Abstract
This case report describes successful treatment of autoimmune autonomic ganglionopathy (AAG) in a 74-year-old woman by total plasma exchanges (PLEX) and rituximab. Two series of PLEX temporarily, but dramatically improved orthostatic intolerance and hypotension and baroreflex function, in a manner inversely related to ganglionic neuronal nicotinic receptor antibody titer. After rituximab treatment, the antibody titer was decreased modestly but persistently, and the patient had symptomatic and clinical laboratory evidence of continued benefit for at least 10 months, supporting the autoimmune pathogenesis of AAG.
Keywords: Autoimmune autonomic ganglionopathy, Pure autonomic failure, Rituximab, Plasma exchange
Introduction
Autoimmune autonomic ganglionopathy (AAG) is a recently recognized, rare, neurological disorder characterized by pandysautonomia, accompanied by elevated levels of antibodies to ganglionic neuronal nicotinic receptors (nAChRs) [1, 5]. In this disorder, deficient sympathetic noradrenergic activity manifests as orthostatic hypotension; deficient parasympathetic cholinergic activity as constipation, urinary retention, decreased salivation, decreased lacrimation, and impairment of the pupillary light reflex; and deficient sympathetic cholinergic activity as anhidrosis. Based on the clinical presentation of AAG it has been suggested that ganglionic nAChR antibodies disrupt cholinergic synaptic transmission in autonomic ganglia and lead to autonomic failure [1, 4, 5].
Here, we report a case of AAG in which two series of plasma exchanges (PLEX), separated by about 6 months, and one series of rituximab infusion were carried out, with sequential measurements of ganglionic nAChR levels and assessments of relationships of ganglionic nAChR levels with clinical and laboratory findings. As far as we know, this is the first report on rituximab treatment in a subject with AAG.
Case report
A 74-year-old woman was studied at the NIH Clinical Center, under two clinical research protocols approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke.
The patient was healthy and active in November 2004, when she presumably contracted a subacute viral illness characterized by malaise and diarrhea, causing her to be bedridden for several weeks. She developed diarrhea in January 2005, which persisted for several months. In May 2005, she noted the onset of acute episodes of weakness and presyncope that were found to be associated with orthostatic hypotension. These problems increased in frequency and severity. Combined midodrine, fludrocortisone, and prednisone improved these symptoms. Around July 2005, the patient began to note a dry mouth, which was treated successfully with pilocarpine. Treatment with pyridostigmine produced no effect. Prednisone was first prescribed in August 2005, for intense achiness at the back of the neck; the drug relieved this symptom. Lip biopsy in September 2005 revealed pathologic findings consistent with mild Sjogren’s syndrome. CT scanning showed a filling defect in the stomach, and endoscopy and biopsy of a circumferential mass demonstrated a benign tumor. Brain MRI depicted a pituitary mass presumed to be a microadenoma. A bone marrow biopsy was negative for amyloid.
Upon initial evaluation at the NIH in January 2006, the patient’s chief complaints were decreased mobility, dry mouth, and presyncope, which she felt were controlled moderately well with prednisone 10 mg daily, midodrine 2.5 mg twice daily, fludrocortisone 0.1 mg daily, liberal salt intake, levothyroxine 0.137 mg daily, and 5 mg pilocarpine twice daily. She no longer complained of diarrhea, though she did report urinary urgency, urinary frequency, nocturia, and insomnia. Physical examination showed a thin, elderly, pleasant woman who did not appear ill. The skin and oropharynx were dry. The pupils were equal, round, 5 mm in diameter, minimally reactive to light and minimally accommodating. There was a 2/6 intensity systolic murmur at the lower left sternal border. There was no bradykinesia or rigidity, and gait, sensation, cerebellar functions, and limb strength were normal.
The patient had abnormal beat-to-beat blood pressure (Finapres Medical Systems, Amsterdam, The Netherlands) responses to the Valsalva maneuver, consistent with baroreflex-sympathoneural failure. Baroreflex-cardiovagal gain, calculated from the slope of the relationship between cardiac interbeat interval (with one beat delay) and systolic blood pressure during Phase II of the Valsalva maneuver, was 0.05 ms/mm Hg (Fig. 1). Systolic and diastolic blood pressure decreased during passive tilting to 70° upright, by 97 and 34 mm Hg (Fig. 1), and heart rate did not change. These results, coupled with a small norepinephrine response to orthostasis (20 ng/mL), measured in antecubital venous plasma by liquid chromatography with electrochemical detection after batch alumina extraction as described elsewhere [1], confirmed neurogenic orthostatic hypotension, and a presumptive diagnosis of pure autonomic failure (PAF) was made. The patient had normal cardiac 6-[18F] fluorodopamine-derived radioactivity on positron emission tomography, consistent with intact cardiac noradrenergic innervation. The patient had no detectable sweat production in the quantitative sudomotor axon reflex test (QSART); 100 mg/mL of acetylcholine delivered by iontophoresis at 2.0 mA for 5 min, Atlas device and Q-Sweat software (WR Medical Electronics, Stillwater, MN, USA). At the time of the initial testing, the plasma level of ganglionic nAChR antibody, measured by a radioimmunoprecipitation assay, as described elsewhere [5], was 1.44 nmol/L (upper limit of normal 0.05 nmol/L). As a result of these findings, her diagnosis was changed to AAG.
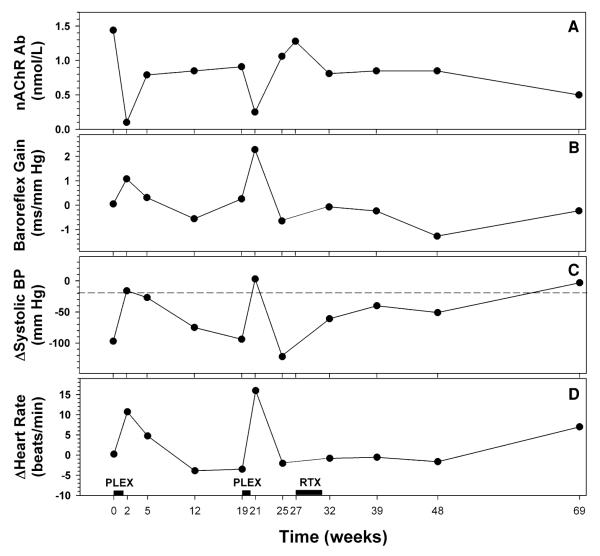
Fig. 1.
Concentration of ganglionic neuronal nicotinic receptor (nAChR) antibody in plasma (a), baroreflex-cardiovagal gain during the Valsalva maneuver (b), change in systolic blood pressure (BP) to orthostasis (c) and change in heart rate to orthostasis (d) in a patient with autoimmune autonomic ganglionopathy over 69 weeks of follow-up. A horizontal dashed line at −20 mm Hg in c indicates the diagnostic threshold for orthostatic hypotension. Therapeutic total plasma exchanges (PLEX) were performed over the course of about 10 days and rituximab (RTX) was administered in four infusions over 4 weeks
The patient enrolled in a clinical research protocol, designed to assess the effects of up to two sessions of PLEX, with frequent follow-up visits for autonomic function testing and blood sampling for levels of ganglionic nAChR antibody. Therapeutic PLEXs were performed every other day over the course of about 10 days (5 PLEX procedures in total per one session), in the Department of Transfusion Medicine at the NIH Clinical Center. PLEX consisted of continuous separation of blood cells from plasma by centrifugation (Spectra Apheresis System, Gambro Lakewood, CO, USA), and continuous return of the blood cells to the circulation in combination with a solution of 5% human serum albumin and saline in approximately 80:20 ratio. The extracorporeal circuit was anticoagulated with citrate at a whole blood to anticoagulant ratio of 13:1. Patient received a “pretreatment” bolus of 200 mL of 5% human serum albumin. The volume of exchange was one plasma volume (40–50 mL/kg of body weight) per procedure. Blood pressure and heart rate were monitored during and after PLEX.
During the first 25 weeks of the follow-up when two courses of PLEXs were performed, the patient was on 20–25 mg of prednisone daily. In January 2007 (week 27, Fig. 1), the patient’s private neurologist initiated treatment with rituximab (Rituxan™, Genentech, San Francisco, CA, USA). Four infusions, each consisting of 275 mg/m2, were administered over 4 weeks. In order to prevent a rituximab infusion reaction, 100 mg of prednisone was also administered. After the rituximab treatment, the prednisone dose was lowered gradually to 10 mg between January and June 2007 (week 27 to week 48), and then to 5 mg between June and September 2007 (week 48 to week 69). Prednisone was discontinued thereafter for at least the next 5 months. During the whole follow-up period the patient had relatively stable, borderline lower serum albumin levels ranging from 3.2 to 3.7 g/dL.
After both courses of total PLEX, performed at weeks 0 and 19, respectively, the patient had an improved pattern of beat-to-beat blood pressure and heart rate changes during and after Valsalva maneuver and an increase in baroreflex-cardiovagal gain (Fig. 1). Plasma norepinephrine response to orthostasis remained low at 61 ng/mL increase from baseline; however, PLEX ameliorated lightheadedness. One week after PLEX, orthostatic hypotension was no longer evident, and heart rate increased by 11 beats/min during orthostasis. The plasma titer of ganglionic nAChR antibody was significantly lower at 0.1 nmol/L (Fig. 1).
Thereafter, effects of PLEX on responses to the Valsalva maneuver and severity of orthostatic hypotension progressively worsened, and the antibody level increased (Fig. 1). A second series of PLEX was performed at week 19, with a similar rapid fall in antibody titer and improvement in baroreflex-cardiovagal gain and amelioration of orthostatic hypotension. Unlike the modest and delayed effect of the first PLEX on sweat production in the QSART, the second PLEX was followed by doubling of the sweat volume 1 week after the second PLEX. By 4 weeks after the second PLEX, however, symptoms returned, antibody levels more than quadrupled, and orthostatic intolerance and hypotension had returned.
After rituximab infusions, flu-like symptoms developed, including dizziness, drowsiness, and nausea. These gradually resolved. During the post-rituximab follow-up period orthostatic intolerance slowly improved, and the patient did not have orthostatic hypotension during follow-up for at least 42 weeks. Baroreflex-cardiovagal gain remained subnormal during the post-rituximab follow-up, and sweat production in the QSART was within the normal range for only two out of four tests in the post-rituximab period. Plasma levels of ganglionic nAChR antibody gradually decreased, to <50% of the initial value at the time of initiation of rituximab treatment. Plasma levels of ganglionic nAChR antibody lower than 0.6 nmol/L were associated with normal systolic blood pressure response to orthostasis (Fig. 2).
Fig. 2.
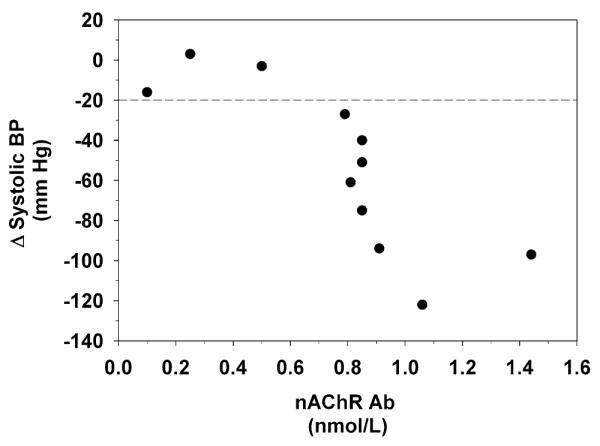
Changes in systolic blood pressure (BP) to orthostasis in relation to concentrations of ganglionic neuronal nicotinic receptor (nAChR) antibody in plasma of a patient with autoimmune autonomic ganglionopathy. A horizontal dashed line at −20 mm Hg indicates the diagnostic threshold for orthostatic hypotension
Discussion
The present findings lend further support to the hypothesis of an autoimmune pathogenetic mechanism in AAG, based on interference with nicotinic receptor function mediating ganglionic neurotransmission [4]. In line with others, our study confirms the dramatic, but transient effect of PLEX as a therapy for AAG [2]. Furthermore, we demonstrate that CD20+ cell depletion using rituximab, a specific monoclonal antibody, may provide a moderate, but sustainable improvement in clinical outcome enabling gradual prednisone withdrawal as well as reduction in plasma concentration of the putative pathogenic antibody. Improvement in blood pressure response to tilting was the major objective indicator of therapeutic effect of rituximab in our study. On the other hand, after rituximab treatment, we failed to detect a similar improvement in other objective indicators of baroreflex function such as baroreflex-cardiovagal gain. Limitations of rituximab treatment include high cost of the treatment compared to traditional immunosuppressive treatments, potential side effects and an inconvenience from repeated intravenous administration of the drug.
The relatively modest decrease in the antibody titer in the first 4 weeks after rituximab treatment contrasted with marked improvement in subjective symptoms and orthostatic hypotension in our patient. The paradoxical finding can be explained by cellular mechanisms of action of rituximab. Depletion of CD20-bearing B cells may significantly affect not only mature B cells, and differentiated immunoglobulin-secreting cells but also other immune cells [3]. Analogously, in AAG, the pathogenetic mechanism might not be solely limited to ganglionic nAChR antibody. Thus, an active role of other immune cells regulated by CD20+ B cells should be considered in the pathogenesis of AAG.
Autoimmune autonomic ganglionopathy is a severe and potentially treatable disorder; however, due to its rarity, clinical similarity with other forms of autonomic failure, and unavailability of diagnostic tools for routine, specific diagnosis, the condition typically is not diagnosed for many months after the patient seeks medical attention. Our patient carried a clinical diagnosis of PAF until 6-[18F]fluorodopamine PET scanning revealed intact cardiac sympathetic innervation. Neuroimaging combined with assay of ganglionic nAChR antibody seems to provide a robust diagnostic plan to diagnose AAG in patients who seem to have PAF.
The results during 18 months of follow-up in this case suggest that once the diagnosis of AAG has been made, relatively simple monitoring of baroreflex-cardiovagal gain during supine rest and blood pressure responses to orthostasis can track disease status. Values for these parameters mirrored spontaneous or therapy-induced fluctuations of ganglionic nAChR antibody levels as well as the patient’s symptoms.
An improvement in orthostatic hypotension symptoms observed after PLEX procedure can be theoretically attributed to acute changes in plasma volume since the procedure includes replacement of fluids and serum albumin. Although changes in plasma volume were not monitored in the study, relatively stable serum albumin concentrations during the follow-up period suggest that PLEXs had no significant effect on orthostatic hypotension symptoms.
In conclusion, total PLEX temporarily improved clinical and laboratory abnormalities in this patient with AAG. Rituximab resulted in minor decreases in antibody level and persistent symptomatic improvement over at least several months.
Acknowledgments
The authors acknowledge Dr. Kaho Wong, MD who administered rituximab to the patient. The authors thank clinical support personnel of the Clinical Neurocardiology Section, including Sandra Pechnik, RN, LaToya Sewell, CRNP, and Tereza Jenkins. The study was supported by the intramural research program of the NINDS.
Contributor Information
Richard Imrich, Clinical Neurocardiology Section, National Institute of Neurological Disorders and Stroke, Bethesda, MD 20892-1620, USA; Center for Molecular Medicine, Institute of Experimental Endocrinology, Vlarska 3-7, 83101 Bratislava, Slovakia.
Steven Vernino, Department of Neurology, University of Texas Southwestern Medical Center, Dallas, TX 75390-9036, USA.
Basil A. Eldadah, Clinical Neurocardiology Section, National Institute of Neurological Disorders and Stroke, Bethesda, MD 20892-1620, USA
Courtney Holmes, Clinical Neurocardiology Section, National Institute of Neurological Disorders and Stroke, Bethesda, MD 20892-1620, USA.
David S. Goldstein, Clinical Neurocardiology Section, National Institute of Neurological Disorders and Stroke, Bethesda, MD 20892-1620, USA
References
- 1.Goldstein DS, Holmes C, Dendi R, Li ST, Brentzel S, Vernino S. Pandysautonomia associated with impaired ganglionic neurotransmission and circulating antibody to the neuronal nicotinic receptor. Clin Auton Res. 2002;12:281–285. doi: 10.1007/s10286-002-0020-3. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder C, Vernino S, Birkenfeld AL, Tank J, Heusser K, Lipp A, Benter T, Lindschau C, Kettritz R, Luft FC, Jordan J. Plasma exchange for primary autoimmune autonomic failure. N Engl J Med. 2005;353:1585–1590. doi: 10.1056/NEJMoa051719. [DOI] [PubMed] [Google Scholar]
- 3.Stubgen JP. B cell-targeted therapy with rituximab and autoimmune neuromuscular disorders. J Neuroimmunol. 2008;204(1–2):1–12. doi: 10.1016/j.jneuroim.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Vernino S, Lindstrom J, Hopkins S, Wang Z, Low PA. Characterization of ganglionic acetylcholine receptor autoantibodies. J Neuroimmunol. 2008;197:63–69. doi: 10.1016/j.jneuroim.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343:847–855. doi: 10.1056/NEJM200009213431204. [DOI] [PubMed] [Google Scholar]