OI. Disparities in genetic testing: thinking outside the BRCA box. J Clin Oncol. 2006;24:2197–203. doi: 10.1200/JCO.2006.05.5889. [DOI] [PubMed] [Google Scholar]
- 61.Lerman C, Shields AE. Genetic testing for cancer susceptibility: the promise and the pitfalls. Nat Rev Cancer. 2004;4:235–41. doi: 10.1038/nrc1301. [DOI] [PubMed] [Google Scholar]
- 62.Vadaparampil ST, Wideroff L, Breen N, Trapido E. The impact of acculturation on awareness of genetic testing for increased cancer risk among Hispanics in the year 2000 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2006;15:618–23. doi: 10.1158/1055-9965.EPI-05-0378. [DOI] [PubMed] [Google Scholar]
- 63.Matthews AK, Cummings S, Thompson S, Wohl V, List M, Olopade O. Genetic testing of African-Americans for susceptibility to inherited cancers: use of focus groups to determine factors contributing to participation. Journal of Psychosocial Oncology. 2000;18:1–19. [Google Scholar]
- 64.Hughes C, Fasaye GA, LaSalle VH, Finch C. Sociocultural influences on participation in genetic risk assessment and testing among African American women. Patient Educ Couns. 2003;51:107–14. doi: 10.1016/s0738-3991(02)00179-9. [DOI] [PubMed] [Google Scholar]
- 65.Sankar P, Wolpe PR, Jones NL, Cho M. How do women decide? Accepting or declining BRCA1/2 testing in a nationwide clinical sample in the United States Community. Genet. 2006;9:78–86. doi: 10.1159/0000XXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kinmonth AL, Reinhard J, Bobrow M, Pauker S. The new genetics. Implications for clinical services in Britain and the United States. Bmj. 1998;316:767–70. doi: 10.1136/bmj.316.7133.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watson EK, Shickle D, Qureshi N, Emery J, Austoker J. The ‘new genetics’ and primary care: GPs’ views on their role and their educational needs. Fam Pract. 1999;16:420–5. doi: 10.1093/fampra/16.4.420. [DOI] [PubMed] [Google Scholar]
- 68.Hayflick SJ, Eiff MP. Role of primary care providers in the delivery of genetics services. Community Genet. 1998;1:18–22. doi: 10.1159/000016131. [DOI] [PubMed] [Google Scholar]
- 69.Suther S, Goodson P. Barriers to the provision of genetic services by primary care physicians: a systematic review of the literature. Genet Med. 2003;5:70–6. doi: 10.1097/01.GIM.0000055201.16487.61. [DOI] [PubMed] [Google Scholar]
- 70.Freedman AN, Wideroff L, Olson L, et al. US physicians’ attitudes toward genetic testing for cancer susceptibility. Am J Med Genet A. 2003;120:63–71. doi: 10.1002/ajmg.a.10192. [DOI] [PubMed] [Google Scholar]
- 71.Burke W, Emery J. Genetics education for primary-care providers. Nat Rev Genet. 2002;3:561–6. doi: 10.1038/nrg845. [DOI] [PubMed] [Google Scholar]
- 72.Emery J, Watson E, Rose P, Andermann A. A systematic review of the literature exploring the role of primary care in genetic services. Fam Pract. 1999;16:426–45. doi: 10.1093/fampra/16.4.426. [DOI] [PubMed] [Google Scholar]
- 73.Wideroff L, Vadaparampil ST, Greene MH, Taplin S, Olson L, Freedman AN. Hereditary breast/ovarian and colorectal cancer genetics knowledge in a national sample of US physicians. J Med Genet. 2005;42:749–55. doi: 10.1136/jmg.2004.030296. [DOI] [PMC free article] [PubMed] [Google Scholar]