Abstract
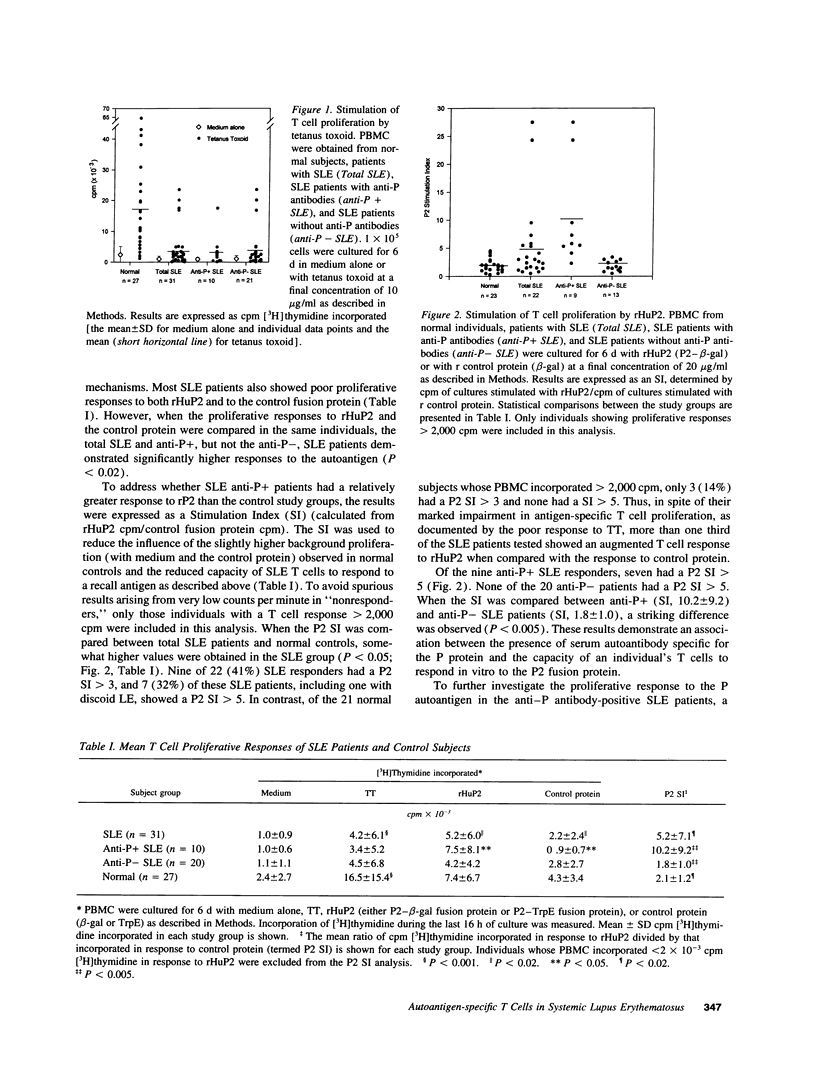
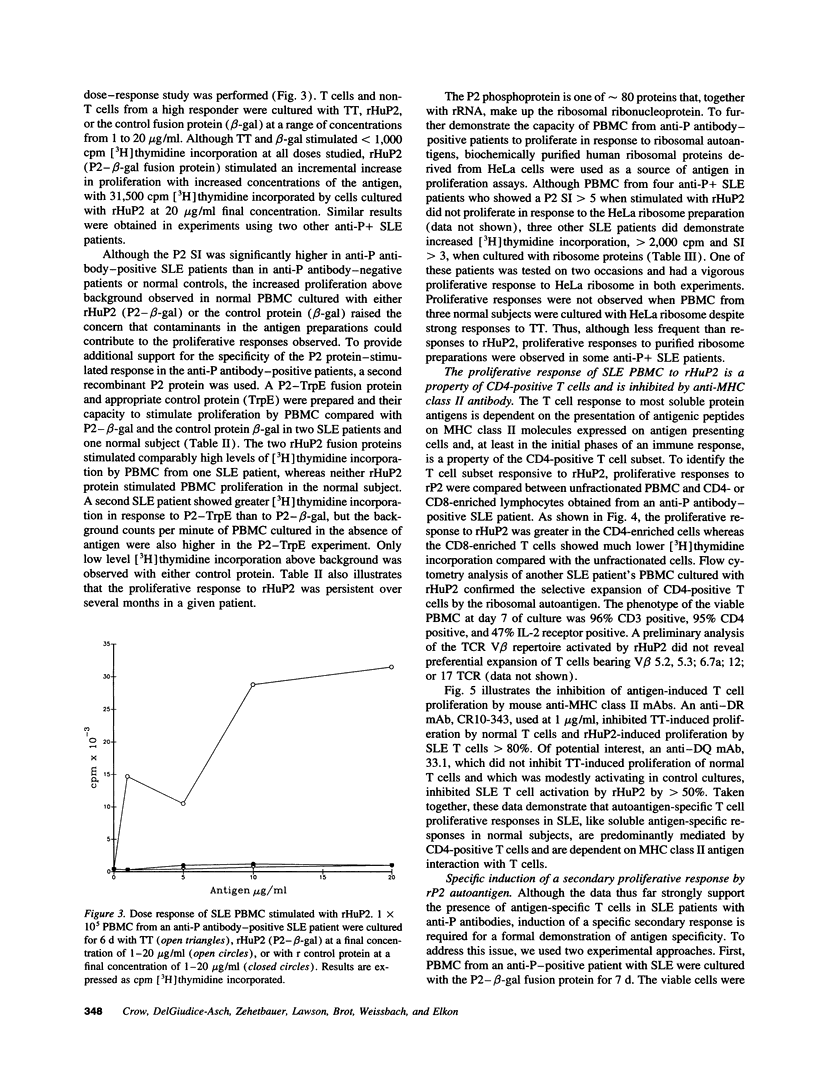
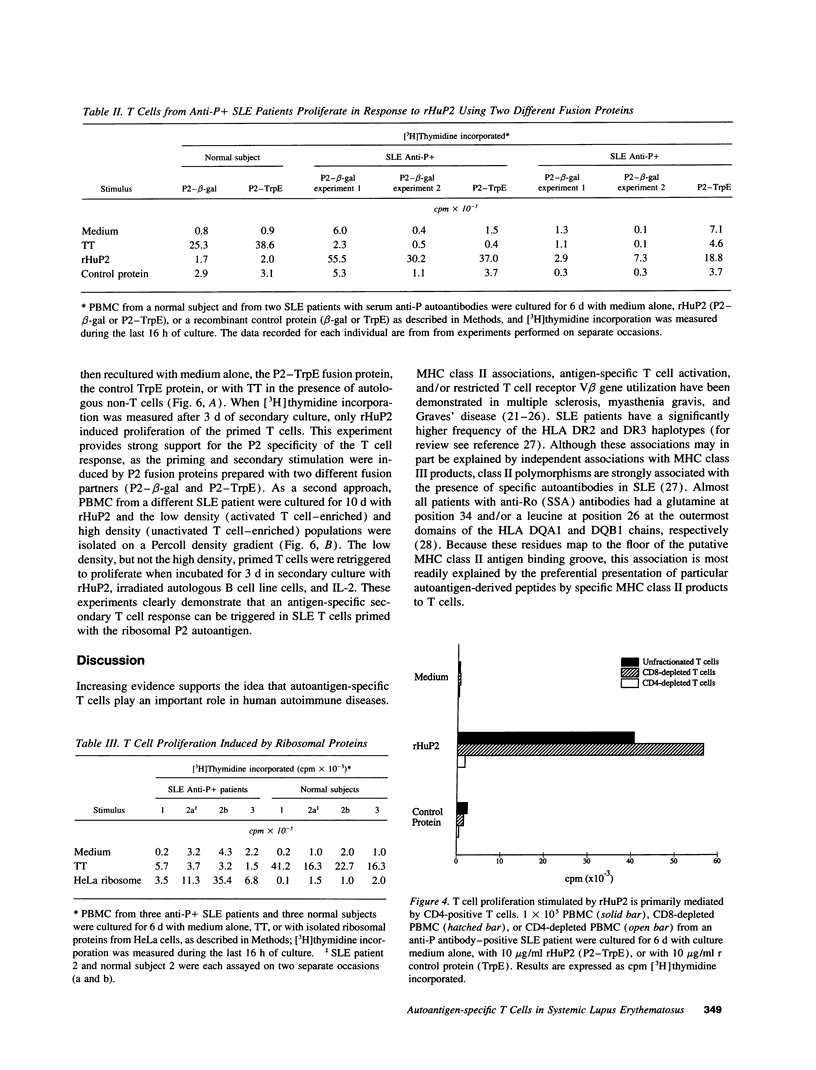
A role for helper T cells in the induction of pathogenic lupus autoantibodies is increasingly supported by data from studies of murine lupus and patients with systemic lupus erythematosus (SLE). However, the poor in vitro function of SLE T cells has hampered the identification and characterization of autoantigen-specific T cells. We used recombinant fusion proteins to study the T cell proliferative response of 31 lupus patients and 27 healthy subjects to a well-characterized SLE autoantigen, the ribosomal P2 protein. Although PBMC from SLE patients showed marked impairment in the proliferative response to the common recall antigen tetanus toxoid when compared with normal subjects, a significantly greater proportion of SLE patients (32%) than normal individuals (0%) showed a T cell response to a recombinant P2 fusion protein. When the SLE patients were subgrouped according to the presence of serum anti-P autoantibody, 7 of 10 anti-P antibody-positive patients, but 0 of 20 anti-P antibody-negative SLE patients, demonstrated > 2,000 cpm [3H]thymidine incorporation and a P2 stimulation index > 5. The specificity of the T cell proliferative response for the P2 protein was confirmed by studies using a second recombinant human P2 fusion protein and by the specific activation of P2-primed T cells by recombinant P2 in secondary cultures. Moreover, the T cell proliferative response to the P2 autoantigen was mediated by CD4-positive T cells and was inhibited by anti-MHC class II antibodies. These data demonstrate the presence of autoantigen-specific T helper cells in patients with SLE and suggest that these T cells drive the production of autoantibodies by B lymphocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Nakagawa T., Swanson M. S., Woodruff T. K., Dreyfuss G. mRNA polyadenylate-binding protein: gene isolation and sequencing and identification of a ribonucleoprotein consensus sequence. Mol Cell Biol. 1986 Aug;6(8):2932–2943. doi: 10.1128/mcb.6.8.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett F. C. Genetic aspects of human lupus. Clin Immunol Immunopathol. 1992 Apr;63(1):4–6. doi: 10.1016/0090-1229(92)90082-y. [DOI] [PubMed] [Google Scholar]
- Bonfa E., Elkon K. B. Clinical and serologic associations of the antiribosomal P protein antibody. Arthritis Rheum. 1986 Aug;29(8):981–985. doi: 10.1002/art.1780290806. [DOI] [PubMed] [Google Scholar]
- Bonfa E., Golombek S. J., Kaufman L. D., Skelly S., Weissbach H., Brot N., Elkon K. B. Association between lupus psychosis and anti-ribosomal P protein antibodies. N Engl J Med. 1987 Jul 30;317(5):265–271. doi: 10.1056/NEJM198707303170503. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carson D. A. The specificity of anti-DNA antibodies in systemic lupus erythematosus. J Immunol. 1991 Jan 1;146(1):1–2. [PubMed] [Google Scholar]
- Chicz R. M., Urban R. G., Gorga J. C., Vignali D. A., Lane W. S., Strominger J. L. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993 Jul 1;178(1):27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J. L., Brot N., Weissbach H., Elkon K. Lupus antiribosomal P antisera contain antibodies to a small fragment of 28S rRNA located in the proposed ribosomal GTPase center. J Exp Med. 1991 Sep 1;174(3):507–514. doi: 10.1084/jem.174.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A., Manheimer-Lory A., Aranow C., Peterson R., Hannigan N., Diamond B. Molecular characterization of a somatically mutated anti-DNA antibody bearing two systemic lupus erythematosus-related idiotypes. J Clin Invest. 1990 May;85(5):1401–1409. doi: 10.1172/JCI114584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K. B., Bonfa E., Brot N. Antiribosomal antibodies in systemic lupus erythematosus. Rheum Dis Clin North Am. 1992 May;18(2):377–390. [PubMed] [Google Scholar]
- Elkon K. B., Bonfa E., Llovet R., Eisenberg R. A. Association between anti-Sm and anti-ribosomal P protein autoantibodies in human systemic lupus erythematosus and MRL/lpr mice. J Immunol. 1989 Sep 1;143(5):1549–1554. [PubMed] [Google Scholar]
- Elkon K. B., Hines J. J., Chu J. L., Parnassa A. Epitope mapping of recombinant HeLa SmB and B' peptides obtained by the polymerase chain reaction. J Immunol. 1990 Jul 15;145(2):636–643. [PubMed] [Google Scholar]
- Elkon K. B., Parnassa A. P., Foster C. L. Lupus autoantibodies target ribosomal P proteins. J Exp Med. 1985 Aug 1;162(2):459–471. doi: 10.1084/jem.162.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatenejad S., Mamula M. J., Craft J. Role of intermolecular/intrastructural B- and T-cell determinants in the diversification of autoantibodies to ribonucleoprotein particles. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):12010–12014. doi: 10.1073/pnas.90.24.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. M., Crow M. K., Tumang J. R., Tumang M., Xu Y. Q., Hodtsev A. S., Cole B. C., Posnett D. N. Characterization of human T cells reactive with the Mycoplasma arthritidis-derived superantigen (MAM): generation of a monoclonal antibody against V beta 17, the T cell receptor gene product expressed by a large fraction of MAM-reactive human T cells. J Exp Med. 1991 Oct 1;174(4):891–900. doi: 10.1084/jem.174.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. M., Posnett D. N., Tumang J. R., Cole B. C., Crow M. K. A potential role for microbial superantigens in the pathogenesis of systemic autoimmune disease. Arthritis Rheum. 1991 Apr;34(4):468–480. doi: 10.1002/art.1780340412. [DOI] [PubMed] [Google Scholar]
- Gharavi A. E., Chu J. L., Elkon K. B. Autoantibodies to intracellular proteins in human systemic lupus erythematosus are not due to random polyclonal B cell activation. Arthritis Rheum. 1988 Nov;31(11):1337–1345. doi: 10.1002/art.1780311101. [DOI] [PubMed] [Google Scholar]
- Gleichmann E., Van Elven E. H., Van der Veen J. P. A systemic lupus erythematosus (SLE)-like disease in mice induced by abnormal T-B cell cooperation. Preferential formation of autoantibodies characteristic of SLE. Eur J Immunol. 1982 Feb;12(2):152–159. doi: 10.1002/eji.1830120210. [DOI] [PubMed] [Google Scholar]
- Gottlieb A. B., Lahita R. G., Chiorazzi N., Kunkel H. G. Immune function in systemic lupus erythematosus. Impairment of in vitro T-cell proliferation and in vivo antibody response to exogenous antigen. J Clin Invest. 1979 May;63(5):885–892. doi: 10.1172/JCI109388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J. A. The lupus autoantigens and the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1986 Apr;29(4):457–460. doi: 10.1002/art.1780290401. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Chu S. X., DeAizpurua H. J., Graham M., Honeyman M. C., Colman P. G. Islet-reactive T cells are a marker of preclinical insulin-dependent diabetes. J Clin Invest. 1992 Apr;89(4):1161–1165. doi: 10.1172/JCI115698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R. W., Takeda Y., Sharp G. C., Lee D. R., Hill D. L., Kaneoka H., Caldwell C. W. Human T cell clones reactive against U-small nuclear ribonucleoprotein autoantigens from connective tissue disease patients and healthy individuals. J Immunol. 1993 Dec 1;151(11):6460–6469. [PubMed] [Google Scholar]
- Koerner T. J., Hill J. E., Myers A. M., Tzagoloff A. High-expression vectors with multiple cloning sites for construction of trpE fusion genes: pATH vectors. Methods Enzymol. 1991;194:477–490. doi: 10.1016/0076-6879(91)94036-c. [DOI] [PubMed] [Google Scholar]
- Kuntz M. M., Innes J. B., Weksler M. E. The cellular basis of the impaired autologous mixed lymphocyte reaction in patients with systemic lupus erythematosus. J Clin Invest. 1979 Jan;63(1):151–153. doi: 10.1172/JCI109270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann P. V., Forsthuber T., Miller A., Sercarz E. E. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992 Jul 9;358(6382):155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- Liblau R., Tournier-Lasserve E., Maciazek J., Dumas G., Siffert O., Hashim G., Bach M. A. T cell response to myelin basic protein epitopes in multiple sclerosis patients and healthy subjects. Eur J Immunol. 1991 Jun;21(6):1391–1395. doi: 10.1002/eji.1830210610. [DOI] [PubMed] [Google Scholar]
- Link H., Olsson O., Sun J., Wang W. Z., Andersson G., Ekre H. P., Brenner T., Abramsky O., Olsson T. Acetylcholine receptor-reactive T and B cells in myasthenia gravis and controls. J Clin Invest. 1991 Jun;87(6):2191–2196. doi: 10.1172/JCI115253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker-Israeli M., Bakke A. C., Kitridou R. C., Gendler S., Gillis S., Horwitz D. A. Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE). J Immunol. 1983 Jun;130(6):2651–2655. [PubMed] [Google Scholar]
- Magsaam J., Gharavi A. E., Parnassa A. P., Weissbach H., Brot N., Elkon K. B. Quantification of lupus anti-ribosome P antibodies using a recombinant P2 fusion protein and determination of the predicted amino acid sequence of the autoantigen in patients' mononuclear cells. Clin Exp Immunol. 1989 May;76(2):165–171. [PMC free article] [PubMed] [Google Scholar]
- Mohan C., Adams S., Stanik V., Datta S. K. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993 May 1;177(5):1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. M., Cram D. S., Coppel R. L., Harrison L. C. T-cell epitopes on the 70-kDa protein of the (U1)RNP complex in autoimmune rheumatologic disorders. J Autoimmun. 1990 Dec;3(6):747–757. doi: 10.1016/s0896-8411(05)80041-1. [DOI] [PubMed] [Google Scholar]
- Okubo M., Yamamoto K., Kato T., Matsuura N., Nishimaki T., Kasukawa R., Ito K., Mizushima Y., Nishioka K. Detection and epitope analysis of autoantigen-reactive T cells to the U1-small nuclear ribonucleoprotein A protein in autoimmune disease patients. J Immunol. 1993 Jul 15;151(2):1108–1115. [PubMed] [Google Scholar]
- Pham B. N., Prin L., Gosset D., Hatron P. Y., Devulder B., Capron A., Dessaint J. P. T lymphocyte activation in systemic lupus erythematosus analysed by proliferative response to nucleoplasmic proteins on nitrocellulose immunoblots. Clin Exp Immunol. 1989 Aug;77(2):168–174. [PMC free article] [PubMed] [Google Scholar]
- Protti M. P., Manfredi A. A., Straub C., Wu X. D., Howard J. F., Jr, Conti-Tronconi B. M. Use of synthetic peptides to establish anti-human acetylcholine receptor CD4+ cell lines from myasthenia gravis patients. J Immunol. 1990 Mar 1;144(5):1711–1720. [PubMed] [Google Scholar]
- Rajagopalan S., Zordan T., Tsokos G. C., Datta S. K. Pathogenic anti-DNA autoantibody-inducing T helper cell lines from patients with active lupus nephritis: isolation of CD4-8- T helper cell lines that express the gamma delta T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7020–7024. doi: 10.1073/pnas.87.18.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reveille J. D., Macleod M. J., Whittington K., Arnett F. C. Specific amino acid residues in the second hypervariable region of HLA-DQA1 and DQB1 chain genes promote the Ro (SS-A)/La (SS-B) autoantibody responses. J Immunol. 1991 Jun 1;146(11):3871–3876. [PubMed] [Google Scholar]
- Sun J. B., Harcourt G., Wang Z. Y., Hawke S., Olsson T., Fredrikson S., Link H. T cell responses to human recombinant acetylcholine receptor-alpha subunit in myasthenia gravis and controls. Eur J Immunol. 1992 Jun;22(6):1553–1559. doi: 10.1002/eji.1830220631. [DOI] [PubMed] [Google Scholar]
- Sun J. B., Olsson T., Wang W. Z., Xiao B. G., Kostulas V., Fredrikson S., Ekre H. P., Link H. Autoreactive T and B cells responding to myelin proteolipid protein in multiple sclerosis and controls. Eur J Immunol. 1991 Jun;21(6):1461–1468. doi: 10.1002/eji.1830210620. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Saiki O., Negoro S., Igarashi T., Kuritani T., Hara H., Suemura M., Kishimoto S. Decreased expression of interleukin-2 binding molecules (p70/75) in T cells from patients with systemic lupus erythematosus. Arthritis Rheum. 1989 May;32(5):552–559. doi: 10.1002/anr.1780320507. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Uchiumi T., Traut R. R., Elkon K., Kominami R. A human autoantibody specific for a unique conserved region of 28 S ribosomal RNA inhibits the interaction of elongation factors 1 alpha and 2 with ribosomes. J Biol Chem. 1991 Feb 5;266(4):2054–2062. [PubMed] [Google Scholar]



