Abstract
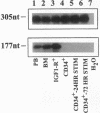
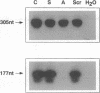
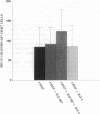
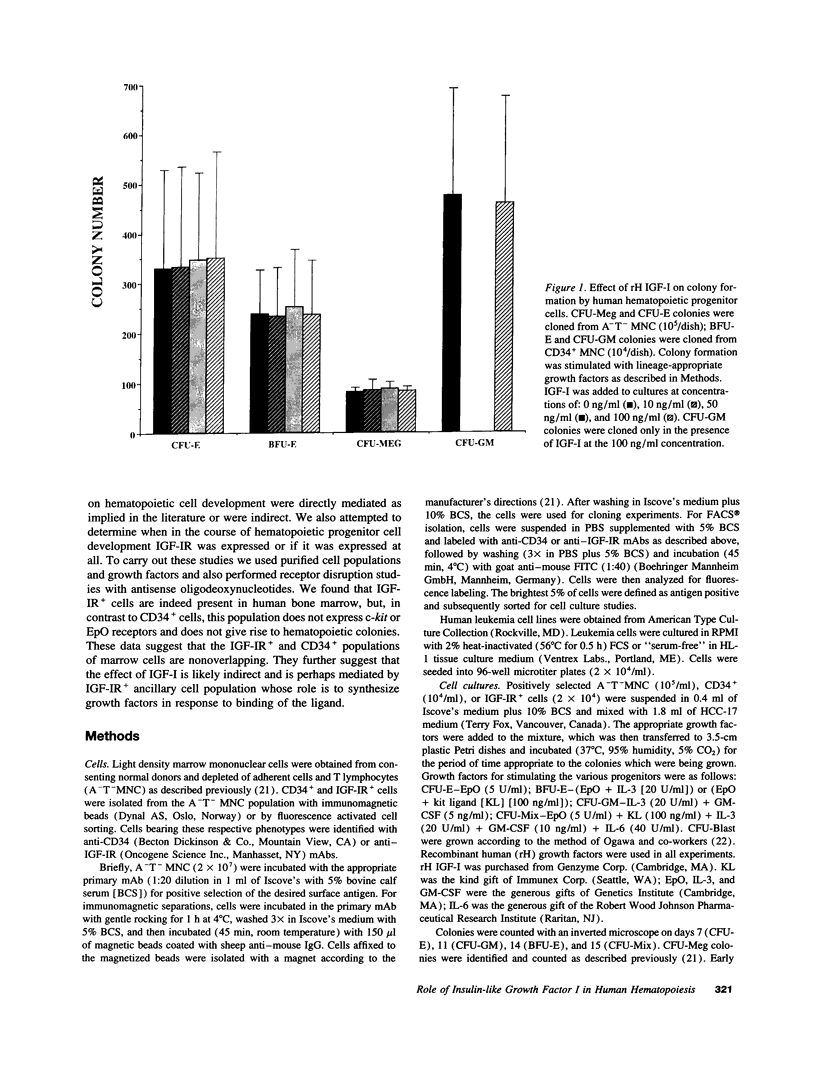
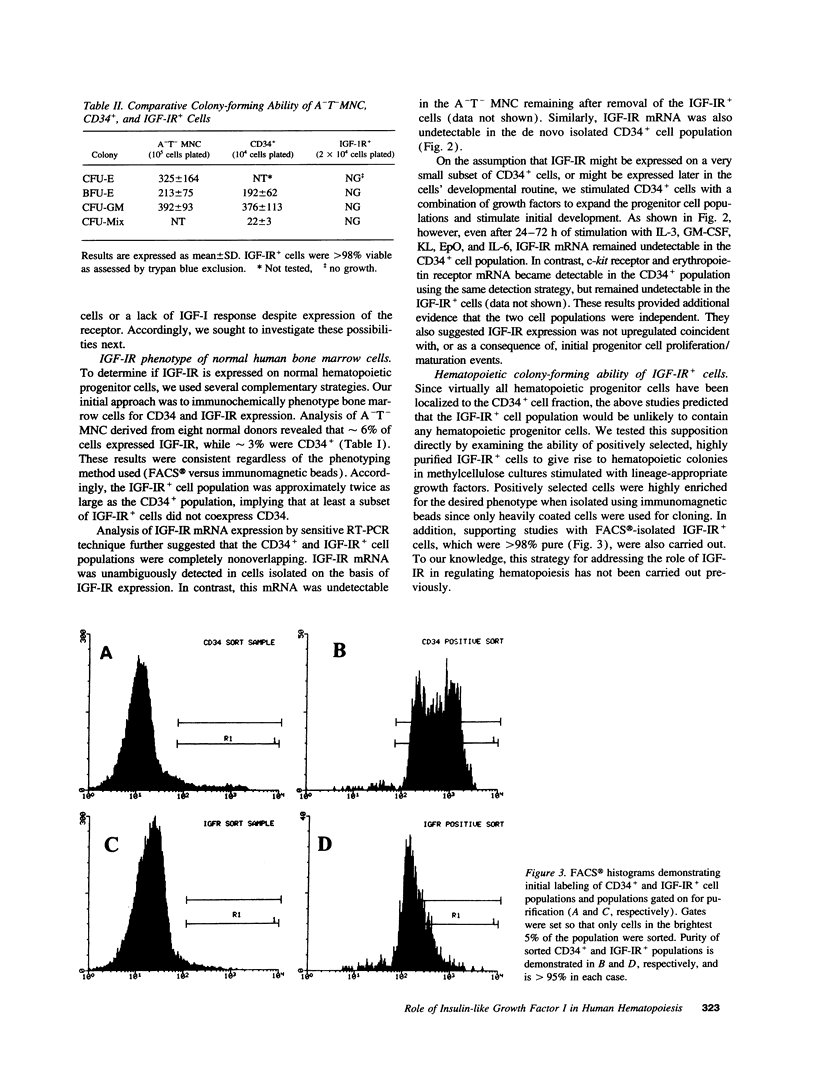
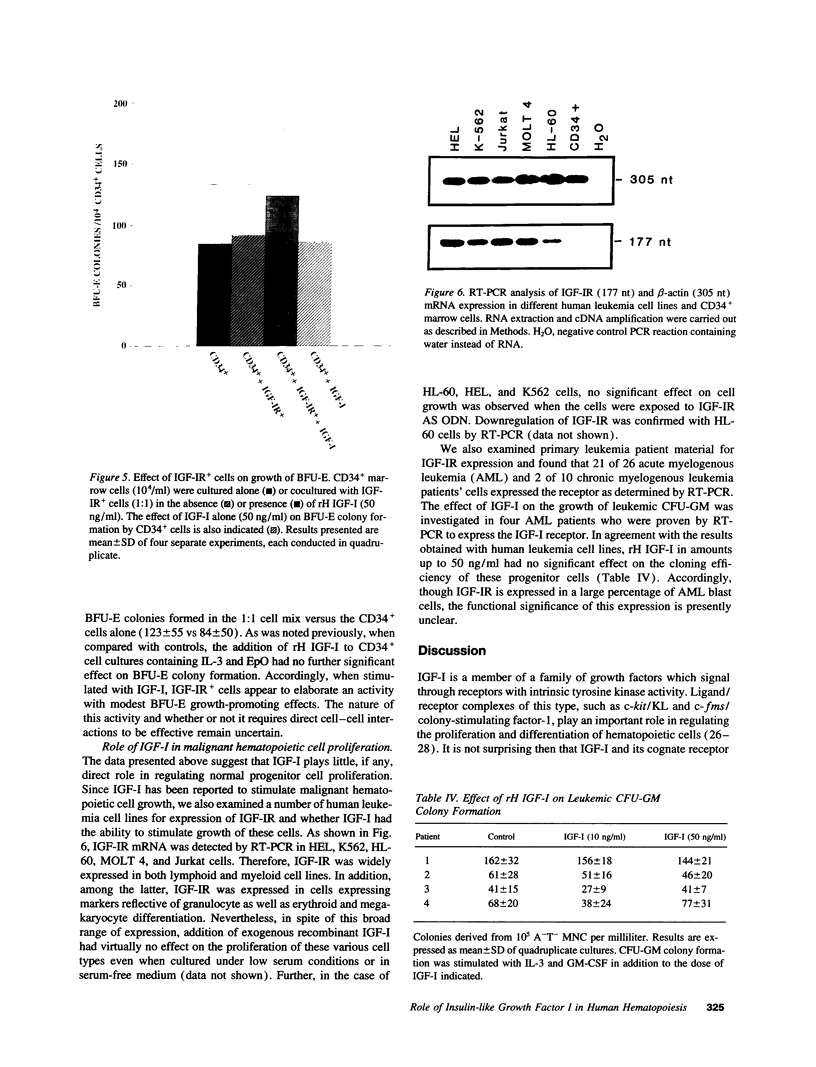
IGF-I has been reported to increase hematopoietic progenitor cell cloning efficiency. To investigate this phenomenon, we studied the IGF-I responsiveness of human marrow cells expressing IGF-I receptor (IGF-IR), a direct strategy not used previously. IGF-IR+ and control CD34+ marrow cells were isolated using immunoaffinity methods. Then, the cells were cloned in methylcellulose containing variable amounts of serum- and lineage-appropriate growth factors supplemented with recombinant human IGF-I. In contrast to CD34+ cells, IGF-IR+ cells never gave rise to CFU-Blast, CFU-Mix, CFU-GM, BFU-E, or CFU-E. To substantiate the suggestion that CD34+ and IGF-IR+ cells were distinct populations, we used reverse transcription PCR to detect IGF-I, EpO, and KIT receptor mRNAs in these cells. The mRNA phenotype of CD34+ cells was EpO (+), KIT (+), and IGF-IR (-), while IGF-IR+ cells were IGF-IR (+), EpO (-), and KIT (-). These results suggested that IGF-IR is either not expressed or expressed at low levels on normal hematopoietic progenitor cells. Functional significance of the latter possibility was tested by exposing CD34+ cells to IGF-IR antisense oligodeoxynucleotides. Colony formation was unaffected by oligodeoxynucleotide disruption of IGF-IR, suggesting that, even if expressed at low level, the receptor's functional significance was doubtful. Nevertheless, when cultured in the presence of IGF-I, IGF-IR+ cells elaborated an activity with mild BFU-E stimulatory effects. Accordingly, if IGF-I plays a role in hematopoietic colony formation, it is probably and results from stimulation of IGF-IR-positive ancillary cells to secrete growth factors. Studies carried out with human leukemia cells yielded similar results.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akahane K., Tojo A., Urabe A., Takaku F. Pure erythropoietic colony and burst formations in serum-free culture and their enhancement by insulin-like growth factor I. Exp Hematol. 1987 Aug;15(7):797–802. [PubMed] [Google Scholar]
- Baker J., Liu J. P., Robertson E. J., Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993 Oct 8;75(1):73–82. [PubMed] [Google Scholar]
- Boyer S. H., Bishop T. R., Rogers O. C., Noyes A. N., Frelin L. P., Hobbs S. Roles of erythropoietin, insulin-like growth factor 1, and unidentified serum factors in promoting maturation of purified murine erythroid colony-forming units. Blood. 1992 Nov 15;80(10):2503–2512. [PubMed] [Google Scholar]
- Clark R., Strasser J., McCabe S., Robbins K., Jardieu P. Insulin-like growth factor-1 stimulation of lymphopoiesis. J Clin Invest. 1993 Aug;92(2):540–548. doi: 10.1172/JCI116621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa P. N., Axelrad A. A. Production of erythropoietic bursts by progenitor cells from adult human peripheral blood in an improved serum-free medium: role of insulinlike growth factor 1. Blood. 1991 Dec 1;78(11):2823–2833. [PubMed] [Google Scholar]
- Cotton E. W., Means R. T., Jr, Cline S. M., Krantz S. B. Quantitation of insulin-like growth factor-I binding to highly purified human erythroid colony-forming units. Exp Hematol. 1991 May;19(4):278–281. [PubMed] [Google Scholar]
- Dai C. H., Krantz S. B., Zsebo K. M. Human burst-forming units-erythroid need direct interaction with stem cell factor for further development. Blood. 1991 Nov 15;78(10):2493–2497. [PubMed] [Google Scholar]
- Dainiak N. Control of hematopoietic cell growth by somatomedins. Exp Hematol. 1993 Oct;21(11):1405–1407. [PubMed] [Google Scholar]
- Estrov Z., Meir R., Barak Y., Zaizov R., Zadik Z. Human growth hormone and insulin-like growth factor-1 enhance the proliferation of human leukemic blasts. J Clin Oncol. 1991 Mar;9(3):394–399. doi: 10.1200/JCO.1991.9.3.394. [DOI] [PubMed] [Google Scholar]
- Feldman L., Frazier J. G., Sytkowski A. J. B-lymphocyte-derived burst-promoting activity is a pleiotropic erythroid colony-stimulating factor, E-CSF. Exp Hematol. 1992 Nov;20(10):1223–1228. [PubMed] [Google Scholar]
- Florini J. R., Ewton D. Z., Falen S. L., Van Wyk J. J. Biphasic concentration dependency of stimulation of myoblast differentiation by somatomedins. Am J Physiol. 1986 May;250(5 Pt 1):C771–C778. doi: 10.1152/ajpcell.1986.250.5.C771. [DOI] [PubMed] [Google Scholar]
- Fu Y. K., Arkins S., Wang B. S., Kelley K. W. A novel role of growth hormone and insulin-like growth factor-I. Priming neutrophils for superoxide anion secretion. J Immunol. 1991 Mar 1;146(5):1602–1608. [PubMed] [Google Scholar]
- Gibson L. F., Piktel D., Landreth K. S. Insulin-like growth factor-1 potentiates expansion of interleukin-7-dependent pro-B cells. Blood. 1993 Nov 15;82(10):3005–3011. [PubMed] [Google Scholar]
- Hooghe R., Delhase M., Vergani P., Malur A., Hooghe-Peters E. L. Growth hormone and prolactin are paracrine growth and differentiation factors in the haemopoietic system. Immunol Today. 1993 May;14(5):212–214. doi: 10.1016/0167-5699(93)90165-h. [DOI] [PubMed] [Google Scholar]
- Huang S., Terstappen L. W. Formation of haematopoietic microenvironment and haematopoietic stem cells from single human bone marrow stem cells. Nature. 1992 Dec 24;360(6406):745–749. doi: 10.1038/360745a0. [DOI] [PubMed] [Google Scholar]
- Humbel R. E. Insulin-like growth factors I and II. Eur J Biochem. 1990 Jul 5;190(3):445–462. doi: 10.1111/j.1432-1033.1990.tb15595.x. [DOI] [PubMed] [Google Scholar]
- Kiess W., Malozowski S., Gelato M., Butenand O., Doerr H., Crisp B., Eisl E., Maluish A., Belohradsky B. H. Lymphocyte subset distribution and natural killer activity in growth hormone deficiency before and during short-term treatment with growth hormone releasing hormone. Clin Immunol Immunopathol. 1988 Jul;48(1):85–94. doi: 10.1016/0090-1229(88)90159-6. [DOI] [PubMed] [Google Scholar]
- Kooijman R., Willems M., De Haas C. J., Rijkers G. T., Schuurmans A. L., Van Buul-Offers S. C., Heijnen C. J., Zegers B. J. Expression of type I insulin-like growth factor receptors on human peripheral blood mononuclear cells. Endocrinology. 1992 Nov;131(5):2244–2250. doi: 10.1210/endo.131.5.1425423. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Jelkmann W., Bauer C. A new candidate for the regulation of erythropoiesis. Insulin-like growth factor I. FEBS Lett. 1982 Nov 22;149(1):105–108. doi: 10.1016/0014-5793(82)81081-8. [DOI] [PubMed] [Google Scholar]
- Landreth K. S., Narayanan R., Dorshkind K. Insulin-like growth factor-I regulates pro-B cell differentiation. Blood. 1992 Sep 1;80(5):1207–1212. [PubMed] [Google Scholar]
- Leary A. G., Hirai Y., Kishimoto T., Clark S. C., Ogawa M. Survival of hemopoietic progenitors in the G0 period of the cell cycle does not require early hemopoietic regulators. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4535–4538. doi: 10.1073/pnas.86.12.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. P., Baker J., Perkins A. S., Robertson E. J., Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993 Oct 8;75(1):59–72. [PubMed] [Google Scholar]
- Merchav S., Tatarsky I., Hochberg Z. Enhancement of erythropoiesis in vitro by human growth hormone is mediated by insulin-like growth factor I. Br J Haematol. 1988 Nov;70(3):267–271. doi: 10.1111/j.1365-2141.1988.tb02480.x. [DOI] [PubMed] [Google Scholar]
- Merchav S., Tatarsky I., Hochberg Z. Enhancement of human granulopoiesis in vitro by biosynthetic insulin-like growth factor I/somatomedin C and human growth hormone. J Clin Invest. 1988 Mar;81(3):791–797. doi: 10.1172/JCI113385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. J., Tsarfaty G., Longo D. L. Growth hormone exerts hematopoietic growth-promoting effects in vivo and partially counteracts the myelosuppressive effects of azidothymidine. Blood. 1992 Sep 15;80(6):1443–1447. [PubMed] [Google Scholar]
- Nicola N. A. Hemopoietic cell growth factors and their receptors. Annu Rev Biochem. 1989;58:45–77. doi: 10.1146/annurev.bi.58.070189.000401. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Pepe M. G., Ginzton N. H., Lee P. D., Hintz R. L., Greenberg P. L. Receptor binding and mitogenic effects of insulin and insulinlike growth factors I and II for human myeloid leukemic cells. J Cell Physiol. 1987 Nov;133(2):219–227. doi: 10.1002/jcp.1041330204. [DOI] [PubMed] [Google Scholar]
- Pietrzkowski Z., Sell C., Lammers R., Ullrich A., Baserga R. Roles of insulinlike growth factor 1 (IGF-1) and the IGF-1 receptor in epidermal growth factor-stimulated growth of 3T3 cells. Mol Cell Biol. 1992 Sep;12(9):3883–3889. doi: 10.1128/mcb.12.9.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak M. Z., Hijiya N., Catani L., DeRiel K., Luger S. M., McGlave P., Gewirtz A. M. Acute- and chronic-phase chronic myelogenous leukemia colony-forming units are highly sensitive to the growth inhibitory effects of c-myb antisense oligodeoxynucleotides. Blood. 1992 Apr 15;79(8):1956–1961. [PubMed] [Google Scholar]
- Ratajczak M. Z., Luger S. M., DeRiel K., Abrahm J., Calabretta B., Gewirtz A. M. Role of the KIT protooncogene in normal and malignant human hematopoiesis. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1710–1714. doi: 10.1073/pnas.89.5.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak M. Z., Luger S. M., Gewirtz A. M. The c-kit proto-oncogene in normal and malignant human hematopoiesis. Int J Cell Cloning. 1992 Jul;10(4):205–214. doi: 10.1002/stem.5530100403. [DOI] [PubMed] [Google Scholar]
- Reiss K., Porcu P., Sell C., Pietrzkowski Z., Baserga R. The insulin-like growth factor 1 receptor is required for the proliferation of hemopoietic cells. Oncogene. 1992 Nov;7(11):2243–2248. [PubMed] [Google Scholar]
- Sanders M., Sorba S., Dainiak N. Insulin-like growth factors stimulate erythropoiesis in serum-substituted umbilical cord blood cultures. Exp Hematol. 1993 Jan;21(1):25–30. [PubMed] [Google Scholar]
- Sara V. R., Hall K. Insulin-like growth factors and their binding proteins. Physiol Rev. 1990 Jul;70(3):591–614. doi: 10.1152/physrev.1990.70.3.591. [DOI] [PubMed] [Google Scholar]
- Sawada K., Krantz S. B., Dessypris E. N., Koury S. T., Sawyer S. T. Human colony-forming units-erythroid do not require accessory cells, but do require direct interaction with insulin-like growth factor I and/or insulin for erythroid development. J Clin Invest. 1989 May;83(5):1701–1709. doi: 10.1172/JCI114070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C., Steiner T., Froesch E. R. Insulin-like growth factor I supports differentiation of cultured osteoblast-like cells. FEBS Lett. 1984 Jul 23;173(1):48–52. doi: 10.1016/0014-5793(84)81015-7. [DOI] [PubMed] [Google Scholar]
- Stuart C. A., Meehan R. T., Neale L. S., Cintron N. M., Furlanetto R. W. Insulin-like growth factor-I binds selectively to human peripheral blood monocytes and B-lymphocytes. J Clin Endocrinol Metab. 1991 May;72(5):1117–1122. doi: 10.1210/jcem-72-5-1117. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]