Abstract
During sexual reproduction in many species, sperm and oocyte secrete diffusible signaling molecules to help orchestrate the biological symphony of fertilization. In the Caenorhabditis elegans gonad, bidirectional signaling between sperm and oocyte is important for guiding sperm to the fertilization site and inducing oocyte maturation. The molecular mechanisms that regulate sperm guidance and oocyte maturation are being delineated. Unexpectedly, these mechanisms are providing insight into human diseases, such as amyotrophic lateral sclerosis, spinal muscular atrophy, and cancer. Here we review sperm and oocyte communication in C. elegans and discuss relationships to human disorders.
Keywords: C. elegans, sperm, oocyte, fertilization, MSP, VAPB, PUFA, eicosanoid, prostaglandin, ALS, SMA, cancer, oocyte maturation, Eph receptor
INTRODUCTION
Fertilization is the union of two haploid gametes to form a diploid embryo. It is essential for sexual reproduction and generates genetic diversity that leads to evolution of species. In metazoans, females produce large immotile gametes called oocytes, whereas males produce small motile sperm (reviewed in Matova and Cooley, 2001). After mating or spawning, sperm must locate oocytes competent for fertilization, either in the female reproductive tract or aquatic environment. Gamete development is dependent on meiosis, a specialized cell cycle program comprising two divisions that result in a haploid genome. Sperm proceed through meiosis uninterrupted, but oocytes arrest during multiple stages following premeiotic DNA replication and meiotic recombination. The first arrest occurs in meiotic prophase, while the oocytes accumulate RNAs, proteins, and nutrients essential for early embryonic development. Meiosis resumes during oocyte maturation, a process that prepares the oocyte for fertilization and embryogenesis (reviewed in Masui and Clarke, 1979; Masui, 1985; Dekel, 2005). Oocyte maturation is temporally coupled to the expulsion of the oocyte from the ovary, termed ovulation. After oocyte maturation and ovulation, oocytes arrest again in meiosis, frequently in metaphase I or metaphase II, depending on the species. Sperm and oocyte fusion induces egg activation, a process that restarts the oocyte meiotic divisions, initiates embryonic development, and prevents polyspermy (reviewed in Runft et al., 2002). Ovulated oocytes that are not fertilized in a timely fashion degenerate, essentially wasting the energy and resources necessary to produce them. Diffusible factors, often secreted by sperm and oocytes themselves, help motile sperm locate oocytes at the correct stage of development.
The molecular machinery that mediates sperm and oocyte communication is largely unexplored, with the exception of several marine species that are amenable to biochemical analysis. For example, the egg jelly of the sea urchin Arbacia punctulata contains a small peptide called resact that functions as a sperm attractant (Ward et al., 1985). In other marine species, amino acid derivatives, steroids, or fatty acids regulate sperm motility (Coll et al., 1994; Riffell et al., 2002; Yoshida et al., 2002). Considerably less is known about factors secreted by sperm. Sperm are thought to influence oocyte maturation and ovulation in a variety of species, but the molecular mechanisms are not understood (reviewed in Masui and Clarke, 1979).
Secreted signaling molecules such as Wnts, Hedgehogs, insulin-like peptides, and TGFβ ligands are fundamental for animal growth and development. These signals and their evolutionarily conserved transduction mechanisms are used reiteratively during development to regulate cell proliferation, differentiation, apoptosis, and migration. It is tempting to speculate that sperm and oocyte communicate using conserved mechanisms important for regulating migration and proliferation during earlier development. While the sampling of sperm chemoattractants identified in marine animals does not support this view, the current scope is too narrow to make conclusions. Marine animals release their gametes into aquatic environments that contain gametes from an array of species. In these environments, there may be strong selective pressure to use novel factors that do not attract sperm from incompatible genotypes, limiting cross hybridization. The pressure may not be as strong for animals with internal fertilization, because species-specific behavioral and physical differences can prevent mating.
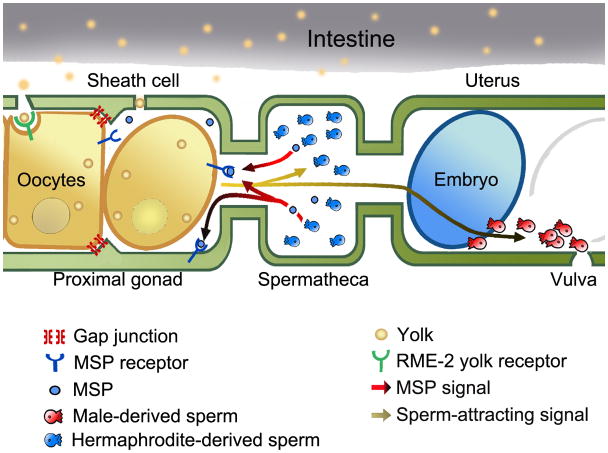
The nematode C. elegans is becoming an important model for discovering the molecular mechanisms by which sperm and oocyte communicate within the reproductive tract (reviewed in Singson, 2001; Greenstein, 2005; Yamamoto et al., 2005). Prior to fertilization, sperm secrete a protein hormone called MSP (major sperm protein) to stimulate oocyte maturation and ovarian sheath cell contraction, which act together to facilitate ovulation (Miller et al., 2001) (Figure 1). MSPs are the founding members of a new class of secreted hormones that may be important for human motor neuron survival (Tsuda et al., 2008). A missense mutation in the MSP domain of the human gene vapb is associated with amyotrophic lateral sclerosis (ALS) and late-onset spinal muscular atrophy (SMA) (Nishimura et al., 2004). There is accumulating evidence that MSP domains of C. elegans, Drosophila, and mammalian VAPB homologs function as secreted ligands for Eph receptors and other receptors, as previously shown for MSPs (Tsuda et al., 2008). The pathogenic mutation functions as a dominant negative, causes intracellular VAPB aggregation, and prevents MSP domain secretion in Drosophila cells. Eph receptors bind to membrane-associated ligands called ephrins and are implicated in diverse processes, including angiogenesis, morphogenesis, neuron migration, and insulin secretion (reviewed in Brantley-Sieders and Chen, 2004; Pasquale, 2005; Pasquale, 2008).
Figure 1. The C. elegans proximal gonad and reproductive tract.
Oocytes secrete signals derived from PUFAs to regulate sperm guidance to the spermatheca, the site of fertilization. Recent data provide strong evidence that these sperm guidance signals are novel F-series prostaglandins (J. Edmonds, J. Prasain, and M. Miller, unpublished data). Sperm, in turn, secrete the protein hormone MSP to induce oocyte maturation and ovarian sheath cell contraction. The maturing oocyte signals for dilation of the spermathecal valve, promoting ovulation into the spermatheca.
C. elegans oocytes also communicate with sperm (Figure 1). Oocytes secrete factors derived from polyunsaturated fatty acids (PUFAs) to guide sperm to the spermatheca, the site of fertilization (Kubagawa et al., 2006). In humans, PUFAs are precursors of signaling molecules called eicosanoids (reviewed in Funk, 2001; Zamaria, 2004). Leukotrienes and prostaglandins are two eicosanoid classes implicated in diverse actions throughout the human body. For example, the leukotriene LTB4 and prostaglandin PGD2 help guide immune cells to sites of infection, likely by acting as chemoattractants (Goodarzi et al., 2003; Tager et al., 2003; Luster and Tager, 2004). Nonsteroidal anti-inflammatory drugs (NSAIDs), such as aspirin and ibuprofen specifically inhibit prostaglandin synthesis by targeting cyclooxygenase enzymes (Vane, 1971; Vane et al., 1998). Prostaglandins are important for fertilization, but the mechanisms are not well understood (Lim et al., 1997; Langenbach et al., 1999).
This review focuses on sperm and oocyte communication mechanisms that regulate C. elegans fertilization. We summarize the current understanding of MSP and PUFA signaling and discuss how these mechanisms might provide insight into human fertility, as well as diseases like ALS, SMA, and cancer. The signaling mechanisms that occur during sperm and oocyte fusion have been reviewed elsewhere and are not included here (Singson, 2001; Runft et al., 2002; Singson et al., 2008).
SPERM TO OOCYTE COMMUNICATION
In the Caenorhabditis genus, hermaphroditism has likely evolved from gonochorism (separate female and male sexes) multiple times (Kiontke et al., 2004; Haag, 2005). C. elegans is a facultative hermaphroditic species consisting of hermaphrodites and males. Hermaphrodites are modified females with germ lines that produce sperm during larval development before transitioning to oocytes, exclusively (Hirsh et al., 1976; reviewed in Ellis and Schedl, 2007; Ellis, 2008). During the first ovulation, spermatids in the proximal gonad enter the spermatheca and become motile spermatozoa (L’Hernault, 2006; L’Hernault, 2009). C. elegans sperm, like those of most nematodes, crawl across substrates using a pseudopod of MSP filaments (Klass and Hirsh, 1981; Roberts and Stewart, 2000; Bottino et al., 2002). The distal hermaphrodite gonad contains mitotic germ cells whose daughters move proximally and enter meiosis. The molecular mechanisms that regulate germ cell proliferation, meiotic entry, and recombination have been studied extensively and are reviewed elsewhere (reviewed in Kimble and Crittenden, 2005; Hansen and Schedl, 2006; Kimble and Crittenden, 2007). Oocyte development and fertilization occur in an assembly line that can be viewed in the intact animal in real time. Smooth muscle-like ovarian sheath cells surround the developing oocytes, form gap junctions with them, and contract to facilitate ovulation (Hall et al., 1999) (Figure 1). The oocyte adjacent to the spermatheca undergoes oocyte maturation and ovulation approximately every 25 minutes when sperm are present (McCarter et al., 1999). Oocyte maturation is considered a transition from G2 to M phase of the cell cycle and consists of nuclear envelope breakdown, cytoskeletal rearrangement, and meiotic spindle assembly. During ovulation, sheath cells contract intensely, the spermathecal valve dilates, and the maturing oocyte enters the spermatheca, where it is fertilized (Figure 1). Fertilized eggs begin embryonic development in the uterus, before being expelled through the vulva into the external environment.
In a wide range of species with internal fertilization, oocyte maturation is linked to sperm presence (Masui and Clarke, 1979; Masui, 1985). In 1979, Ward and Carrel showed that C. elegans oocyte production is stimulated by sperm or seminal fluid (Ward and Carrel, 1979). Twenty years later, McCarter et al. demonstrated that sperm presence stimulates oocyte maturation and basal sheath cell contraction, while the maturing oocyte induces spermathecal valve dilation and a transient rise in sheath contraction (McCarter et al., 1999). When sperm are absent, oocytes arrest in diakinesis of meiotic prophase. Because the C. elegans hermaphrodite gonad produces sperm before oocytes, oocyte maturation and ovulation occur constitutively in adults until sperm depletion. In mutant hermaphrodites that do not produce sperm and gonochoristic Caenorhabditids, oocytes arrest in meiotic prophase until mating occurs and sperm crawl to the spermatheca.
Sperm in the spermatheca are physically separated from oocytes by a valve-like constriction. The ability of sperm to induce oocyte maturation without contact led to the hypothesis that sperm secrete a diffusible factor(s). A biochemical approach was used to identify sperm-derived factors that could promote oocyte maturation and sheath contraction in the absence of sperm (Miller et al., 2001). Substances were introduced by microinjection into the reproductive tracts of unmated females, which were generated using null mutations in fog-2 and fog-3 (feminization of germline) genes (Schedl and Kimble, 1988; Ellis and Kimble, 1995). Injected molecules diffuse throughout the extracellular regions of the uterus, spermatheca, and proximal gonad. Oocyte maturation and ovarian contraction inducers from other animals, such as 1-methyladenine and oxytocin, had no effect in C. elegans gonads. In contrast, a conditioned medium made from purified spermatids stimulated oocyte maturation, sheath contraction, and ovulation. The coordinated responses were identical to those seen after mating, except that fertilization did not occur because sperm were not present. Fractionation of the conditioned medium using reversed-phase high-performance liquid chromatography indicated that oocyte maturation and sheath contraction activities co-purify on multiple columns. Matrix-assisted laser desorption/ionization mass spectrometry showed that a complex mixture of polypeptides ranging from 14,121-14,147 daltons was present in the active fractions. Tryptic peptide mapping and sequencing representative fragments with post source decay mass spectrometry identified the bioactive polypeptides as the MSPs. Microinjecting nanomolar concentrations of purified sperm-derived or recombinant MSPs into unmated female gonads stimulates oocyte maturation and sheath contraction responses that mimic sperm presence. Furthermore, extracellular MSP was able to activate the oocyte mitogen-activated protein kinase (MAPK) MPK-1 (Miller et al., 2001). Injecting antibodies against MSP could slow oocyte maturation rates in the presence of sperm. These results provided the first compelling evidence that MSPs are secreted hormones. More recently, extracellular MSP has been shown to regulate oocyte microtubule reorganization, oocyte growth, and large ribonucleoprotein foci disassembly (Harris et al., 2006; Jud et al., 2008; Govindan et al., 2009).
THE BI-FUNCTIONAL MSPS
MSPs are the most abundant proteins in nematode sperm originally identified by Klass and Hirsh in 1981 (Klass and Hirsh, 1981). A single MSP polypeptide is 126 amino acids, after N-terminal methionine cleavage, and contains an acetylated alanine residue at the first position. The role of acetylation is not understood. In C. elegans, approximately 28 genes encode MSP isoforms that are nearly identical, differing at most by four amino acids (Burke and Ward, 1983). These variants are the result of massive gene duplication events that occurred independently during several lineages of Caenorhabditids (Ward et al., 1988; Miller et al., 2004). The C. elegans genome contains three large clusters of MSP loci, one on chromosome II and two on chromosome IV. These regions also contain large numbers of other genes with reproductive functions, including a disproportionate frequency of genes involved in MSP signal transduction (Miller et al., 2003; Miller et al., 2004). The aggregated pattern of reproductive genes is consistent with a selective process. A major disadvantage of the MSP gene expansion is that it has limited functional studies using genetic methods.
Studies by several labs spanning decades demonstrated that MSPs function as cytoskeletal proteins that mediate sperm locomotion (reviewed in Bottino et al., 2002; Smith, 2006). Nematode sperm crawl over short distances using a pseudopod. Actin and myosin are undetectable in mature spermatozoa, indicating that motility occurs without these structural proteins (Nelson et al., 1982; Sepsenwol et al., 1989). MSPs comprise about 85% of the total protein in the pseudopod, an extraordinary enrichment mediated by a specialized trafficking process during spermiogenesis (reviewed in L’Hernault, 1997). X-ray crystallography indicates that MSP folds into an immunoglobulin-like seven-stranded β sandwich, termed the MSP domain, which is structurally related to the N-terminal domain of the bacterial chaperonin, PapD (Bullock et al., 1996; Baker et al., 2002). MSP forms a filamentous network in the pseudopod built from MSP dimers and helical subfilaments. The assembly of MSP filaments at the pseudopod’s leading edge and disassembly at the trailing edge drives locomotion.
The discovery that MSPs have a second function as a hormone was surprising because of the cytoskeletal function and lack of a signal sequence for secretion. Sperm are highly specialized cells without ribosomes (reviewed in L’Hernault, 1997; L’Hernault, 2006). The protein secretion machinery, including the endoplasmic reticulum and golgi system, is either absent or highly derived (an unusual secretory structure called the membranous organelle or MO forms from the golgi during spermatogenesis). Protein hormones cannot be synthesized de novo and must be secreted by an unconventional mechanism, two major mechanistic challenges. The ability of MSPs to form tightly packed filaments is a potential solution to the first challenge. Filament formation may enable sperm to store large quantities of MSP for long-term secretion. It is tempting to speculate that MSP polymerization and secretion have coevolved with the cytoskeletal role. An important study by Kosinski et al. provides support for this idea (Kosinski et al., 2005). MSP secretion occurs by the budding of novel vesicles that have inner and outer membranes with MSP sandwiched between them. These extracellular vesicles lyse, either passively or actively, to generate long-range MSP gradients for signaling. Budding protrusions from the cell body contain MSP, but not the MSD proteins that counteract MSP filament assembly. Based on these and other observations, MSP was proposed to generate the protrusive force for its own vesicular export (Kosinski et al., 2005).
THE MSP SIGNAL TRANSDUCTION MECHANISM
As a first step toward understanding MSP signal transduction, an in situ binding assay was developed to visualize MSP receptors (Miller et al., 2003). In this assay, dissected gonads were incubated with purified MSP conjugated to fluorescein (MSP-FITC) and washed. MSP-FITC is biologically active, indicating that fluorescein conjugation does not interfere with signaling. MSP-FITC binds extensively to the plasma membranes of oocytes and sheath cells in the proximal gonad. Binding to oocytes saturates at concentrations greater than 50 nM and is out-competed with an excess of unlabeled MSP. Furthermore, the oocyte secretory pathway is necessary for binding. Binding to spermatozoa, male gonads, oocyte precursors, unfertilized oocytes, and spermathecal and uterine walls is not detectable. Whether MSP-FITC binds at low levels to any of these cell types is not clear. These results demonstrate that MSP binds to cell surface receptors expressed in oocytes and surrounding sheath cells (Miller et al., 2003) (Figures 1 and 2).
Figure 2. Working model of the MSP signal transduction mechanism that controls oocyte maturation rates.
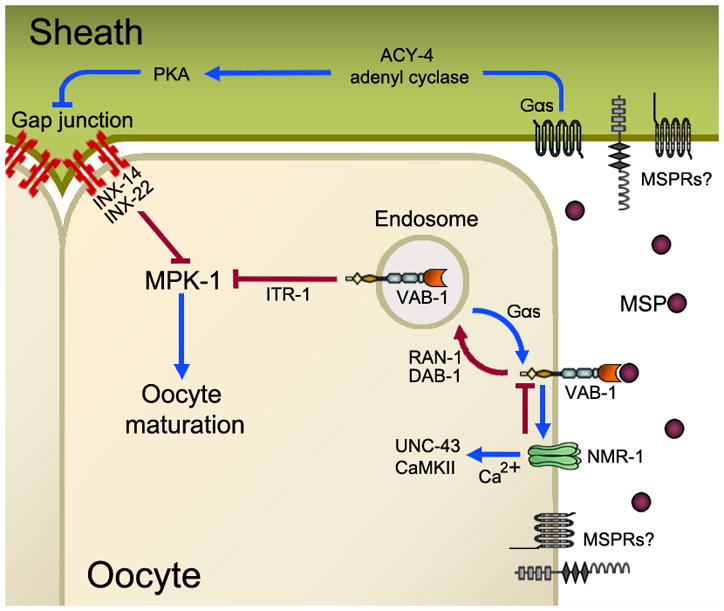
MSP binds to multiple receptors (MSPRs) expressed on oocyte and sheath cell surfaces. The only identified MSP receptor to date is the VAB-1 Eph receptor. MSP binding to MSPRs stimulates a sheath Gαs-adenylate cyclase cascade that antagonizes sheath/oocyte gap junctional signaling. Downstream of these gap junctions in oocytes is the MPK-1 MAP Kinase. VAB-1 and possibly other MSPRs in oocytes regulate the activity of an NMDA-subtype glutamate receptor comprised of the NMR-1 subunit. Ca2+ influx through this glutamate receptor is thought to regulate UNC-43 CaMKII activity. UNC-43 functions redundantly to promote oocyte maturation, but the mechanism is not well understood. VAB-1 trafficking from the cell surface to recycling endosomes is important for regulating maturation rates. The inositol triphosphate receptor ITR-1 may act downstream of VAB-1 to inhibit MPK-1 activation. Red lines indicate inhibitory pathways, while blue lines indicate stimulatory pathways. See text for further details.
Govindan et al. identified a sheath cell Gαs-adenylate cyclase cascade that is critical for oocyte maturation (Govindan et al., 2006; Govindan et al., 2009). gsa-1 encodes a Gαs heterotrimeric G protein subunit that functions upstream of acy-4 adenylate cyclase and kin-2, the regulatory subunit of protein kinase A (Govindan et al., 2009). Genetic mosaic analysis indicates that gsa-1 activity in sheath cells is required for MSP to promote oocyte maturation. Gαs-adenylate cyclase signaling antagonizes a mechanism dependent on sheath/oocyte gap junctions (Figure 2). The innexins INX-14 and INX-22 localize to plaque-like structures at the sheath/oocyte interface, where they are thought to form gap junction channels (Govindan et al., 2006; Whitten and Miller, 2007; Govindan et al., 2009). INX-14 and INX-22 negatively regulate MAPK MPK-1, but the molecular mechanism is not well understood. MPK-1 activity is primarily regulated by phosphorylation (Lee et al., 2007). Delays in oocyte maturation are observed in the temperature-sensitive mpk-1(ga111) mutant, with the delayed oocytes resembling diakinesis-arrested oocytes in unmated females (Lee et al., 2007). These data support a critical role for oocyte MPK-1 phosphorylation in MSP-induced oocyte maturation. The emerging picture is that signal transduction events in sheath cells and oocytes are critical for oocyte maturation. MSP-FITC binding sites are enriched at plaque-like structures at the sheath/oocyte interface, raising the possibility that MSP receptors neighbor gap junctions (Govindan et al., 2009). The simplest interpretation of the current data is that MSP stimulates sheath Gαs signaling to induce oocyte maturation. Whether MSP directly binds to an unidentified G protein-coupled receptor that stimulates Gαs or binds to another receptor class that indirectly stimulates Gαs is not clear.
The MSP-FITC binding assay was used in conjunction with RNAi to screen for receptor candidates required for binding to gonads. This screen identified the VAB-1 Eph receptor tyrosine kinase (EphR) as an MSP receptor (Miller et al., 2003). Multiple biochemical experiments have demonstrated that VAB-1 is a bona fide MSP receptor, although co-receptors or cofactors may influence binding affinity (Miller et al., 2003; Govindan et al., 2006; Tsuda et al., 2008). Both binding and genetic data indicate that there are additional MSP receptors expressed in the proximal gonad (Miller et al., 2003). A continuation of the MSP receptor screen has identified several additional receptor candidates (S. M. Han and M. A. Miller, unpublished results). The current model is that MSP binds to multiple receptors expressed on oocyte and sheath surfaces to promote sheath contraction, oocyte maturation, oocyte growth, microtubule rearrangement, and large ribonucleoprotein foci disassembly. MSP receptors may have overlapping, partially redundant functions or largely separate functions. Multiple MSP receptors might enable tight control of ovulation rates, ensuring that oocytes are not wasted when sperm numbers fluctuate. This scenario may be particularly important in gonochoristic species.
Multiple immunocytochemical and transgenic experiments have shown that VAB-1 expression in oocytes mirrors MSP-FITC binding sites (Miller et al., 2003; Corrigan et al., 2005; Cheng et al., 2008; Brisbin et al., 2009). The evidence for sheath expression is not as strong because sheath cells are thin and closely apposed to oocyte membranes (Miller et al., 2003). Genetic studies have shown that VAB-1 has multiple roles in regulating oocyte maturation and MPK-1 activation (Miller et al., 2003). vab-1 null mutants have high oocyte maturation rates in the presence of MSP, indicating that VAB-1 is not required for oocyte maturation. VAB-1 is required to inhibit MPK-1 activation in developing oocytes and to down-regulate oocyte maturation rates when hermaphrodites run out of sperm. These functions require the ephrin EFN-2 and PTEN homolog DAF-18, which binds the VAB-1 intracellular region (Miller et al., 2003; Brisbin et al., 2009). In unmated females, VAB-1 acts in parallel to gap junctions to inhibit MPK-1 activation and oocyte maturation (Miller et al., 2003), functions that appear to be largely independent of ephrins (Cheng et al., 2008). Taken together, the data support the model that MSP binding to VAB-1 antagonizes ephrin-dependent and ephrin-independent inhibitory mechanisms. Biochemical studies with mammalian proteins support the hypothesis that MSP domains compete with ephrins for EphR binding (Tsuda et al., 2008).
In a recent study, Cheng et al. used a transgenic approach to investigate VAB-1 trafficking in oocytes (Cheng et al., 2008). In the absence of MSP, VAB-1::GFP localizes to an endocytic-recycling compartment, suggesting that VAB-1 inhibits oocyte maturation while in endosomes. Endosomal localization is independent of ephrins, but is antagonized by MSP. VAB-1::GFP trafficking to recycling endosomes requires the cytoplasmic adaptor protein Disabled DAB-1 and GTPase RAN-1, both of which interact with the VAB-1 intracellular domain (Figure 2). Inactivation of endosomal recycling regulators causes a VAB-1-dependent reduction in the oocyte maturation rate. These results support the model that MSP promotes trafficking of VAB-1 and possibly other MSP receptors to the cell surface. GSA-1 and sheath/oocyte gap junctions are critical for VAB-1::GFP trafficking (Figure 2). gsa-1 RNAi causes VAB-1::GFP to accumulate in endosomes in the presence of MSP, resembling the pattern seen in unmated females. In contrast, loss of gap junctions in unmated females causes VAB-1::GFP to localize to the cell surface, resembling the pattern seen in hermaphrodites. Thus, cross talk between gap junction and VAB-1 pathways is important for regulating oocyte maturation rates.
There are at least two possible models that can explain the current data. In the first, MSP antagonizes VAB-1 signaling to promote oocyte maturation. VAB-1 does not regulate sheath Gαs-adenylate cyclase signaling; instead, it modulates oocyte maturation rates through a parallel mechanism. In the second model, MSP antagonizes VAB-1-dependent inhibition in oocytes and acts redundantly with another MSP receptor(s) to stimulate Gαs-adenylate cyclase signaling. This model predicts that VAB-1 has negative and positive roles in regulating oocyte maturation. Indirect support for the second model comes from studies in mammals and C. elegans. Mammalian Eph receptors and ephrins regulate bidirectional signaling networks that involve cascades with opposite effects (reviewed in Palmer and Klein, 2003; Arvanitis and Davy, 2008; Pasquale, 2008). These networks can include cross talk with gap junctions, G protein-coupled receptors, and NMDA-subtype glutamate receptors. In C. elegans, the NMR-1 NMDA receptor subunit functions in oocytes to negatively regulate oocyte maturation (Corrigan et al., 2005). NMR-1 loss causes a substantial increase in the oocyte maturation rate of unmated females, an increase that is largely dependent on VAB-1. The simplest interpretation of these data is that NMR-1 down-regulates VAB-1, which induces oocyte maturation when activated in excess. Genetic evidence suggests that VAB-1 and NMR-1 act in a complex, as demonstrated biochemically for mammalian EphRs and NMDA receptors (Dalva et al., 2000). MSP, VAB-1, and NMR-1 control activity of the Ca2+/calmodulin-dependent protein kinase II (CaMKII) UNC-43, which functions redundantly to promote oocyte maturation (Corrigan et al., 2005) (Figure 2). Whether UNC-43 functions upstream or downstream of sheath Gαs-adenylate cyclase signaling is not known. These data highlight the complexity of VAB-1’s role in regulating oocyte maturation rates.
The abundant expression of MSP receptors on the oocyte surface suggests that they play a key role in regulating oocyte maturation rates. There is accumulating evidence that numerous proteins function in oocytes to control oocyte maturation. These proteins include cell surface receptors, such as VAB-1 and NMR-1, as well as downstream effectors, such as UNC-43, the inositol triphosphate receptor ITR-1, the 14-3-3 homolog PAR-5, and the Rho/Rac-family guanine nucleotide exchange factor (GEF) VAV-1 (Miller et al., 2003; Corrigan et al., 2005; Govindan et al., 2006). Currently, the mechanism(s) by which MSP receptors couple to sheath Gαs-adenylate cyclase signaling is not understood. One possibility is that sheath MSP receptors stimulate Gαs signaling and oocyte receptors modulate this response. Another possibility is that oocyte receptors stimulate sheath Gαs activity indirectly by an unknown mechanism. Nevertheless, sheath Gαs activation appears to occur early in the MSP signaling response. Within 15 minutes after extracellular MSP addition, the MLC-4 regulatory light chain of non-muscle myosin becomes phosphorylated throughout the gonad (Nadarajan et al., 2009). Phosphorylation is dependent on GSA-1 (Govindan et al., 2009). MPK-1 activation and oocyte maturation occur approximately 10–25 minutes later. The delays suggest that the transduction mechanism involves multiple time-consuming steps, possibly including post-transcriptional regulation. The zinc finger domain-containing proteins OMA-1 and OMA-2 are redundantly required for oocyte maturation (Detwiler et al., 2001). Although the biochemical function of these proteins is not clear, they may act as translational regulators. In summary, MSP binding to sheath and oocyte receptors regulates a complex communication network between these cells that controls oocyte maturation rates (Figure 2).
MSP stimulates an increase in the basal sheath contraction rate, a response that occurs within minutes after MSP injection (Miller et al., 2001). Oocytes are not required, indicating that sheath MSP receptors are sufficient to promote contraction (McCarter et al., 1999; Corrigan et al., 2005). Sheath contraction is an actomyosin-dependent process that does not involve input from the nervous system. A signal transduction cascade comprising the heterotrimeric G protein subunit Gαq EGL-30, phospholipase Cγ PLC-3, and inositol triphosphate receptor ITR-1 is critical for sheath contraction (Yin et al., 2004; Corrigan et al., 2005; Govindan et al., 2009). Since ITR-1 is an intracellular Ca2+ channel (Baylis et al., 1999), it seems likely that cytosolic Ca2+ oscillations control contractile activity. Cell surface Ca2+ channels may play a modulatory role, as the glutamate receptor subunit NMR-1 acts upstream of CaMKII UNC-43 in sheath cells to inhibit MSP-induced basal contraction (Corrigan et al., 2005). Glutamate receptors are cell surface Ca2+ channels that regulate CaMKII activity at mammalian synapses (Merrill et al., 2005). In addition, sheath/oocyte gap junctions modulate sheath contraction rates (Whitten and Miller, 2007). The role of VAB-1 in sheath contraction is controversial. Three studies have reported lower basal sheath contraction rates in vab-1 mutant and RNAi hermaphrodites (Miller et al., 2003; Yin et al., 2004; Corrigan et al., 2005), while another study found that contractions were normal in vab-1 null mutants, except under certain conditions (Govindan et al., 2009). Mammalian EphR signaling promotes recruitment of the proto-oncogene VAV2 to the EphR intracellular domain and stimulates VAV2 GEF activity (Cowan et al., 2005). In C. elegans, VAV-1 is required for basal sheath contraction, but there are no reported links between the VAB-1 EphR and VAV-1 (Norman et al., 2005). The current working model is that MSP regulates sheath cell Ca2+ dynamics to control basal contraction rates.
MSP DOMAINS AND HUMAN MOTOR NEURON DEGENERATION
The human genome contains five predicted genes that encode proteins with an MSP domain, including two VAMP/synaptobrevin-associated proteins (VAPs) called VAPA and VAPB (Weir et al., 1998; Nishimura et al., 1999) (Figure 3). VAPC is a splicing variant of VAPB consisting of the N-terminal half of the MSP domain. VAPs have been investigated the most and are implicated in several human diseases, but their functions are still poorly understood (Table 1). In 2004, Nishimura et al. discovered an autosomal dominant mutation (P56S) in the VAPB MSP domain is associated with amyotrophic lateral sclerosis (ALS) and late-onset spinal muscular atrophy (SMA) (Nishimura et al., 2004). ALS, also known as Lou Gehrig’s disease, and SMA are progressive muscle disorders caused by motor neuron degeneration (reviewed in Cleveland and Rothstein, 2001; Wang and Lunn, 2008). The clinical features of ALS pathogenesis are progressive muscle weakness, atrophy, and spasticity, which re ect the degeneration of upper and lower motor neurons in the cortex, brainstem, and spinal cord (reviewed in Rowland, 1998). Respiratory muscle failure is most often the fatal event. The causes and mechanisms responsible for ALS pathogenesis are hotly debated and most ALS cases occur sporadically without a known genetic basis. Multiple mechanisms have been proposed to explain motor neuron injury, including altered mitochondrial function, glutamate excitotoxicity, altered calcium homeostasis, altered proteosomal function, impaired axoplasmic transport, and neuroinflammation (reviewed in Boillee et al., 2006; Pasinelli and Brown, 2006).
Figure 3. Domain organization of selected MSP domain-containing proteins in C. elegans and humans.
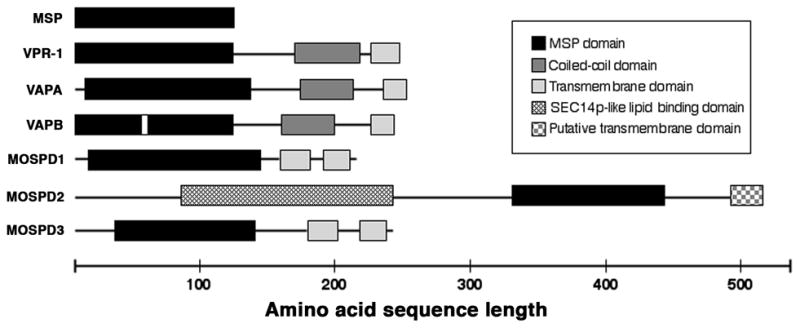
The C. elegans genome encodes over sixty predicted proteins with an MSP domain, including approximately 28 MSPs and the VAP homolog VPR-1. The human genome encodes five predicted proteins with an MSP domain, including two VAP homologs. A P56S mutation (white box) in human VAPB MSP domain is associated with ALS and late-onset SMA. Recent evidence supports the model that animal VAP MSP domains are cleaved from the transmembrane regions and secreted (Tsuda et al., 2008). MSPs share approximately 20–25% identity with VAP MSP domains. MSP domains from human MOSPD proteins share limited sequence identity with MSPs and VAP MSP domains (<25%), but the presence of key highly conserved residues are consistent with inclusion in the MSP family (Marchler-Bauer et al., 2009). VAP homologs are found in fungi, plants, and animals. MSPs are highly conserved in diverse nematodes (>60% identity), but have not been identified outside of nematodes. Putative MOSPD2 homologs have been identified in mammals, chickens, zebrafish, Drosophila, and C. elegans (B0336.11).
Table 1.
Selected human disease associations of VAPs, Eph receptors, and prostaglandins.
| Factor | Human Disease Associations | Selected References |
|---|---|---|
| VAPA | Niemann-pick type C disease, Bipolar disorder, Hepatitis C virus propagation | Tu et al., 1999; Lohoff et al., 2008; Rocha et al., 2009 |
| VAPB | Amyotrophic lateral sclerosis, Spinal muscular atrophy, Hepatitis C virus propagation | Nishimura et al., 2004; Kang et al., 2009 |
| VAPCa | Hepatitis C virus propagation | Kukihara et al., 2009 |
| EphRs | Craniofrontonasal syndrome, Alzheimer’s disease, Cancer | Reviewed in Pasquale et al., 2008; Simon et al., 2009 |
| PGD2 | Asthma, Allergic rhinitis, Alzheimer’s disease, Duchenne muscular dystrophy | Hardy et al., 1984; Arimura et al., 2001; Reviewed in Pettipher, 2008; Mohri et al., 2007; Mohri et al., 2009 |
| PGE2 | Colorectal cancer, Endometriosis, Infertility, Rheumatoid arthritis | Sheng et al., 2001; McCoy et al., 2002; Takahashi et al., 2006; Banu et al., 2008 |
| PGF2α | Labour-associated disorders, Atherogenesis | Fischer et al., 2008; Yu et al., 2009 |
| PGI2 | Cardiovascular disease, Rheumatoid arthritis | Honda et al., 2006; Arehart et al. 2008 |
VAPC is a splicing variant of VAPB consisting of the N-terminal half of the MSP domain.
Abbreviations: VAP, VAMP/synaptobrevin-associated protein; EphR, Eph receptor; PG, prostaglandin; TBX, thromboxane.
VAPB contains an N-terminal MSP domain, a coiled-coil motif, and a C-terminal transmembrane-spanning region (Figure 3). The P56S mutation occurs in a conserved linker region between two beta strands in the immunoglobulin-like fold. VAPB expression is reduced in sporadic ALS patients and a mouse model, suggesting that it plays a widespread role in pathogenesis (Anagnostou et al., 2008). VAP homologs have been identified in yeast, C. elegans, Drosophila, Aplysia, and mammals. These proteins are broadly expressed in animal tissues and localize to the endoplasmic reticulum, golgi, and plasma membrane, depending on the cell type (Kagiwada et al., 1998; Lapierre et al., 1999; Soussan et al., 1999; Skehel et al., 2000; Tsuda et al., 2008). Studies in yeast and cultured mammalian cells have implicated VAPs in lipid metabolism and transport, vesicle trafficking, targeting FFAT-containing (two phenylalanines in an acidic tract) proteins to intracellular organelles, maintaining organelle structural integrity, and the unfolded protein response (Wyles et al., 2002; Lev et al., 2008; Prosser et al., 2008). While the precise role of VAPs is not understood, these studies suggest that VAPs have complex intracellular functions.
Most studies have not considered the possibility that VAPs have an extracellular signaling function. Initial evidence for a signaling function came from Drosophila. Fly dVAP regulates the number and size of neuromuscular junction boutons in a dosage-dependent manner and influences the microtubule architecture (Pennetta et al., 2002). The role of dVAP in microtubule reorganization is interesting because MSP regulates the oocyte microtubule cytoskeleton. Tsuda et al. showed that dVAP localizes to the neuronal cell body, but not to the neuromuscular junction in wild-type flies, suggesting that dVAP functions at a distance (Tsuda et al., 2008). In wing imaginal discs, dVAP is found throughout the cytoplasm, as well as in the extracellular space. Fly extracts contain full-length dVAP and multiple cleavage products between 13–17 kDa, roughly the size of the MSP domain. Similar size VAP fragments are observed in C. elegans and mammalian cells (Gkogkas et al., 2008; Tsuda et al., 2008). These results, together with studies of MSP, led to the hypothesis that the VAP MSP domain is cleaved from the transmembrane domain and secreted. This hypothesis was confirmed using wing disc cells and Drosophila S2 cultured cells. Moreover, human blood serum contains a VAPB fragment of ~17–18 kDa that is likely to contain the MSP domain (Tsuda et al., 2008). The data support the model that VAP MSP domains are secreted by an unconventional mechanism, similar to C. elegans MSPs.
The hypothesis that VAP MSP domains have an extracellular signaling function was tested in C. elegans gonads (Tsuda et al., 2008). Microinjected MSP domains from the C. elegans VAP homolog VPR-1, Drosophila dVAP, and human VAPB can induce oocyte maturation and sheath contraction in unmated female gonads, despite low sequence similarity to MSPs (~20–25% identity). The VPR-1 MSP domain is active over the same low nM concentration range as MSP. Furthermore, fluorescein-labeled VAP MSP domains specifically bind to oocyte and sheath cell surface receptors. MSP-FITC binding can be out-competed with an excess of human VAPB MSP domain, indicating that both proteins bind to common receptors (Tsuda et al., 2008). While these experiments are compelling, it will be important to test VAP MSP domains for signaling activity in other paradigms. The ability to produce functional MSP domains in bacteria should make tests in cultured cells straightforward.
The discovery that MSP binds to the VAB-1 EphR paved the way for identifying the first VAP receptor. Pull-down experiments demonstrate that VPR-1 MSP domains bind to VAB-1 EphR ectodomains and human VAPB MSP domains bind to mouse EphA4 receptor ectodomains (Tsuda et al., 2008). Furthermore, VAPB MSP and mouse ephrinB2 compete with each other for EphA4 binding, consistent with an antagonistic relationship. The possibility that MSP domain and EphR interactions require a cofactor or co-receptor for high affinity binding can be eliminated with the present data. Genetic analyses in C. elegans and Drosophila demonstrate that VAPs and EphRs function in common processes in vivo (Tsuda et al., 2008). For example, neuronal dVAP overexpression causes muscle myofibril defects that are suppressed by EphR mutations. The emerging picture is that MSP domains are evolutionarily conserved ligands for EphRs and other receptors.
There is increasing evidence that ALS is a systemic disorder. ALS patients can present with dyslipidemia, insulin resistance, hypermetabolism, and mitochondrial abnormalities in cells inside and outside of the central nervous system (Dupuis et al., 2004a; Dupuis et al., 2004b; Boillee et al., 2006; Dupuis et al., 2008; Dupuis et al., 2009). However, the role of VAPB signaling in ALS pathogenesis is uncertain. Genetic and molecular evidence from multiple labs suggest that P56S is a dominant negative mutation (Teuling et al., 2007; Ratnaparkhi et al., 2008; Tsuda et al., 2008). In fly cells, P56S causes a failure to secrete the MSP domain, the accumulation of wild-type and mutant VAP in cytoplasmic inclusions, and an unfolded protein response (Tsuda et al., 2008). EphRs comprise the largest subfamily of receptor tyrosine kinases in mammals and have been implicated in angiogenesis, morphogenesis, neuron migration, and motor function (Poliakov et al., 2004; Kuijper et al., 2007; Pasquale, 2008). Abnormal EphR signaling is associated with several human diseases (Table 1), although no studies to date have linked human EphRs to ALS. However, multiple ephrins and EphRs, including EphA4 and A7, are expressed throughout the adult nervous system and in skeletal muscle (Lai et al., 2001). EphRs influence synaptic glutamate receptor clustering, which is implicated in ALS pathogenesis (Dalva et al., 2000; Takasu et al., 2002; Boillee et al., 2006). It is, therefore, possible that abnormal VAPB signaling through EphRs influences ALS pathogenesis. Regardless, the discovery that VAPs are EphR ligands could provide insight into diverse processes and human diseases.
THE EVOLUTION OF MSP DOMAIN FUNCTIONS
The MSP domain is an ancient structure found in a surprising array of fungi, plant, and animal proteins (reviewed in Tarr and Scott, 2005) (Figure 3). MSP genes are a characteristic feature of nematode genomes, but they have not been identified outside of nematodes. The VAPs appear to be the ancestral prototype from which MSP domain-containing proteins derive because VAPs are encoded in yeast, plant, nematode, arthropod, and vertebrate genomes. The most likely historical scenario is that nematode MSPs originated from a duplicated vap gene early in nematode evolution. The coiled-coil motif and transmembrane-spanning region were lost and sperm-specific expression was acquired. Therefore, VAPs are critical to understanding the evolution of MSP domain functions.
Studies of VAPs in yeast, plants, and mammals point to a role in intracellular lipid metabolism and transport, raising the possibility that this function is ancestral (Kagiwada et al., 1998; Wyles et al., 2002; Loewen and Levine, 2005; Lev et al., 2008). However, there is still considerable uncertainty regarding the mechanism and its biological role. It seems unlikely that plant and yeast VAPs have a hormonal function given their lack of EphRs, though this hypothesis has not been directly tested. Evidence is accumulating that nematode, arthropod, and mammalian VAPs share an extracellular signaling activity with MSPs (Tsuda et al., 2008). EphRs have been found in the earliest metazoans, indicating that VAPs and EphRs have been together for several hundred million years (Suga et al., 2001). The most parsimonious scenario based on the current data is that VAP MSP domains acquired their hormonal function before nematodes split from the last common ancestor of nematodes, arthropods, and mammals. MSPs would have acquired their cytoskeletal function after the nematode split. Since sperm cannot synthesize proteins de novo, the acquisition of filament forming ability might allow MSP to be stored and secreted over time. The evolution of MSP domain functions has important implications for human development and disease. If animal VAPs have an ancient hormonal function, then MSP domain-containing proteins could comprise a large new family of ligands, similar to the Wnt and TGF-beta families. It will be of considerable interest to determine whether the intracellular and extracellular functions are mechanistically linked and how these seemingly disparate functions are regulated.
OOCYTE TO SPERM COMMUNICATION
Successful fertilization and embryonic development require that sperm locate and fuse with the oocyte. Research in marine species has provided considerable insight into oocyte to sperm signaling. In these organisms, oocytes often secrete sperm chemoattractants that diffuse into the surrounding aquatic environment. The most well studied models are echinoderms, which incorporate sperm-activating peptides into the egg jelly layer (reviewed in Darszon et al., 2008). In the sea urchin A. punctulata, egg jelly contains a 14 amino acid peptide called resact that functions as a sperm attractant (Ward et al., 1985). Resact binds to guanylyl cyclase receptors on the flagellum and stimulates production of 3′, 5′ cyclic guanine monophosphate (cGMP) (Ward et al., 1986; Kaupp et al., 2003). cGMP then binds to K+ selective cyclic nucleotide channels, causing a brief hyperpolarization of the cell membrane that, in turn, activates voltage-dependant Ca2+ channels (Strunker et al., 2006). Increased Ca2+ levels regulate rhythmic beating of the flagella and change the trajectory of sperm toward increasing resact concentrations (Brokaw et al., 1974; Brokaw, 1979; Kaupp et al., 2003). In the sea urchin Strongylocentrotus purpuratus, a different peptide called speract has been identified as a sperm chemoattractant (Hansbrough and Garbers, 1981). Speract is synthesized by the follicle cells, not the oocytes and is incorporated into egg jelly (Darszon et al., 2008). In other echinoderms such as starfish, sperm-activating peptides are secreted by oocytes (Matsumoto et al., 1999). A mixture of peptides called asterosaps from the starfish Asterias amurensis appear to function as sperm chemoattractants (Nishigaki et al., 1996; Bohmer et al., 2005). As with resact, asterosaps modulate cGMP and intracellular calcium levels to regulate sperm motility (Matsumoto et al., 2003).
Outside of echinoderms, sperm chemoattractants from several species have been identified. Scleractinian coral oocytes secrete unsaturated fatty alcohols, red abalone oocytes secrete the L-isomer of tryptophan, and ascidian oocytes secrete a novel sulfated steroid (Coll et al., 1994; Riffell et al., 2002; Yoshida et al., 2002). The egg jelly of the African clawed frog Xenopus laevis contains a 21-kDa protein called allurin that is a sperm chemoattractant (Olson et al., 2001). Allurin is a member of the cysteine-rich secretory protein family found in diverse metazoans. Like speract, allurin is secreted by the accessory cells that surround oocytes (Xiang et al., 2004). These studies illustrate that marine species use a wide array of molecules, secreted by oocytes or the surrounding gonadal cells, to regulate sperm motility. With the possible exception of allurin, the factors identified to date are not members of evolutionarily conserved families with homologous functions. In aquatic environments, there may be strong selective pressure to use novel factors that do not attract sperm from other species.
The molecular processes that guide sperm to maturing oocytes inside the female reproductive tract are largely unknown. Evidence is accumulating that mammalian oocytes and surrounding cumulus cells secrete sperm chemoattractants (reviewed in Eisenbach and Giojalas, 2006; Oren-Benaroya et al., 2008). Follicular fluid has been identified as one source of sperm chemoattractants in humans, mice, and rabbits (Ralt et al., 1991; Oliveira et al., 1999; Fabro et al., 2002). Sun et al. investigated the role of the oocyte-cumulus complex in sperm chemoattraction using media conditioned with either mature oocytes or cumulus cells (Sun et al., 2005). Their results showed both cells are sources of sperm chemoattractants. Similarly, Guidobaldi et al. demonstrate cumulus cells of rabbits secrete progesterone to attract sperm (Guidobaldi et al., 2008). The signal transduction events occurring in sperm following chemoattractant binding are poorly understood, but may involve the human OR-17-4 olfactory receptor. OR-17-4 localizes to the sperm flagellum and has been shown to activate an adenylyl cyclase cascade that affects sperm trajectory (Spehr et al., 2003). The endogenous ligand for OR-17-4 is not known. The major limitation with mammalian studies is that sperm guidance is analyzed in vitro. What happens inside the fallopian tube is a mystery. Sperm guidance in Drosophila is not well understood either. Two studies showed that the nonselective cation channel Pkd2, which is expressed in the sperm tail, is required for fertility (Gao et al., 2003; Watnick et al., 2003). The mutant sperm are motile, but fail to swim into the female storage organs after mating. How sperm find this organ and what signals, if any, regulate Pkd2 activity are not clear.
C. elegans is emerging as an attractive system to explore the mechanisms that guide motile sperm to the fertilization site (Kubagawa et al., 2006). The epidermis is transparent, allowing sperm guidance to be observed in live animals. In young adults, nonmotile spermatids accumulate in the proximal gonad while oogenesis is occurring (Hirsh et al., 1976). During the first ovulation, the maturing oocyte pushes the spermatids into the spermatheca, where they rapidly become motile spermatozoa (Ward and Carrel, 1979; Ward et al., 1983). Sperm displaced into the uterus as the fertilized egg leaves the spermatheca must crawl back into the spermatheca to fertilize the next maturing oocyte (reviewed in Singson, 2001; L’Hernault, 2006). Nearly all self-derived sperm fertilize an oocyte, suggesting that the ability of sperm to locate the spermatheca is highly efficient (Ward and Carrel, 1979; Kadandale and Singson, 2004). During mating, spermatids are injected through the vulva, become activated, and crawl across the uterine walls and developing embryos to the spermatheca, where they displace hermaphrodite sperm (Ward and Carrel, 1979; Ward et al., 1983; L’Hernault, 2006) (Figure 1). Ward and Carrel were the first to visualize sperm motility in the uterus, but were unable to determine whether sperm motility was directed or random (Ward and Carrel, 1979). Monitoring the motility of a single sperm among the large numbers inseminated is difficult using conventional microscopy. However, there has long been speculation that the spermatheca is the source of a sperm attractant.
Fluorescent dyes have been used to stain male sperm, enabling easy visualization in the hermaphrodite reproductive tract (Ward and Carrel, 1979; Hill and L’Hernault, 2001; Kosinski et al., 2005; Kubagawa et al., 2006). A method was developed using MitoTracker CMXRos (MT) to track male sperm within the uterus and measure their migration parameters (Kubagawa et al., 2006; Whitten and Miller, 2007) (Figure 4A and B). When MT-labeled males are mated to non-labeled hermaphrodites, fluorescent sperm accumulate in the spermatheca over time. From time-lapse videos, migration velocity, directional velocity toward the spermatheca, and reversal frequency are measured. MT does not appear to interfere with sperm function and MT sperm migrate at velocities similar to non-labeled sperm. The following events have been observed from live imaging and time-lapse videos. During copulation, sperm are deposited throughout the uterus with most positioned near the vulva (Figure 1). Sperm deposited at the vulva can migrate toward the anterior or posterior spermatheca. Once a commitment is made, sperm move through the uterus rarely reversing their direction. The average directional velocity is less than the average velocity, primarily due to migration around fertilized eggs. One hour after mating, ninety-three percent of sperm reach the spermatheca, where a bottleneck occurs while sperm search for the spermatheca-uterine valve (Figure 4A and B). Sperm reverse their direction often in this bottleneck, but remain in a closely packed mass as each sperm passes through the valve. These observations indicated that sperm possess an efficient mechanism for targeting the spermatheca.
Figure 4. Sperm guidance in C. elegans.
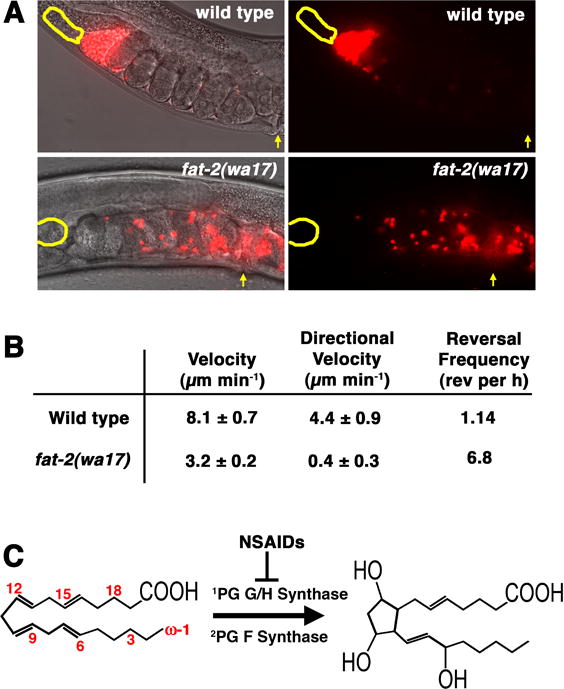
(A) Sperm guidance is monitored in vivo using a novel tracking assay. When mitotracker-labeled males are mated to non-labeled hermaphrodites, sperm (red) accumulate at the spermatheca (yellow outline) over time. One hour after mating, over 93% of sperm have migrated from the vulva (yellow arrow) to the spermatheca. In some anesthetized hermaphrodites, sperm do not enter the spermatheca because the spermatheca-uterine valve is blocked. When wild-type males are mated to PUFA-deficient fat-2(wa17) mutant hermaphrodites, sperm fail to accumulate at the spermatheca efficiently (Kubagawa et al., 2006). (B) Sperm motility values are measured from time-lapse videos taken shortly after mating. Directional velocity toward the spermatheca is measured by creating a straight line through the uterus from the vulva to the spermatheca. A reversal is defined as occurring when the angle generated from a sperm trace during three consecutive 30s frames is less than 90 degrees. Motility values are from wild-type sperm (Kubagawa et al., 2006). (C) In mammals, the omega-6 PUFA arachidonic acid can be converted into prostaglandin F2α by prostaglandin G/H synthase and prostaglandin F synthase. Numbers indicate carbon positions from the omega (ω) end. PG, prostaglandin.
Within the spermatheca, sperm are physically separated from oocytes in the proximal gonad by a tight constriction (Hirsh et al., 1976). Observation of sperm behavior within the spermatheca suggests that sperm migrate toward oocytes (Kubagawa et al., 2006). For instance, sperm density is greatest at the distal end of the spermatheca and sperm appear to continuously compete for position nearest the constriction. In transmission electron micrographs, pseudopods of sperm closest to the constriction often point toward it (Ward and Carrel, 1979; Kosinski et al., 2005). The two most plausible models are that either oocytes or distal spermathecal cells secrete a sperm attractant(s). Evidence for the former hypothesis comes from analysis of mutant hermaphrodites defective in oocyte production (Kubagawa et al., 2006). In these mutants, wild-type sperm fail to target the spermatheca efficiently and migrate in the uterus with reduced velocity, no directional velocity, and high reversal frequency. Therefore, it seems likely that oocytes secrete a factor(s) that stimulates sperm velocity and directional velocity. However, the possibility that the spermatheca plays a role in the metabolism or transport of this factor cannot be excluded.
PUFAS AS SIGNAL PRECURSORS
PUFAs contain at least two double bonds in their carbon backbone (Figure 4C). Desaturase enzymes insert double bonds into the carbon chain, while the elongation system increases the length of the chain (reviewed in Shanklin and Cahoon, 1998; Tocher et al., 1998). In mammals, the 20-carbon PUFAs arachidonic acid and eicosapentaenoic acid are precursors of signaling molecules called eicosanoids, which include the prostaglandins and leukotrienes (Funk, 2001) (Figure 4C). Prostaglandins, in particular, are important for mammalian fertilization and function as chemoattractants for immune cells (Lim et al., 1997; Tamba et al., 2008). C. elegans synthesizes a variety of PUFAs, including arachidonic acid and eicosapentaenoic acid, from monounsaturated and saturated fatty acids provided in their E. coli diet (Watts and Browse, 2002; Watts, 2009). PUFA biosynthetic pathways have been characterized at the genetic and biochemical levels, creating an infrastructure for investigating PUFA functions.
The link between PUFAs and sperm guidance came from the serendipitous discovery that rme-2 mutant hermaphrodites have severe non-autonomous sperm guidance defects (Kubagawa et al., 2006). RME-2 is a low-density lipoprotein receptor homolog specifically expressed in oocytes, where it mediates yolk endocytosis (Grant and Hirsh, 1999). The rme-2 mutant sperm guidance defects led to the hypothesis that yolk contains a sperm attractant precursor. Yolk is a macromolecular complex consisting of lipids and lipid-binding proteins called vitellogenins, which are not secreted (Sharrock et al., 1990). Thus, yolk lipids are the strongest candidates for a sperm attractant precursor. Thin layer and gas chromatography of purified yolk complexes indicates that they contain significant quantities of PUFAs, present as free fatty acids and esterified to phosphatidylcholine and phosphatidylethanolamine (Kubagawa et al., 2006). A much smaller fraction of PUFAs is found in triacylglycerols, the major form used for energy storage. In mammals, PUFAs are liberated from phosphatidylcholine and phosphatidylethanolamine during eicosanoid synthesis. Another low-density lipoprotein receptor-like protein called EGG-1 may be important for sperm guidance, but EGG-1 is not required for yolk uptake and its potential role in sperm guidance is not understood (Kadandale et al., 2005).
A direct requirement for PUFAs in sperm guidance was shown through genetic analysis of fat-2 and fat-3 genes (Kubagawa et al., 2006). fat-2 encodes a 12-desaturase that synthesizes 18- and 20-carbon PUFAs (Watts and Browse, 2002). Wild-type sperm in fat-2 mutants migrate with reduced velocity, little directional velocity, and high reversal frequency (Figure 4). The fat-2 mutant sperm motility values are similar to those values from rme-2 mutants. fat-3 mutants lack 6-desaturase activity and consequently, fail to produce most 20-carbon PUFAs, such as arachidonic acid and eicosapentaenoic acid (Watts and Browse, 2002; Lesa et al., 2003). Like fat-2 mutant hermaphrodites, fat-3 mutants have severe non-autonomous sperm guidance defects (Kubagawa et al., 2006). The sperm guidance defects in fat mutants can be rescued by PUFA supplementation either in the diet or through direct PUFA injection into the gonad. Moreover, injecting PUFA-containing yolk complexes into fat-2 mutant gonads can rescue the sperm targeting defects. Oocytes are the only cell-type that uptakes yolk (Grant and Hirsh, 1999), indicating that oocyte PUFAs are sufficient to rescue the sperm guidance defects of PUFA-deficient fat-2 mutants. Mutant hermaphrodites with sperm guidance defects have brood size reductions, due to loss of self-derived sperm from the uterus during egg laying. Taken together, these data demonstrate that oocyte 20-carbon PUFAs are essential for sperm guidance.
In addition to eicosanoid precursors, PUFAs are components of phospholipids necessary to create a fluid membrane environment. Several lines of evidence support the model that oocyte PUFAs are sperm attractant precursors. First, a 1–2% increase in total membrane PUFAs by exogenous supplementation is sufficient to rescue the sperm guidance defects of PUFA-deficient mutants (Kubagawa et al., 2006). Rescue with such low PUFA concentrations is more consistent with a role in eicosanoid synthesis than in membrane fluidity. Second, multiple enzymes with potential roles in eicosanoid metabolism are required non-autonomously to regulate sperm guidance. These enzymes include cytochrome P450s and predicted prostaglandin synthases expressed in oocytes (Kubagawa et al., 2006). Finally, analysis of bioactive lipid extracts using liquid chromatography tandem mass spectrometry indicates that C. elegans generates a series of novel F-series prostaglandins (J. Edmonds, J. Prasain, and M. Miller, unpublished data). The synthesis of multiple structurally similar F-series prostaglandins is dependent on FAT-3 and RME-2 function. Microinjecting nanomolar concentrations of human F-series prostaglandins stimulates sperm motility as well as wild-type lipid extracts in PUFA-deficient mutants. Therefore, oocyte PUFAs appear to be precursors of novel F-series prostaglandins that regulate sperm guidance.
The following model has been proposed for sperm guidance (Figure 1). FAT-2 and FAT-3 desaturase enzymes convert monounsaturated fatty acids into PUFAs in the intestine, the site of fat metabolism and yolk synthesis. PUFAs are incorporated into yolk complexes in two major forms, esterified to phospholipids and unesterified. Yolk is released into the pseudocoelom and flows to the gonad. The RME-2 LDL receptor mediates yolk endocytosis and PUFA transport, providing oocytes with a source of PUFAs for synthesizing prostaglandins. F-series prostaglandins are secreted into the reproductive tract, where they function to guide sperm to the spermatheca.
The mechanism by which sperm transduce prostaglandin signals to regulate motility is not understood. In mammals, prostaglandins bind to G protein-coupled receptors, the DP2 chemoattractant receptor, and nuclear peroxisome proliferator activated receptors. Sperm are transcriptionally inactive, so it is unlikely that nuclear hormone receptors play a role in guidance. While the C. elegans genome lacks clear homologs of mammalian cell surface prostaglandin receptors, a number of putative G protein-coupled receptors are expressed during spermatogenesis (Reinke et al., 2000; Reinke et al., 2004). Downstream of the receptor(s), prostaglandins are likely to regulate MSP filament assembly in the pseudopod. Sperm migration occurs by extension of the leading edge of the pseudopod, attachment to the substrate, and retraction of the cell body (reviewed in Bottino et al., 2002; Smith, 2006). Assembly of new MSP fibers at the leading edge and disassembly at the trailing edge produce a treadmilling motion that correlates with the migration rate. It is tempting to speculate that prostaglandins regulate MSP secretion, which might be linked to filament dynamics. In any event, the signal transduction mechanisms that control sperm guidance are an important area for future investigation.
PUFAS IN HUMAN FERTILITY AND DISEASE
Mammals lack the desaturase enzymes necessary to convert monounsaturated fatty acids into PUFAs. The 18-carbon PUFAs linoleic and linolenic acids are called essential fatty acids and must be provided in the diet (Burr and Burr, 1929; Burr and Burr, 1930). Plants are important dietary sources because of their ability to synthesize PUFAs (reviewed in Wallis et al., 2002). Mammals are capable of desaturating and elongating linoleic acid to produce arachidonic acid and linolenic acid to produce eicosapentaenoic acid. Omega-6 fatty acids, such as arachidonic acid contain a double bond at the 6th carbon in the chain, whereas omega-3 fatty acids, such as eicosapentaenoic acid contain a double bond at the 3rd carbon (Figure 4C). Fat exclusion from the diet of rats causes growth retardation, reproductive defects, scaly skin, kidney lesions, and excessive water consumption (Burr and Burr, 1929; Burr and Burr, 1930). In humans, dietary intake of omega-3 and omega-6 PUFAs is associated with protection against or treatment of conditions such as rheumatoid arthritis, multiple sclerosis, coronary artery disease, and infertility (reviewed in Zamaria, 2004; Breslow, 2006). Arachidonic and eicosapentaenoic acids are precursors of prostaglandins, thromboxanes, leukotrienes, and other eicosanoids (Figure 4C).
Prostaglandins promote a wide range of cellular effects, including chemotaxis, smooth muscle contraction, proliferation, and platelet aggregation (reviewed in Funk, 2001). The rate-limiting step in mammalian prostaglandin synthesis is the conversion of arachidonic acid into prostaglandin H2 by prostaglandin G/H synthase or cyclooxygenase (Figure 4C). NSAIDs, such as aspirin, ibuprofen, and naproxen specifically block cyclooxygenase activity, thereby inhibiting prostaglandin synthesis (Vane, 1971; Vane et al., 1998). The C. elegans genome lacks prostaglandin G/H synthase homologs, indicating that prostaglandin synthesis initiates by a novel mechanism. Defining this mechanism is an important goal for future investigation.
Prostaglandins are critical for mammalian reproduction. Excess NSAID consumption in women is associated with luteinized unruptured follicle syndrome and infertility that can be reversed by drug withdrawal (Smith et al., 1996). Studies in mammalian models have shown that prostaglandins are important for ovulation, fertilization, implantation, and parturition (Langenbach et al., 1995; Lim et al., 1997; Gross et al., 1998; Lim et al., 1999; Matsumoto et al., 2001; Tamba et al., 2008). The molecular basis for the fertilization defects, in particular, is not well understood and may involve multiple abnormalities. A possibility consistent with current data is that sperm fail to efficiently locate ovulated oocytes in the oviduct. However, monitoring sperm guidance in the mammalian reproductive tract is not currently possible, making this idea difficult to test. in vitro studies suggest that prostaglandins can influence human sperm motility, but it is not clear whether this occurs in vivo (Aitken and Kelly, 1985; Gottlieb et al., 1988). There are many important, yet unanswered questions relating to prostaglandin functions and mechanisms of action during mammalian reproduction. A major limiting factor is the enormous complexity of the reproductive system at the cellular and molecular levels.
In addition to infertility, abnormal prostaglandin signaling is associated with other pathological conditions, including cancer, asthma, cardiovascular disease, and rheumatoid arthritis (reviewed in Funk, 2001; Ulrich et al., 2006; Wang et al., 2007; Harizi et al., 2008; Smyth et al., 2009) (Table 1). Prostaglandin G/H synthase expression is elevated in colorectal, breast, lung, prostate, stomach, pancreas, and urinary bladder cancers. Elevated prostaglandin activity is proposed to increase angiogenesis and proliferation, decrease apoptosis, and promote metastasis. Epidemiologic studies have shown that low doses of aspirin are associated with reduced risk of colon cancer fatality (Thun et al., 1991). Clinical trials investigating the effects of NSAIDs on various cancers are ongoing. A major problem is that NSAIDs inhibit synthesis of a wide range of prostaglandin types, some of which are beneficial. Further research on prostaglandins might identify new drug targets for treating infertility, cancer, and other pathologies.
CONCLUSIONS AND PERSPECTIVES
In C. elegans, sperm and oocyte communicate using two distinct hormone types, protein hormones called MSPs and lipid hormones called prostaglandins (Figure 1). Sperm secrete MSPs to induce oocyte maturation, thereby preparing the oocyte for fertilization and embryogenesis. MSP domains are found in proteins from a wide range of species. While the jury is still out regarding the extent to which these proteins function as extracellular signals, it is likely that the common ancestor of nematodes, arthropods, and mammals had a VAP homolog with signaling activity. The discovery that MSP domains in C. elegans and mammals bind to Eph receptors is provocative. Eph receptors are among the oldest receptors in metazoans and comprise the largest class of receptor tyrosine kinases in humans. To attract sperm to the fertilization site, oocytes convert PUFAs into novel F-series prostaglandins that are released into the reproductive tract. In mammals, prostaglandins are important for fertilization, but the molecular mechanisms are not well understood. Based on the studies summarized here, we suggest that C. elegans sperm and oocyte communication mechanisms derive from ancestral mechanisms present in early-diverging animals. These mechanisms could have been coopted during C. elegans evolution from other developmental circuits or they could have been present in sperm and oocyte from C. elegans ancestors.
The outlook for the future is bright. A young field is often judged by the new questions it poses. Here are several important questions for future studies. What are the remaining MSP receptors? Is the MSP signaling mechanism evolutionarily conserved and if so, what processes does it regulate? What role does the human VAPB MSP domain play in ALS and SMA pathogenesis? In contrast to MSPs, the hormonal function of prostaglandins has been established for over fifty years. Nevertheless, there are many important questions to address. For instance, how do prostaglandins regulate sperm motility? Are prostaglandins conserved sperm guidance factors? Answers to these question and others will require hard work, creative interdisciplinary approaches, and inquisitive minds. The answers will bring forth new questions and the cycle will continue.
Acknowledgments
We thank Elizabeth Turnipseed for comments on the manuscript and Ian Chin-Sang for sharing results. This work was supported by the NIH (R01GM085105 to MAM, including an ARRA administrative supplement) and the American Cancer Society (RSG-06-151-01-DDC).
Funded by: NIH; Grant Number: GM085105
American Cancer Society; Grant Number: RSG-06-151-01-DDC
References
- Aitken RJ, Kelly RW. Analysis of the direct effects of prostaglandins on human sperm function. J Reprod Fertil. 1985;73:139–146. doi: 10.1530/jrf.0.0730139. [DOI] [PubMed] [Google Scholar]
- Anagnostou G, Akbar MT, Paul P, Angelinetta C, Steiner TJ, de Belleroche J. Vesicle associated membrane protein B (VAPB) is decreased in ALS spinal cord. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Arehart E, Stitham J, Asselbergs FW, Douville K, MacKenzie T, Fetalvero KM, Gleim S, Kasza Z, Rao Y, Martel L, Segel S, Robb J, Kaplan A, Simons M, Powell RJ, Moore JH, Rimm EB, Martin KA, Hwa J. Acceleration of cardiovascular disease by a dysfunctional prostacyclin receptor mutation: potential implications for cyclooxygenase-2 inhibition. Circ Res. 2008;102:986–993. doi: 10.1161/CIRCRESAHA.107.165936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura A, Yasui K, Kishino J, Asanuma F, Hasegawa H, Kakudo S, Ohtani M, Arita H. Prevention of allergic inflammation by a novel prostaglandin receptor antagonist, S-5751. J Pharmacol Exp Ther. 2001;298:411–419. [PubMed] [Google Scholar]
- Arvanitis D, Davy A. Eph/ephrin signaling: networks. Genes Dev. 2008;22:416–429. doi: 10.1101/gad.1630408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AM, Roberts TM, Stewart M. 2.6 A resolution crystal structure of helices of the motile major sperm protein (MSP) of Caenorhabditis elegans. J Mol Biol. 2002;319:491–499. doi: 10.1016/S0022-2836(02)00294-2. [DOI] [PubMed] [Google Scholar]
- Banu SK, Lee J, Speights VO, Jr, Starzinski-Powitz A, Arosh JA. Cyclooxygenase-2 regulates survival, migration, and invasion of human endometriotic cells through multiple mechanisms. Endocrinology. 2008;149:1180–1189. doi: 10.1210/en.2007-1168. [DOI] [PubMed] [Google Scholar]
- Baylis HA, Furuichi T, Yoshikawa F, Mikoshiba K, Sattelle DB. Inositol 1,4,5-trisphosphate receptors are strongly expressed in the nervous system, pharynx, intestine, gonad and excretory cell of Caenorhabditis elegans and are encoded by a single gene (itr-1) J Mol Biol. 1999;294:467–476. doi: 10.1006/jmbi.1999.3229. [DOI] [PubMed] [Google Scholar]
- Bohmer M, Van Q, Weyand I, Hagen V, Beyermann M, Matsumoto M, Hoshi M, Hildebrand E, Kaupp UB. Ca2+ spikes in the flagellum control chemotactic behavior of sperm. Embo J. 2005;24:2741–2752. doi: 10.1038/sj.emboj.7600744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Bottino D, Mogilner A, Roberts T, Stewart M, Oster G. How nematode sperm crawl. J Cell Sci. 2002;115:367–384. doi: 10.1242/jcs.115.2.367. [DOI] [PubMed] [Google Scholar]
- Brantley-Sieders DM, Chen J. Eph receptor tyrosine kinases in angiogenesis: from development to disease. Angiogenesis. 2004;7:17–28. doi: 10.1023/B:AGEN.0000037340.33788.87. [DOI] [PubMed] [Google Scholar]
- Breslow JL. n-3 fatty acids and cardiovascular disease. Am J Clin Nutr. 2006;83:1477S–1482S. doi: 10.1093/ajcn/83.6.1477S. [DOI] [PubMed] [Google Scholar]
- Brisbin S, Liu J, Boudreau J, Peng J, Evangelista M, Chin-Sang ID. A Role for C. elegans Eph RTK Signaling in PTEN Regulation. Developmental Cell. 2009;17:459–469. doi: 10.1016/j.devcel.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Brokaw CJ. Calcium-induced asymmetrical beating of triton-demembranated sea urchin sperm flagella. J Cell Biol. 1979;82:401–411. doi: 10.1083/jcb.82.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokaw CJ, Josslin R, Bobrow L. Calcium ion regulation of flagellar beat symmetry in reactivated sea urchin spermatozoa. Biochem Biophys Res Commun. 1974;58:795–800. doi: 10.1016/s0006-291x(74)80487-0. [DOI] [PubMed] [Google Scholar]
- Bullock TL, Roberts TM, Stewart M. 2.5 A resolution crystal structure of the motile major sperm protein (MSP) of Ascaris suum. J Mol Biol. 1996;263:284–296. doi: 10.1006/jmbi.1996.0575. [DOI] [PubMed] [Google Scholar]
- Burke DJ, Ward S. Identification of a large multigene family encoding the major sperm protein of Caenorhabditis elegans. J Mol Biol. 1983;171:1–29. doi: 10.1016/s0022-2836(83)80312-x. [DOI] [PubMed] [Google Scholar]
- Burr GO, Burr MM. A new deficiency disease produced by the rigid exclusion of fat from the diet. J Biol Chem. 1929;82:345–367. doi: 10.1111/j.1753-4887.1973.tb06008.x. [DOI] [PubMed] [Google Scholar]
- Burr GO, Burr MM. On the nature and role of the fatty acids essential for nutrition. J Biol Chem. 1930;86:587–621. [Google Scholar]
- Cheng H, Govindan JA, Greenstein D. Regulated trafficking of the MSP/Eph receptor during oocyte meiotic maturation in C. elegans. Curr Biol. 2008;18:705–714. doi: 10.1016/j.cub.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- Coll JC, Bowden BF, Meehan GV, Konig GM, Carroll AR, Tapiolas DM, Alino PM, Heaton A, De Nys R, Leone PA, Maida M, Aceret TL, Willis RH, Babcock RC, Willis BL, Florian Z, Clayton MN, Miller RL. Chemical aspects of mass spawning in corals. I. Sperm-attractant molecules in the eggs of the scleractinian coral Montipora digitata. Marine Biology. 1994;118:177–182. [Google Scholar]
- Corrigan C, Subramanian R, Miller MA. Eph and NMDA receptors control Ca2+/calmodulin-dependent protein kinase II activation during C. elegans oocyte meiotic maturation. Development. 2005;132:5225–5237. doi: 10.1242/dev.02083. [DOI] [PubMed] [Google Scholar]
- Cowan CW, Shao YR, Sahin M, Shamah SM, Lin MZ, Greer PL, Gao S, Griffith EC, Brugge JS, Greenberg ME. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron. 2005;46:205–217. doi: 10.1016/j.neuron.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Darszon A, Guerrero A, Galindo BE, Nishigaki T, Wood CD. Sperm-activating peptides in the regulation of ion fluxes, signal transduction and motility. Int J Dev Biol. 2008;52:595–606. doi: 10.1387/ijdb.072550ad. [DOI] [PubMed] [Google Scholar]
- Dekel N. Cellular, biochemical and molecular mechanisms regulating oocyte maturation. Mol Cell Endocrinol. 2005;234:19–25. doi: 10.1016/j.mce.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Detwiler MR, Reuben M, Li X, Rogers E, Lin R. Two zinc finger proteins, OMA-1 and OMA-2, are redundantly required for oocyte maturation in C. elegans. Dev Cell. 2001;1:187–199. doi: 10.1016/s1534-5807(01)00026-0. [DOI] [PubMed] [Google Scholar]
- Dupuis L, Corcia P, Fergani A, Gonzalez De Aguilar JL, Bonnefont-Rousselot D, Bittar R, Seilhean D, Hauw JJ, Lacomblez L, Loeffler JP, Meininger V. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology. 2008;70:1004–1009. doi: 10.1212/01.wnl.0000285080.70324.27. [DOI] [PubMed] [Google Scholar]
- Dupuis L, Gonzalez de Aguilar JL, Echaniz-Laguna A, Eschbach J, Rene F, Oudart H, Halter B, Huze C, Schaeffer L, Bouillaud F, Loeffler JP. Muscle mitochondrial uncoupling dismantles neuromuscular junction and triggers distal degeneration of motor neurons. PLoS One. 2009;4:e5390. doi: 10.1371/journal.pone.0005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis L, Gonzalez de Aguilar JL, Oudart H, de Tapia M, Barbeito L, Loeffler JP. Mitochondria in amyotrophic lateral sclerosis: a trigger and a target. Neurodegener Dis. 2004a;1:245–254. doi: 10.1159/000085063. [DOI] [PubMed] [Google Scholar]
- Dupuis L, Oudart H, Rene F, Gonzalez de Aguilar JL, Loeffler JP. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc Natl Acad Sci U S A. 2004b;101:11159–11164. doi: 10.1073/pnas.0402026101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbach M, Giojalas LC. Sperm guidance in mammals - an unpaved road to the egg. Nat Rev Mol Cell Biol. 2006;7:276–285. doi: 10.1038/nrm1893. [DOI] [PubMed] [Google Scholar]
- Ellis R, Schedl T. Sex determination in the germ line. WormBook. 2007:1–13. doi: 10.1895/wormbook.1.82.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RE. Sex determination in the Caenorhabditis elegans germ line. Curr Top Dev Biol. 2008;83:41–64. doi: 10.1016/S0070-2153(08)00402-X. [DOI] [PubMed] [Google Scholar]
- Ellis RE, Kimble J. The fog-3 gene and regulation of cell fate in the germ line of Caenorhabditis elegans. Genetics. 1995;139:561–577. doi: 10.1093/genetics/139.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabro G, Rovasio RA, Civalero S, Frenkel A, Caplan SR, Eisenbach M, Giojalas LC. Chemotaxis of capacitated rabbit spermatozoa to follicular fluid revealed by a novel directionality-based assay. Biol Reprod. 2002;67:1565–1571. doi: 10.1095/biolreprod.102.006395. [DOI] [PubMed] [Google Scholar]
- Fischer DP, Hutchinson JA, Farrar D, O’Donovan PJ, Woodward DF, Marshall KM. Loss of prostaglandin F2alpha, but not thromboxane, responsiveness in pregnant human myometrium during labour. J Endocrinol. 2008;197:171–179. doi: 10.1677/JOE-07-0494. [DOI] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Gao Z, Ruden DM, Lu X. PKD2 cation channel is required for directional sperm movement and male fertility. Curr Biol. 2003;13:2175–2178. doi: 10.1016/j.cub.2003.11.053. [DOI] [PubMed] [Google Scholar]
- Gkogkas C, Middleton S, Kremer AM, Wardrope C, Hannah M, Gillingwater TH, Skehel P. VAPB interacts with and modulates the activity of ATF6. Hum Mol Genet. 2008;17:1517–1526. doi: 10.1093/hmg/ddn040. [DOI] [PubMed] [Google Scholar]
- Goodarzi K, Goodarzi M, Tager AM, Luster AD, von Andrian UH. Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nat Immunol. 2003;4:965–973. doi: 10.1038/ni972. [DOI] [PubMed] [Google Scholar]
- Gottlieb C, Svanborg K, Eneroth P, Bygdeman M. Effect of prostaglandins on human sperm function in vitro and seminal adenosine triphosphate content. Fertil Steril. 1988;49:322–327. doi: 10.1016/s0015-0282(16)59723-4. [DOI] [PubMed] [Google Scholar]
- Govindan JA, Cheng H, Harris JE, Greenstein D. Galphao/i and Galphas signaling function in parallel with the MSP/Eph receptor to control meiotic diapause in C. elegans. Curr Biol. 2006;16:1257–1268. doi: 10.1016/j.cub.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Govindan JA, Nadarajan S, Kim S, Starich TA, Greenstein D. Somatic cAMP signaling regulates MSP-dependent oocyte growth and meiotic maturation in C. elegans. Development. 2009;136:2211–2221. doi: 10.1242/dev.034595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B, Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol Biol Cell. 1999;10:4311–4326. doi: 10.1091/mbc.10.12.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein D. Control of oocyte meiotic maturation and fertilization. WormBook. 2005:1–12. doi: 10.1895/wormbook.1.53.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross GA, Imamura T, Luedke C, Vogt SK, Olson LM, Nelson DM, Sadovsky Y, Muglia LJ. Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc Natl Acad Sci U S A. 1998;95:11875–11879. doi: 10.1073/pnas.95.20.11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidobaldi HA, Teves ME, Unates DR, Anastasia A, Giojalas LC. Progesterone from the cumulus cells is the sperm chemoattractant secreted by the rabbit oocyte cumulus complex. PLoS One. 2008;3:e3040. doi: 10.1371/journal.pone.0003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag ES. The evolution of nematode sex determination: C. elegans as a reference point for comparative biology. WormBook. 2005:1–14. doi: 10.1895/wormbook.1.120.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DH, Winfrey VP, Blaeuer G, Hoffman LH, Furuta T, Rose KL, Hobert O, Greenstein D. Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev Biol. 1999;212:101–123. doi: 10.1006/dbio.1999.9356. [DOI] [PubMed] [Google Scholar]
- Hansbrough JR, Garbers DL. Speract. Purification and characterization of a peptide associated with eggs that activates spermatozoa. J Biol Chem. 1981;256:1447–1452. [PubMed] [Google Scholar]
- Hansen D, Schedl T. The regulatory network controlling the proliferation-meiotic entry decision in the Caenorhabditis elegans germ line. Curr Top Dev Biol. 2006;76:185–215. doi: 10.1016/S0070-2153(06)76006-9. [DOI] [PubMed] [Google Scholar]
- Hardy CC, Robinson C, Tattersfield AE, Holgate ST. The bronchoconstrictor effect of inhaled prostaglandin D2 in normal and asthmatic men. N Engl J Med. 1984;311:209–213. doi: 10.1056/NEJM198407263110401. [DOI] [PubMed] [Google Scholar]
- Harizi H, Corcuff JB, Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med. 2008;14:461–469. doi: 10.1016/j.molmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Harris JE, Govindan JA, Yamamoto I, Schwartz J, Kaverina I, Greenstein D. Major sperm protein signaling promotes oocyte microtubule reorganization prior to fertilization in Caenorhabditis elegans. Dev Biol. 2006;299:105–121. doi: 10.1016/j.ydbio.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Hill KL, L’Hernault SW. Analyses of reproductive interactions that occur after heterospecific matings within the genus Caenorhabditis. Dev Biol. 2001;232:105–114. doi: 10.1006/dbio.2000.0136. [DOI] [PubMed] [Google Scholar]
- Hirsh D, Oppenheim D, Klass M. Development of the reproductive system of Caenorhabditis elegans. Dev Biol. 1976;49:200–219. doi: 10.1016/0012-1606(76)90267-0. [DOI] [PubMed] [Google Scholar]
- Honda T, Segi-Nishida E, Miyachi Y, Narumiya S. Prostacyclin-IP signaling and prostaglandin E2-EP2/EP4 signaling both mediate joint inflammation in mouse collagen-induced arthritis. J Exp Med. 2006;203:325–335. doi: 10.1084/jem.20051310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jud MC, Czerwinski MJ, Wood MP, Young RA, Gallo CM, Bickel JS, Petty EL, Mason JM, Little BA, Padilla PA, Schisa JA. Large P body-like RNPs form in C. elegans oocytes in response to arrested ovulation, heat shock, osmotic stress, and anoxia and are regulated by the major sperm protein pathway. Dev Biol. 2008;318:38–51. doi: 10.1016/j.ydbio.2008.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadandale P, Singson A. Oocyte production and sperm utilization patterns in semi-fertile strains of Caenorhabditis elegans. BMC Dev Biol. 2004;4:3. doi: 10.1186/1471-213X-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadandale P, Stewart-Michaelis A, Gordon S, Rubin J, Klancer R, Schweinsberg P, Grant BD, Singson A. The Egg Surface LDL Receptor Repeat-Containing Proteins EGG-1 and EGG-2 Are Required for Fertilization in Caenorhabditis elegans. Curr Biol. 2005;15:2222–2229. doi: 10.1016/j.cub.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Kagiwada S, Hosaka K, Murata M, Nikawa J, Takatsuki A. The Saccharomyces cerevisiae SCS2 gene product, a homolog of a synaptobrevin-associated protein, is an integral membrane protein of the endoplasmic reticulum and is required for inositol metabolism. J Bacteriol. 1998;180:1700–1708. doi: 10.1128/jb.180.7.1700-1708.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SM, Jun HJ, Lim YS, Choi SH, Hwang SB. Characterization of monoclonal antibodies against the nonstructural 5A protein of hepatitis C virus. Arch Virol. 2009;154:843–851. doi: 10.1007/s00705-009-0386-9. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Solzin J, Hildebrand E, Brown JE, Helbig A, Hagen V, Beyermann M, Pampaloni F, Weyand I. The signal flow and motor response controling chemotaxis of sea urchin sperm. Nat Cell Biol. 2003;5:109–117. doi: 10.1038/ncb915. [DOI] [PubMed] [Google Scholar]
- Kimble J, Crittenden SL. Germline proliferation and its control. WormBook. 2005:1–14. doi: 10.1895/wormbook.1.13.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol. 2007;23:405–433. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]
- Kiontke K, Gavin NP, Raynes Y, Roehrig C, Piano F, Fitch DH. Caenorhabditis phylogeny predicts convergence of hermaphroditism and extensive intron loss. Proc Natl Acad Sci U S A. 2004;101:9003–9008. doi: 10.1073/pnas.0403094101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass MR, Hirsh D. Sperm isolation and biochemical analysis of the major sperm protein from C. elegans. Dev Biol. 1981;84:299–312. doi: 10.1016/0012-1606(81)90398-5. [DOI] [PubMed] [Google Scholar]
- Kosinski M, McDonald K, Schwartz J, Yamamoto I, Greenstein D. C. elegans sperm bud vesicles to deliver a meiotic maturation signal to distant oocytes. Development. 2005;132:3357–3369. doi: 10.1242/dev.01916. [DOI] [PubMed] [Google Scholar]
- Kubagawa HM, Watts JL, Corrigan C, Edmonds JW, Sztul E, Browse J, Miller MA. Oocyte signals derived from polyunsaturated fatty acids control sperm recruitment in vivo. Nat Cell Biol. 2006;8:1143–1148. doi: 10.1038/ncb1476. [DOI] [PubMed] [Google Scholar]
- Kuijper S, Turner CJ, Adams RH. Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc Med. 2007;17:145–151. doi: 10.1016/j.tcm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Kukihara H, Moriishi K, Taguwa S, Tani H, Abe T, Mori Y, Suzuki T, Fukuhara T, Taketomi A, Maehara Y, Matsuura Y. Human VAP-C negatively regulates hepatitis C virus propagation. J Virol. 2009;83:7959–7969. doi: 10.1128/JVI.00889-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Hernault SW. Spermatogenesis. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. ELEGANS II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 271–294. [PubMed] [Google Scholar]
- L’Hernault SW. Spermatogenesis. WormBook. 2006:1–14. doi: 10.1895/wormbook.1.85.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Hernault SW. The genetics and cell biology of spermatogenesis in the nematode C. elegans. Mol Cell Endocrinol. 2009;306:59–65. doi: 10.1016/j.mce.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Lai KO, Ip FC, Cheung J, Fu AK, Ip NY. Expression of Eph receptors in skeletal muscle and their localization at the neuromuscular junction. Mol Cell Neurosci. 2001;17:1034–1047. doi: 10.1006/mcne.2001.0997. [DOI] [PubMed] [Google Scholar]
- Langenbach R, Loftin C, Lee C, Tiano H. Cyclooxygenase knockout mice: models for elucidating isoform-specific functions. Biochem Pharmacol. 1999;58:1237–1246. doi: 10.1016/s0006-2952(99)00158-6. [DOI] [PubMed] [Google Scholar]
- Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Lee CA, Goulding EH, Kluckman KD, Kim HS, Smithies O. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- Lapierre LA, Tuma PL, Navarre J, Goldenring JR, Anderson JM. VAP-33 localizes to both an intracellular vesicle population and with occludin at the tight junction. J Cell Sci. 1999;112 (Pt 21):3723–3732. doi: 10.1242/jcs.112.21.3723. [DOI] [PubMed] [Google Scholar]
- Lee MH, Ohmachi M, Arur S, Nayak S, Francis R, Church D, Lambie E, Schedl T. Multiple functions and dynamic activation of MPK-1 extracellular signal-regulated kinase signaling in Caenorhabditis elegans germline development. Genetics. 2007;177:2039–2062. doi: 10.1534/genetics.107.081356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesa GM, Palfreyman M, Hall DH, Clandinin MT, Rudolph C, Jorgensen EM, Schiavo G. Long chain polyunsaturated fatty acids are required for efficient neurotransmission in C. elegans. J Cell Sci. 2003;116:4965–4975. doi: 10.1242/jcs.00918. [DOI] [PubMed] [Google Scholar]
- Lev S, Ben Halevy D, Peretti D, Dahan N. The VAP protein family: from cellular functions to motor neuron disease. Trends Cell Biol. 2008;18:282–290. doi: 10.1016/j.tcb.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Lim H, Gupta RA, Ma WG, Paria BC, Moller DE, Morrow JD, DuBois RN, Trzaskos JM, Dey SK. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev. 1999;13:1561–1574. doi: 10.1101/gad.13.12.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- Loewen CJ, Levine TP. A highly conserved binding site in vesicle-associated membrane protein-associated protein (VAP) for the FFAT motif of lipid-binding proteins. J Biol Chem. 2005;280:14097–14104. doi: 10.1074/jbc.M500147200. [DOI] [PubMed] [Google Scholar]
- Lohoff FW, Weller AE, Bloch PJ, Nall AH, Ferraro TN, Berrettini WH. Association between polymorphisms in the vesicle-associated membrane protein-associated protein A (VAPA) gene on chromosome 18p and bipolar disorder. J Neural Transm. 2008;115:1339–1345. doi: 10.1007/s00702-008-0093-9. [DOI] [PubMed] [Google Scholar]
- Luster AD, Tager AM. T-cell trafficking in asthma: lipid mediators grease the way. Nat Rev Immunol. 2004;4:711–724. doi: 10.1038/nri1438. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, He S, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Liebert CA, Liu C, Lu F, Lu S, Marchler GH, Mullokandov M, Song JS, Tasneem A, Thanki N, Yamashita RA, Zhang D, Zhang N, Bryant SH. CDD: specific functional annotation with the Conserved Domain Database. Nucleic Acids Res. 2009;37:D205–210. doi: 10.1093/nar/gkn845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y. Meiotic arrest in animal oocytes. In: Monroy A, Metz CB, editors. Biology of Fertilization. Orlando: Academic Press; 1985. pp. 189–219. [Google Scholar]
- Masui Y, Clarke HJ. Oocyte maturation. Int Rev Cytol. 1979;57:185–282. doi: 10.1016/s0074-7696(08)61464-3. [DOI] [PubMed] [Google Scholar]
- Matova N, Cooley L. Comparative aspects of animal oogenesis. Dev Biol. 2001;231:291–320. doi: 10.1006/dbio.2000.0120. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Ma W, Smalley W, Trzaskos J, Breyer RM, Dey SK. Diversification of cyclooxygenase-2-derived prostaglandins in ovulation and implantation. Biol Reprod. 2001;64:1557–1565. doi: 10.1095/biolreprod64.5.1557. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Briones AV, Nishigaki T, Hoshi M. Sequence analysis of cDNAs encoding precursors of starfish asterosaps. Dev Genet. 1999;25:130–136. doi: 10.1002/(SICI)1520-6408(1999)25:2<130::AID-DVG7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Solzin J, Helbig A, Hagen V, Ueno S, Kawase O, Maruyama Y, Ogiso M, Godde M, Minakata H, Kaupp UB, Hoshi M, Weyand I. A sperm-activating peptide controls a cGMP-signaling pathway in starfish sperm. Dev Biol. 2003;260:314–324. doi: 10.1016/s0012-1606(03)00236-7. [DOI] [PubMed] [Google Scholar]
- McCarter J, Bartlett B, Dang T, Schedl T. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol. 1999;205:111–128. doi: 10.1006/dbio.1998.9109. [DOI] [PubMed] [Google Scholar]
- McCoy JM, Wicks JR, Audoly LP. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J Clin Invest. 2002;110:651–658. doi: 10.1172/JCI15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill MA, Chen Y, Strack S, Hell JW. Activity-driven postsynaptic translocation of CaMKII. Trends Pharmacol Sci. 2005;26:645–653. doi: 10.1016/j.tips.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Miller MA, Cutter AD, Yamamoto I, Ward S, Greenstein D. Clustered organization of reproductive genes in the C. elegans genome. Curr Biol. 2004;14:1284–1290. doi: 10.1016/j.cub.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Miller MA, Nguyen VQ, Lee MH, Kosinski M, Schedl T, Caprioli RM, Greenstein D. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science. 2001;291:2144–2147. doi: 10.1126/science.1057586. [DOI] [PubMed] [Google Scholar]
- Miller MA, Ruest PJ, Kosinski M, Hanks SK, Greenstein D. An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans. Genes Dev. 2003;17:187–200. doi: 10.1101/gad.1028303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri I, Aritake K, Taniguchi H, Sato Y, Kamauchi S, Nagata N, Maruyama T, Taniike M, Urade Y. Inhibition of prostaglandin D synthase suppresses muscular necrosis. Am J Pathol. 2009;174:1735–1744. doi: 10.2353/ajpath.2009.080709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri I, Kadoyama K, Kanekiyo T, Sato Y, Kagitani-Shimono K, Saito Y, Suzuki K, Kudo T, Takeda M, Urade Y, Murayama S, Taniike M. Hematopoietic prostaglandin D synthase and DP1 receptor are selectively upregulated in microglia and astrocytes within senile plaques from human patients and in a mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2007;66:469–480. doi: 10.1097/01.jnen.0000240472.43038.27. [DOI] [PubMed] [Google Scholar]
- Nadarajan S, Govindan JA, McGovern M, Hubbard EJ, Greenstein D. MSP and GLP-1/Notch signaling coordinately regulate actomyosin-dependent cytoplasmic streaming and oocyte growth in C. elegans. Development. 2009;136:2223–2234. doi: 10.1242/dev.034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson GA, Roberts TM, Ward S. Caenorhabditis elegans spermatozoan locomotion: amoeboid movement with almost no actin. J Cell Biol. 1982;92:121–131. doi: 10.1083/jcb.92.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishigaki T, Chiba K, Miki W, Hoshi M. Structure and function of asterosaps, sperm-activating peptides from the jelly coat of starfish eggs. Zygote. 1996;4:237–245. doi: 10.1017/s0967199400003154. [DOI] [PubMed] [Google Scholar]
- Nishimura AL, Mitne-Neto M, Silva HC, Richieri-Costa A, Middleton S, Cascio D, Kok F, Oliveira JR, Gillingwater T, Webb J, Skehel P, Zatz M. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet. 2004;75:822–831. doi: 10.1086/425287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Hayashi M, Inada H, Tanaka T. Molecular cloning and characterization of mammalian homologues of vesicle-associated membrane protein-associated (VAMP-associated) proteins. Biochem Biophys Res Commun. 1999;254:21–26. doi: 10.1006/bbrc.1998.9876. [DOI] [PubMed] [Google Scholar]
- Norman KR, Fazzio RT, Mellem JE, Espelt MV, Strange K, Beckerle MC, Maricq AV. The Rho/Rac-family guanine nucleotide exchange factor VAV-1 regulates rhythmic behaviors in C. elegans. Cell. 2005;123:119–132. doi: 10.1016/j.cell.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Oliveira RG, Tomasi L, Rovasio RA, Giojalas LC. Increased velocity and induction of chemotactic response in mouse spermatozoa by follicular and oviductal fluids. J Reprod Fertil. 1999;115:23–27. doi: 10.1530/jrf.0.1150023. [DOI] [PubMed] [Google Scholar]
- Olson JH, Xiang X, Ziegert T, Kittelson A, Rawls A, Bieber AL, Chandler DE. Allurin, a 21-kDa sperm chemoattractant from Xenopus egg jelly, is related to mammalian sperm-binding proteins. Proc Natl Acad Sci U S A. 2001;98:11205–11210. doi: 10.1073/pnas.211316798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren-Benaroya R, Orvieto R, Gakamsky A, Pinchasov M, Eisenbach M. The sperm chemoattractant secreted from human cumulus cells is progesterone. Hum Reprod. 2008;23:2339–2345. doi: 10.1093/humrep/den265. [DOI] [PubMed] [Google Scholar]
- Palmer A, Klein R. Multiple roles of ephrins in morphogenesis, neuronal networking, and brain function. Genes Dev. 2003;17:1429–1450. doi: 10.1101/gad.1093703. [DOI] [PubMed] [Google Scholar]
- Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Pennetta G, Hiesinger P, Fabian-Fine R, Meinertzhagen I, Bellen H. Drosophila VAP-33A directs bouton formation at neuromuscular junctions in a dosage-dependent manner. Neuron. 2002;35:291–306. doi: 10.1016/s0896-6273(02)00769-9. [DOI] [PubMed] [Google Scholar]
- Pettipher R. The roles of the prostaglandin D(2) receptors DP(1) and CRTH2 in promoting allergic responses. Br J Pharmacol. 2008;153(Suppl 1):S191–199. doi: 10.1038/sj.bjp.0707488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Prosser DC, Tran D, Gougeon PY, Verly C, Ngsee JK. FFAT rescues VAPA-mediated inhibition of ER-to-Golgi transport and VAPB-mediated ER aggregation. J Cell Sci. 2008 doi: 10.1242/jcs.028696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralt D, Goldenberg M, Fetterolf P, Thompson D, Dor J, Mashiach S, Garbers DL, Eisenbach M. Sperm attraction to a follicular factor(s) correlates with human egg fertilizability. Proc Natl Acad Sci U S A. 1991;88:2840–2844. doi: 10.1073/pnas.88.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnaparkhi A, Lawless GM, Schweizer FE, Golshani P, Jackson GR. A Drosophila model of ALS: human ALS-associated mutation in VAP33A suggests a dominant negative mechanism. PLoS ONE. 2008;3:e2334. doi: 10.1371/journal.pone.0002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke V, Gil IS, Ward S, Kazmer K. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development. 2004;131:311–323. doi: 10.1242/dev.00914. [DOI] [PubMed] [Google Scholar]
- Reinke V, Smith HE, Nance J, Wang J, Van Doren C, Begley R, Jones SJ, Davis EB, Scherer S, Ward S, Kim SK. A global profile of germline gene expression in C. elegans. Mol Cell. 2000;6:605–616. doi: 10.1016/s1097-2765(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Riffell JA, Krug PJ, Zimmer RK. Fertilization in the sea: the chemical identity of an abalone sperm attractant. J Exp Biol. 2002;205:1439–1450. doi: 10.1242/jeb.205.10.1439. [DOI] [PubMed] [Google Scholar]
- Roberts TM, Stewart M. Acting like actin. The dynamics of the nematode major sperm protein (msp) cytoskeleton indicate a push-pull mechanism for amoeboid cell motility. J Cell Biol. 2000;149:7–12. doi: 10.1083/jcb.149.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha N, Kuijl C, van der Kant R, Janssen L, Houben D, Janssen H, Zwart W, Neefjes J. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J Cell Biol. 2009;185:1209–1225. doi: 10.1083/jcb.200811005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LP. Diagnosis of amyotrophic lateral sclerosis. J Neurol Sci. 1998;160(Suppl 1):S6–24. doi: 10.1016/s0022-510x(98)00193-2. [DOI] [PubMed] [Google Scholar]
- Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertilization: where it all begins. Dev Biol. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- Schedl T, Kimble J. fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics. 1988;119:43–61. doi: 10.1093/genetics/119.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepsenwol S, Ris H, Roberts TM. A unique cytoskeleton associated with crawling in the amoeboid sperm of the nematode, Ascaris suum. J Cell Biol. 1989;108:55–66. doi: 10.1083/jcb.108.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanklin J, Cahoon EB. Desaturation and Related Modifications of Fatty Acids1. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:611–641. doi: 10.1146/annurev.arplant.49.1.611. [DOI] [PubMed] [Google Scholar]
- Sharrock WJ, Sutherlin ME, Leske K, Cheng TK, Kim TY. Two distinct yolk lipoprotein complexes from Caenorhabditis elegans. J Biol Chem. 1990;265:14422–14431. [PubMed] [Google Scholar]
- Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276:18075–18081. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- Simon AM, de Maturana RL, Ricobaraza A, Escribano L, Schiapparelli L, Cuadrado-Tejedor M, Perez-Mediavilla A, Avila J, Del Rio J, Frechilla D. Early Changes in Hippocampal Eph Receptors Precede the Onset of Memory Decline in Mouse Models of Alzheimer’s Disease. J Alzheimers Dis. 2009 doi: 10.3233/JAD-2009-1096. [DOI] [PubMed] [Google Scholar]
- Singson A. Every sperm is sacred: fertilization in Caenorhabditis elegans. Dev Biol. 2001;230:101–109. doi: 10.1006/dbio.2000.0118. [DOI] [PubMed] [Google Scholar]
- Singson A, Hang JS, Parry JM. Genes required for the common miracle of fertilization in Caenorhabditis elegans. Int J Dev Biol. 2008;52:647–656. doi: 10.1387/ijdb.072512as. [DOI] [PubMed] [Google Scholar]
- Skehel PA, Fabian-Fine R, Kandel ER. Mouse VAP33 is associated with the endoplasmic reticulum and microtubules. Proc Natl Acad Sci U S A. 2000;97:1101–1106. doi: 10.1073/pnas.97.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G, Roberts R, Hall C, Nuki G. Reversible ovulatory failure associated with the development of luteinized unruptured follicles in women with inflammatory arthritis taking non-steroidal anti-inflammatory drugs. Br J Rheumatol. 1996;35:458–462. doi: 10.1093/rheumatology/35.5.458. [DOI] [PubMed] [Google Scholar]
- Smith H. Sperm motility and MSP. WormBook. 2006:1–8. doi: 10.1895/wormbook.1.68.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res. 2009;50(Suppl):S423–428. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussan L, Burakov D, Daniels MP, Toister-Achituv M, Porat A, Yarden Y, Elazar Z. ERG30, a VAP-33-related protein, functions in protein transport mediated by COPI vesicles. J Cell Biol. 1999;146:301–311. doi: 10.1083/jcb.146.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299:2054–2058. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- Strunker T, Weyand I, Bonigk W, Van Q, Loogen A, Brown JE, Kashikar N, Hagen V, Krause E, Kaupp UB. A K+-selective cGMP-gated ion channel controls chemosensation of sperm. Nat Cell Biol. 2006;8:1149–1154. doi: 10.1038/ncb1473. [DOI] [PubMed] [Google Scholar]
- Suga H, Katoh K, Miyata T. Sponge homologs of vertebrate protein tyrosine kinases and frequent domain shufflings in the early evolution of animals before the parazoan-eumetazoan split. Gene. 2001;280:195–201. doi: 10.1016/s0378-1119(01)00784-3. [DOI] [PubMed] [Google Scholar]
- Sun F, Bahat A, Gakamsky A, Girsh E, Katz N, Giojalas LC, Tur-Kaspa I, Eisenbach M. Human sperm chemotaxis: both the oocyte and its surrounding cumulus cells secrete sperm chemoattractants. Hum Reprod. 2005;20:761–767. doi: 10.1093/humrep/deh657. [DOI] [PubMed] [Google Scholar]
- Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, Friedrich EB, Carafone AD, Gerszten RE, Luster AD. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol. 2003;4:982–990. doi: 10.1038/ni970. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Morrow JD, Wang H, Dey SK. Cyclooxygenase-2-derived prostaglandin E(2) directs oocyte maturation by differentially influencing multiple signaling pathways. J Biol Chem. 2006;281:37117–37129. doi: 10.1074/jbc.M608202200. [DOI] [PubMed] [Google Scholar]
- Takasu MA, Dalva MB, Zigmond RE, Greenberg ME. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science. 2002;295:491–495. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- Tamba S, Yodoi R, Segi-Nishida E, Ichikawa A, Narumiya S, Sugimoto Y. Timely interaction between prostaglandin and chemokine signaling is a prerequisite for successful fertilization. Proc Natl Acad Sci U S A. 2008;105:14539–14544. doi: 10.1073/pnas.0805699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr DE, Scott AL. MSP domain proteins. Trends Parasitol. 2005;21:224–231. doi: 10.1016/j.pt.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Teuling E, Ahmed S, Haasdijk E, Demmers J, Steinmetz MO, Akhmanova A, Jaarsma D, Hoogenraad CC. Motor neuron disease-associated mutant vesicle-associated membrane protein-associated protein (VAP) B recruits wild-type VAPs into endoplasmic reticulum-derived tubular aggregates. J Neurosci. 2007;27:9801–9815. doi: 10.1523/JNEUROSCI.2661-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun MJ, Namboodiri MM, Heath CW., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- Tocher DR, Leaver MJ, Hodgson PA. Recent advances in the biochemistry and molecular biology of fatty acyl desaturases. Prog Lipid Res. 1998;37:73–117. doi: 10.1016/s0163-7827(98)00005-8. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Han SM, Yang Y, Tong C, Lin YQ, Mohan K, Haueter C, Zoghbi A, Harati Y, Kwan J, Miller MA, Bellen HJ. The amyotrophic lateral sclerosis 8 protein VAPB is cleaved, secreted, and acts as a ligand for Eph receptors. Cell. 2008;133:963–977. doi: 10.1016/j.cell.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Gao L, Shi ST, Taylor DR, Yang T, Mircheff AK, Wen Y, Gorbalenya AE, Hwang SB, Lai MM. Hepatitis C virus RNA polymerase and NS5A complex with a SNARE-like protein. Virology. 1999;263:30–41. doi: 10.1006/viro.1999.9893. [DOI] [PubMed] [Google Scholar]
- Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006;6:130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Wallis JG, Watts JL, Browse J. Polyunsaturated fatty acid synthesis: what will they think of next? Trends Biochem Sci. 2002;27:467. doi: 10.1016/s0968-0004(02)02168-0. [DOI] [PubMed] [Google Scholar]
- Wang CH, Lunn MR. Spinal muscular atrophy: advances in research and consensus on care of patients. Curr Treat Options Neurol. 2008;10:420–428. doi: 10.1007/s11940-008-0044-7. [DOI] [PubMed] [Google Scholar]
- Wang MT, Honn KV, Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev. 2007 doi: 10.1007/s10555-007-9096-5. [DOI] [PubMed] [Google Scholar]
- Ward GE, Brokaw CJ, Garbers DL, Vacquier VD. Chemotaxis of Arbacia punctulata spermatozoa to resact, a peptide from the egg jelly layer. J Cell Biol. 1985;101:2324–2329. doi: 10.1083/jcb.101.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward GE, Moy GW, Vacquier VD. Phosphorylation of membrane-bound guanylate cyclase of sea urchin spermatozoa. J Cell Biol. 1986;103:95–101. doi: 10.1083/jcb.103.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S, Burke DJ, Sulston JE, Coulson AR, Albertson DG, Ammons D, Klass M, Hogan E. Genomic organization of major sperm protein genes and pseudogenes in the nematode Caenorhabditis elegans. J Mol Biol. 1988;199:1–13. doi: 10.1016/0022-2836(88)90374-9. [DOI] [PubMed] [Google Scholar]
- Ward S, Carrel JS. Fertilization and sperm competition in the nematode Caenorhabditis elegans. Dev Biol. 1979;73:304–321. doi: 10.1016/0012-1606(79)90069-1. [DOI] [PubMed] [Google Scholar]
- Ward S, Hogan E, Nelson GA. The initiation of spermiogenesis in the nematode Caenorhabditis elegans. Dev Biol. 1983;98:70–79. doi: 10.1016/0012-1606(83)90336-6. [DOI] [PubMed] [Google Scholar]
- Watnick TJ, Jin Y, Matunis E, Kernan MJ, Montell C. A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr Biol. 2003;13:2179–2184. doi: 10.1016/j.cub.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Watts JL. Fat synthesis and adiposity regulation in Caenorhabditis elegans. Trends Endocrinol Metab. 2009;20:58–65. doi: 10.1016/j.tem.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JL, Browse J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:5854–5859. doi: 10.1073/pnas.092064799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir ML, Klip A, Trimble WS. Identification of a human homologue of the vesicle-associated membrane protein (VAMP)-associated protein of 33 kDa (VAP-33): a broadly expressed protein that binds to VAMP. Biochem J. 1998;333 (Pt 2):247–251. doi: 10.1042/bj3330247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten SJ, Miller MA. The role of gap junctions in Caenorhabditis elegans oocyte maturation and fertilization. Dev Biol. 2007;301:432–446. doi: 10.1016/j.ydbio.2006.08.038. [DOI] [PubMed] [Google Scholar]
- Wyles JP, McMaster CR, Ridgway ND. VAMP-associated protein-A (VAP-A) interacts with the oxysterol binding protein (OSBP) to modify export from the endoplasmic reticulum. J Biol Chem. 2002;21:21. doi: 10.1074/jbc.M201191200. [DOI] [PubMed] [Google Scholar]
- Xiang X, Burnett L, Rawls A, Bieber A, Chandler D. The sperm chemoattractant “allurin” is expressed and secreted from the Xenopus oviduct in a hormone-regulated manner. Dev Biol. 2004;275:343–355. doi: 10.1016/j.ydbio.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Yamamoto I, Kosinski ME, Greenstein D. Start me up: Cell signaling and the journey from oocyte to embryo in C. elegans. Dev Dyn. 2005 doi: 10.1002/dvdy.20662. [DOI] [PubMed] [Google Scholar]
- Yin X, Gower NJ, Baylis HA, Strange K. Inositol 1,4,5-trisphosphate signaling regulates rhythmic contractile activity of myoepithelial sheath cells in Caenorhabditis elegans. Mol Biol Cell. 2004;15:3938–3949. doi: 10.1091/mbc.E04-03-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Murata M, Inaba K, Morisawa M. A chemoattractant for ascidian spermatozoa is a sulfated steroid. Proc Natl Acad Sci U S A. 2002;99:14831–14836. doi: 10.1073/pnas.242470599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Lucitt MB, Stubbe J, Cheng Y, Friis UG, Hansen PB, Jensen BL, Smyth EM, FitzGerald GA. Prostaglandin F2alpha elevates blood pressure and promotes atherosclerosis. Proc Natl Acad Sci U S A. 2009;106:7985–7990. doi: 10.1073/pnas.0811834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamaria N. Alteration of polyunsaturated fatty acid status and metabolism in health and disease. Reprod Nutr Dev. 2004;44:273–282. doi: 10.1051/rnd:2004034. [DOI] [PubMed] [Google Scholar]