Abstract
The α and β tubulins compose the microtubule cytoskeleton which is involved in many cellular processes such as vesicular transport. The photoreceptor cells in the retina are neurons specialized for phototransduction. Here we report a novel interaction between tubulin and the photoreceptor cGMP phosphodiesterase (PDE6) gamma subunit (PDEγ). The specificity and molecular details of the PDEγ:tubulin interaction were analyzed through the experiments of pull down, microtubule co-sedimentation, and NMR spectroscopy. The tubulin-interacting site was identified to be in the PDEγ C-terminal I67-G85 region, and the interaction interface appeared to be distinct from those with the other PDEγ targets in phototransduction. We also observed that PDEγ interacted with tubulin in a GTP-dependent manner. Our findings offer implications for non-phototransduction role(s) of PDEγ in the photoreceptor neurons.
Keywords: Photoreceptor cells, tubulin/microtubules, PDE6, PDEγ
Microtubules, a major component of the cytoskeleton, participate in numerous cellular processes ranging from cell division, organelle positioning, intracellular transport, to neuronal differentiation [5]. They usually consist of laterally associated protofilaments, each made up of α/β-tubulin dimers that are able to self-assemble in a GTP-dependent way. There are a large number of tubulin/microtubule-interacting proteins that do not necessarily share sequence homology or structural similarity. While many of them regulate microtubule stability/dynamics, some undertake intracellular protein transport such as kinesins and dyneins. Noticeably, many of the microtubule-associated proteins contain intrinsically disordered domains, for example, MAP2 and tau [4], doublecortin and RP1 [12], TPPP/p25 [14], α-synuclein [21], and stathmin [19]. In this study, we have identified another intrinsically disordered tubulin/microtubule-interacting protein, the γ-subunit (PDEγ) of the photoreceptor cGMP phosphodiesterase (PDE6) [8].
The rod photoreceptor PDE6 is composed of two similar catalytic subunits (PDEαβ) and two identical inhibitory PDEγ subunits. PDEγ is a small protein of 87 amino acids containing two distinct functional domains: the positively charged central domain (residues G19-G49) and a negatively charged but relatively hydrophobic C-terminal domain (T62-I87) (for review see [8]). The canonical function of PDEγ in phototransduction has been well defined. PDEγ keeps PDE6 inactive in the dark but regulates the turn-on as well as turn-off of visual signaling upon photoresponse, via interactions with PDEαβ, the transducin α subunit (Gαt) and the regulator of G protein signaling (RGS9-1). Crystal structures revealed distinct PDEγ C-terminal interactions with the chimeric PDE5/6 catalytic domain [2], Gαt and the RGS9-1 catalytic core [16]. An NMR study indicated that some structural elements for interacting with these targets were preconfigured in the free PDEγ molecules in solution although PDEγ was overall disordered [18].
Consistent with the feature of intrinsically disordered proteins that they can interact with distinct partners to achieve various functions, increasing evidence implicates non-phototransduction PDEγ targets [8]. A recent study reported that the N-terminal proline rich region of PDEγ interacts with PACSIN, suggesting a possible role of PDEγ in endocytosis in the photoreceptor cell [10]. We were therefore motivated to find additional PDEγ-interacting proteins in the retinal photoreceptors.
Methods
Bovine and mouse retinal homogenates were prepared according to the method described previously [6]. Biotinylated PDEγ (Btn-PDEγ) was generated by covalently linking maleimide-PEO2-biotin (Pierce) to the single cysteine placed at the PDEγ N-terminus. The peptide Btn-Bz30 containing a PDEγ N-terminal polycationic sequence (BtnG19-Q32) was custom-synthesized at the University of Wisconsin Peptide Synthesis Facility. Btn-CytC (cytochrome C) and Btn-BSA were prepared by first reacting maleimide-PEO2-biotin with pure proteins and then removing the unreacted biotin molecules with a G-50 spin column (Amersham).
Pull down from retinal homogenates was performed using the streptavidin beads (Pierce) pre-bound with Btn-PDEγ. The beads were first incubated with retinal homogenates for 1 h at 4°C in buffer A (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1mM EDTA, 10 mM MgSO4, 50 μM AlCl3, 30 mM NaF, and 50 μM GDP), and then washed 3× with the same buffer. Proteins on the beads were eluted using the SDS/DTT sample buffer and resolved on a 15% SDS gel. In the control, equal amounts of retina homogenates were incubated with the streptavidin beads with no Btn-PDEγ bound.
Western blotting was performed with antibody dilutions as follows: Anti-PDEα (Affinity Bioreagents), 1,000 fold; anti-tubulin-α (Sigma-Aldrich), 10,000 fold; anti-Gαt (Affinity Bioreagents), 5,000 fold.
For pulling down microtubules, purified bovine brain tubulin (Cytoskeleton, purity >99%) was used. Tubulin polymerization was performed according to the manufacturer’s instruction, and Taxol was added to stabilize microtubules. For each pull down reaction, 5 μg of microtubules was added to 1 μl of streptavidin beads with 2 μg Btn-PDEγ pre-bound. Following incubation of the reactions for 30 min at room temperature in the BST01 buffer (80 mM PIPES, pH 7.0, 1 mM EGTA, 1 mM MgCl2) supplemented with AlF4−-GDP, the beads were washed 3× with 50 μl of buffer each time.
Microtubule co-sedimentation assays were performed according to the published method [22] with minor modifications. In each reaction, 20 μg of microtubules was incubated with 2 μg of PDEγ for 30 min at room temperature in Buffer A. Microtubules were pelleted by centrifugation at 20,000 ×g for 30 min at room temperature, and then washed twice by repeating centrifugation and removal of the supernatants. The protein pellets were resolved on a SDS gel.
NMR analysis of the tubulin-PDEγ interactions was performed based on the published methods [12, 18]. 15N-labeled PDEγ was prepared as previously described [18]. The NMR experiments were performed at 20°C using a 600-MHz Varian Inova spectrometer equipped with 1H, 15N, and 13C triple-resonance cryogenic probe at the National Magnetic Resonance Facility at Madison, WI (NMRFAM). [1H,15N]-HSQC (heteronuclear single quantum correlation) spectra were first collected for 30 μM 15N-labeled free PDEγ in the buffer containing 10 mM PIPES, 1 mM EGTA, 1 mM Mg2+, 90% H2O/10%D2O, pH 6.2. The data collection was repeated under the same experimental conditions following addition of 10 μM tubulin dimer and 1 mM GTP or GDP.
Results
We have observed a novel PDEγ:tubulin interaction. This finding resulted from the effort to identify additional PDEγ targets in the photoreceptor cells through pull down experiments using Btn-PDEγ (Figure 1A, B, C, D, E).
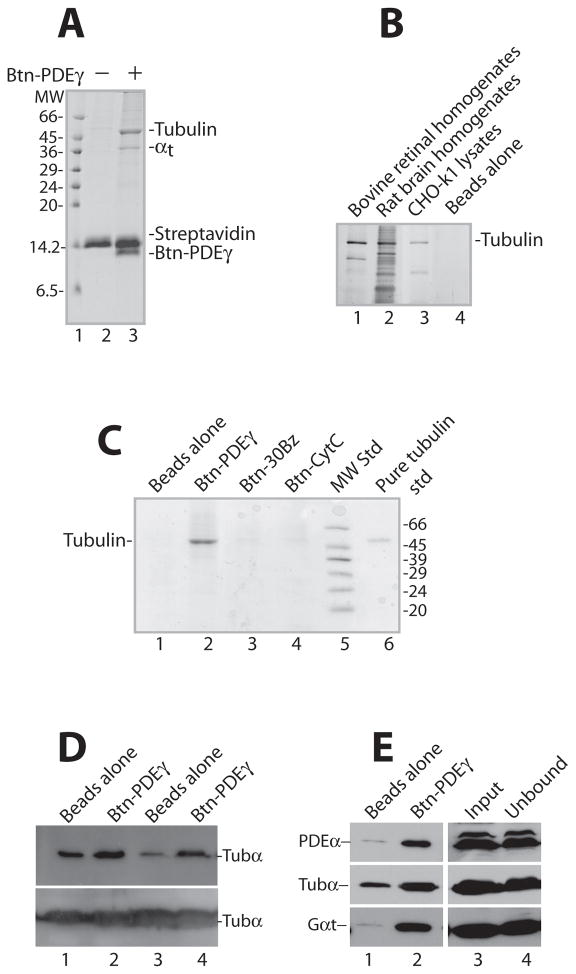
Figure 1. Specific pull down of tubulin by PDEγ from bovine retinal homogenates.
Shown are Coomassie-stained SDS gels (A, B, C) and Western blots (D, E), each is a representative of 2–4 similar experiments. In each reaction pull down from 100 μg retinal homogenates was performed using 2 μg (or otherwise stated) of Btn-PDEγ immobilized on 1 μl streptavidin beads.
A. The tubulin pull down by Btn-PDEγ from retinal homogenates appears as a prominent band of ~55 kDa (lane 3). Lane 2 is the control with no Btn-PDEγ. MW, molecular weight. B. Tubulin was pulled down by Btn-PDEγ from bovine retinal homogenates (lane 1), rat brain homogenates (lane 2), as well as CHO-k1 cell lysates (lane 3). The gel was silver-stained using the Pierce SilverSNAP Stain kit II. C. Btn-PDEγ on the streptavidin beads pulled down tubulin specifically from retinal homogenates (lane 2), as compared to the beads with no Btn-PDEγ (lane 1), the beads bound with Btn-30Bz (lane 3), and the beads bound with Btn-CytC (lane 4). D. Btn-PDEγ specifically pulled down tubulin from both bovine (see lanes 1and 2) and mouse (see lanes 3 and 4) retinal homogenates, as revealed by immunoblotting. The lower panel shows unbound tubulin in the supernatants. E. Immunoblotting of PDEα, tubulin-α (tubα), and transducin-α (Gαt) in the pull down from bovine retinal homogenates. For each reaction 6 μg of Btn-PDEγ and 2 μl of streptavidin beads were used. Lane 1 is the Btn-PDEγ minus control; lane 2 is pull down on the Btn-PDEγ beads; lane 3 is the homogenates used for each reaction; lane 4 is the unbound protein in the supernatant. Semi-quantitation of tubulin pull down relative to input (100%, lane 3): 6.7%, 34.4%, and 58.2% in lanes 1, 2, and 4, respectively.
The major pull down from bovine retinal homogenates appeared as a prominent band of ~55 kDa (A, lane 3; C, lane 2). N-terminal micro-sequencing of this band resulted in two sequences: MRECISIH and MREIVHIQ. Blast search indicated that these sequences match the bovine tubulin α and β subunits, respectively. Apparently, this 55 kDa band contained both α and β-tubulin because these two subunits form a constitutive heterodimer [5].
The specificity of the observed tubulin pull down is manifested by the following facts: 1) There was no prominent tubulin pull down in the control, in which no Btn-PDEγ was bound on the beads (A, lane 2; B, lane 4; C, lane 1). 2) Tubulin was not pulled down by Btn-30Bz (lane 3 in C), indicating that tubulin was pulled down by a specific PDEγ sequence other than the N-terminal polycationic G19-Q32 region. In contrast, arrestin in photoreceptor cells is reported to bind microtubules with its positively charged surface [9]. 3) Tubulin was not pulled down by Btn-CytC either (lane 4 in C), which like PDEγ is also a positively charged protein with an isoelectric point at pH 9.25. This result together with the lack of pull down by Btn-30Bz argues against the possibility that tubulin was pulled down merely due to a nonspecific charge effect. 4) While tubulin is abundant in the retina, actin, another abundant cytoskeleton protein (42 kDa), was not efficiently pulled down (Lane 3 in A). 5) PDEαβ and Gαt, however, were both pulled down, as confirmed by Western blotting (Figure 1E). This result served as a positive control because PDEαβ and Gαt are well-documented PDEγ-interacting proteins [8]. It is unlikely that PDEγ pulled down tubulin indirectly by binding to Gαt because Gαt does not interact with tubulin although Gαs, Gαi and Gαq do interact [3]. 6) In addition, Btn-PDEγ also readily pulled down tubulin from mouse retinal homogenates (Figure 1D, lane 4), rat brain homogenates and CHO-k1 cell lysates (Figure 1B), further supporting a specific PDEγ-tubulin interaction.
We then further confirmed the specific PDEγ:tubulin interaction using pure proteins. As seen in Figure 2A, in comparison to the background control (lane 4), Btn-PDEγ readily pulled down microtubules which were prepared with the pure bovine brain tubulin (lane 2). Btn-BSA which is irrelevant to PDEγ, however, pulled down microtubules only to the background level (lane 6). Btn-CytC did not pull down microtubules efficiently either (lane 5). This is in agreement with the data obtained with retinal homogenates (Figure 1C, lane 4), further eliminating a possibility of nonspecific charge effect in the observed PDEγ:tubulin interaction. Remarkably, when added as competitors, the C-terminal PDEγ peptide PDEγ62-87 but not the N-terminal peptide PDEγ1-61, significantly reduced microtubule pull down (Figure 2B and lane 3 in A). This is also in agreement with the observation that the PDEγ central peptide Btn-30Bz was not able to pull down tubulin from retinal homogenates (Figure 1C, lane 3), suggesting that the PDEγ C-terminal domain was responsible for the observed PDEγ-tubulin interaction. Thus the PDEγ domain-dependence of this interaction shows an additional layer of specificity.
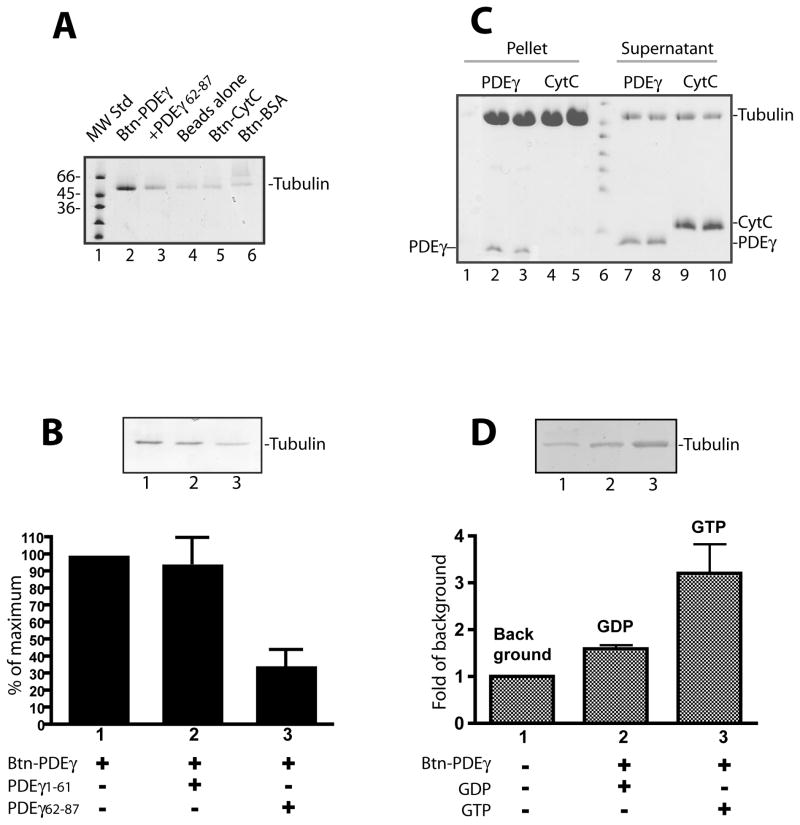
Figure 2. Specific PDEγ:tubulin interaction revealed by assays using pure proteins.
Streptavidin beads pull down assays (A, B, D) or microtubule co-sedimentation assay (C) were performed using pure tubulin proteins.
A. Btn-PDEγ pulled down microtubules specifically (lane 2) as compared with various controls including the beads with no Btn-PDEγ bound (lane 4), the beads with Btn-CytC bound (lane 5), and the beads with Btn-BSA bound (lane 6). In lane 3, the PDEγ C-terminal peptide PDEγ62-87 in a 20 fold molar excess over Btn-PDEγ was added. B. Microtubules pull down by Btn-PDEγ on the streptavidin beads (lane 1) was reduced in the presence of PDEγ62-87 (lane 2) but not PDEγ1-61 (lane 3) in a 20 fold molar excess. Pull down was quantitated as percent of the Coomassie staining in the absence of PDEγ peptides (lane 1). Error bar is a standard deviation from 3 separated experiments. Student’s t-tests indicate that the difference between bars 2 and 1 is insignificant (p>0.5); whereas the difference between bars 3 and 1 is very significant (p<0.1). C. Co-sedimented proteins are shown in lanes 2–5; the supernatants of the corresponding conditions are shown in lanes 7–10. Each condition is duplicates except lane 1, which is the control of PDEγ pellet from the reaction with no microtubules. D. GTP dependence of tubulin pull down by Btn-PDEγ-beads. Tubulin (9 μg) was incubated with beads alone (lane 1) or beads prebound with 1 μg Btn-PDEγ for 1 hr at room temperature in the buffer containing 20 mM HEPES, pH 7.4, 120 mM NaCl, and 5 mM MgCl2, in the presence of 2 mM GDP (lane 2) or 2 mM GTP (lane 3). Pull down was quantitated as fold of the Coomassie staining in the control (lane 1). Error bar is a standard deviation of 2 separate experiments. The difference between the GDP condition and the GTP condition is significance (p= 0.207).
One useful feature of tubulin is that the polymerized form (microtubules) can be readily pelleted down by centrifugation, and appears as visible glassy pellet at the bottom of the micro-centrifuge tube (Cytoskeleton Inc.). Thus the normally formed microtubule matrix is distinguishable from the opaque appearance of possible protein precipitations. Taking advantage of this property, we have further characterized the specificity of the PDEγ-tubulin interaction by co-sedimentation assays. Consistent with the Btn-PDEγ pull down results (Figure 2A), PDEγ co-sedimented with microtubules (Figure 2C, lanes 2 and 3); CytC however, did not (lanes 4 and 5). PDEγ itself as a highly soluble protein was not pelleted in the absence of microtubules (lane 1).
These pull down and co-sedimentation experiments together indicated that tubulin interacted with PDEγ specifically, probably with its C-terminal domain.
Since tubulin is a GTP/GDP binding protein, we examined a possible GTP dependence of the PDEγ:tubulin interaction by pull down assays and NMR spectroscopy. As shown in Figure 2D, while Btn-PDEγ pulled down tubulin 1.6 fold over the background in the presence of GDP, the pull down was 3.2 fold over the background in the presence of GTP, indicating a GTP dependence of the PDEγ:tubulin interaction.
This feature was also revealed by NMR spectroscopy, a method that can provide molecular details in PDEγ interactions [18]. Identification of residues that exhibit a large change in NMR chemical shift or peak intensity upon complex formation offers an informative approach for mapping the protein-protein interaction interface [12, 18]. We have therefore performed the NMR titrations on full-length 15N-labeled PDEγ with pure tubulin (Figure 3). The 1H, 15N-HSQC spectra were collected for free PDEγ (black), PDEγ in the presence of tubulin and GTP (blue), as well as PDEγ in the presence of tubulin and GDP (red). The NMR peaks were assigned based on the published results [18]. The residues of PDEγ in contact with tubulin were identified by analysis of signal broadening.
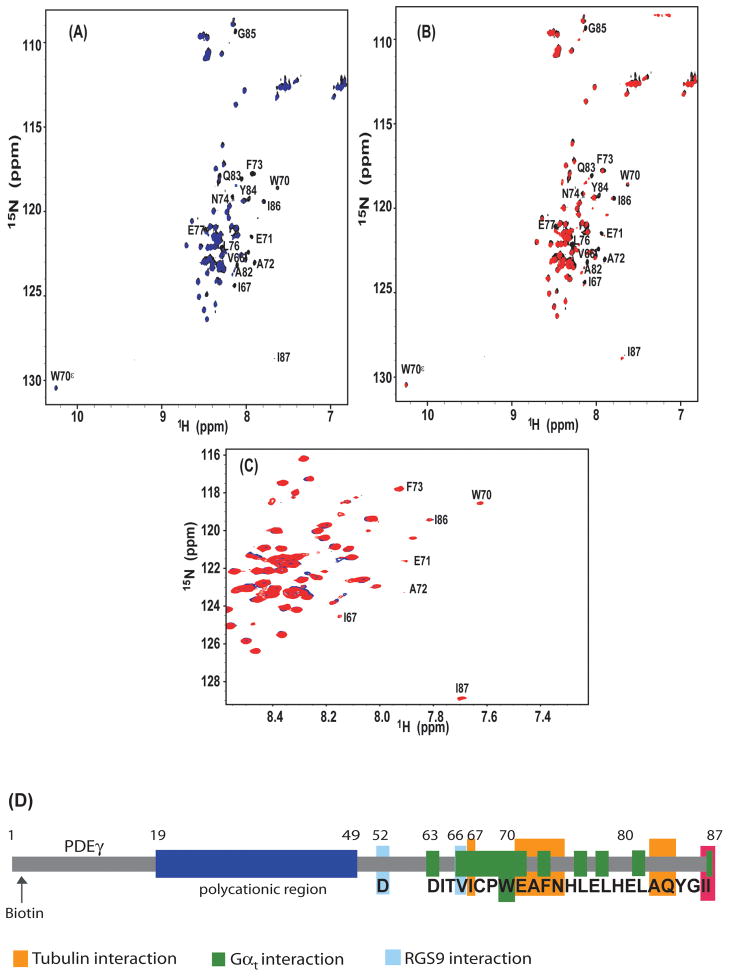
Figure 3. NMR HSQC spectra indicating the PDEγ:tubulin interaction.
The [1H,15N]-HSQC spectra were collected on 30 μM 15N-labeled PDEγ, in the absence (black) or presence of 10 μM tubulin dimer in the buffer containing 10 mM PIPES, 1 mM EGTA, 1 mM Mg2+, 90% H2O/10%D2O, pH 6.2, including either 1 mM GTP (blue) or 1 mM GDP (red).
A. Overlay of the HSQC spectra of free 15N-PDEγ (black) and 15N-PDEγ in the presence of tubulin-GTP (blue). The cross peaks corresponding to the indole group of W70 is denoted as W70ε. B. Overlay of the HSQC spectra of free 15N-PDEγ (black) and 15N-PDEγ in the presence of tubulin-GDP (red). C. Overlay of the HSQC spectra of 15N-PDEγ+tubulin-GTP (blue) and 15N-PDEγ+tubulin-GDP (red). D. A diagram of PDEγ interactions with different partners. The PDEγ residues that showed most significant interactions with tubulin-GTP are highlighted in orange. The polycationic region is shown in blue. I86 and I87 are labeled red. The Gαt-interacting (green) and RGS9-interacting (cyan) residues are shown based on the crystal structure including the C-terminal half of the PDEγ molecule [16].
Inspection of the NMR spectra indicated that, whereas most of the NMR peaks retained nearly identical intensities upon addition of tubulin in the presence of GTP, some PDEγ residues experienced significant signal loss (Figure 3A). When mapped to the sequence of PDEγ, these residues were located to the PDEγ C-terminal I67-G85 region. This is in accord with the observation that the PDEγ C-terminal peptide PDEγ62-87 but not the N-terminal peptide PDEγ1-61 eliminated the tubulin pull down (Figure 2B). Residues that displayed most prominent signal broadening included I67, E71, A72, F73, N74, A82, and Q83. Interestingly, these residues are not critically important for either the Gαt GTPase stimulation [17] or PDEαβ inhibition [7]. By contrast, residues I86 and I87, which are key for the inhibitory PDEγ interaction with the PDE6 catalytic domain [2, 7], exhibited minimum signal broadening. Meanwhile, the indole group of W70, which is critical for binding of PDEγ with Gαt [16, 17], was also unaffected by the presence of tubulin. Thus this NMR study suggests that the PDEγ:tubulin interaction is distinguished from the established PDEγ C-terminal interactions with its visual signaling targets [2, 7, 16] (Figure 3D).
Furthermore, the GDP-bound tubulin appeared to impose less line broadening effect than the GTP-bound tubulin (Figure 3A, B,C), confirming a GTP-dependence of the PDEγ:tubulin interaction observed through pull down assays (Figure 2D).
Discussion
In this study we have identified a novel PDEγ interaction with tubulin/microtubules. This interaction was determined to be specific for the PDEγ C-terminus, and was enhanced in the presence of GTP. The tubulin-interacting interface, however, was distinct from the PDEγ interaction with either transducin, the PDE6 catalytic domain, or the RGS9-1 catalytic core. The uniqueness of the PDEγ:tubulin interaction may provide useful clues for possible non-phototransduction PDEγ functions in the photoreceptor cells.
An important feature of the photoreceptor cell is that all the phototransduction proteins are synthesized in the inner segment, and must be transported through the narrow connecting cilium into the outer segment, the default destination, to perform their functions [1]. Microtubules constitute the “railway” for this inter-segmental transport, with the minus ends anchored in the basal body [15] and the plus ends extended either into the inner segment or through the connecting cilium into the outer segment (Figure S1). The mechanism underlying transport inside the inner segment, from the ER (endoplasmic reticulum) to the basal body, is largely unknown. Recent studies using mouse models led to a hypothesis that PDE6 and other membrane proteins transport along microtubules on cargo vesicles, which are moved from the ER region to the basal body most likely by the minus end-directed dynein II [11].
Our results showed that the PDEγ:tubulin interaction is GTP-dependent (Figure 2D and Figure 3) and is differential from the PDEαβ interaction (Figure 3). We thus speculate that through the interaction with GTP-tubulin, PDEγ may help load the PDE6-carrying vesicles to microtubules and thus facilitate PDE6 transport from ER to the basal body (see the diagram in Figure S1). Since fatty acids are attached to the PDE6 α and β C-termini by a post-translational process on the ER [11], the PDE6 αβγγ heterotetramer may be assembled and then tethered to the membrane of carrier vesicles in the ER region. The PDE6-laden vesicles can then be “docked” through the PDEγ:tubulin interaction to nearby microtubule plus ends where GTP-bound tubulins are accessible [5]. Thus temporary immobilization of the PDE6 vesicle to microtubules should facilitate its efficient recognition by dyneins, probably also with the assistance of the dynactin complex. In this case, PDEγ could reasonably bind tubulin and PDEαβ simultaneously, because it is the PDEγ polycationic region rather than the tubulin-interacting C-terminal domain (Figure 2B and Figure 3) that provides most of the binding strength with PDEαβ [13]. In addition, the key PDE6-inhibiting residues did not interact with tubulin (Figure 3), suggesting that tubulin may not compete with the PDE6 catalytic domain for interaction with the PDEγ C-terminus. Once GTP-tubulin is converted into GDP-tubulin by its GTPase activity [5], PDEγ dissociates from microtubules and the PDE6-carrying vesicles can now be moved along microtubules toward the connecting cilium by the motor proteins.
Microtubule dynamics is a highly regulated event that plays a critical role in neurite extension. The microtubule plus end is stabilized by the loading of GTP-tubulin subunits [5]. Therefore, in a scenario alternative to a possible PDEγ role in PDE6 trafficking, PDEγ which recognizes GTP-tubulin (Figures 2D and 3) may modulate microtubule dynamics and thus regulate the morphogenesis of the photoreceptor synaptic terminal.
In either case, the novel interaction between the PDEγ C-terminus and tubulin that we have observed may provide important implications for understanding the degenerative retinal diseases caused by the lack of PDEγ [20]. Further investigations are warranted to unravel possible cellular functions of the PDEγ:tubulin interaction in the photoreceptor neurons. Distribution of the PDE6 α and β subunits in the retinal outer segment of the PDEγ-knockout mouse should be determined. In the absence of PDEγ, PDE6 may not be able to transport normally from the inner segment to the outer segment, which may partially explain the abnormal development of the rod outer segment prior to a rapid retinal degeneration in the PDEγ-knockout mouse [20]. In addition, the morphology of the rod photoreceptor synaptic terminal in the PDEγ-knockout mouse should also be examined. A lack of possible regulation of microtubule dynamics by PDEγ could lead to abnormal synapse formation.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baker SA, Haeri M, Yoo P, Gospe SM, 3rd, Skiba NP, Knox BE, Arshavsky VY. The outer segment serves as a default destination for the trafficking of membrane proteins in photoreceptors. J Cell Biol. 2008;183:485–98. doi: 10.1083/jcb.200806009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barren B, Gakhar L, Muradov H, Boyd KK, Ramaswamy S, Artemyev NO. Structural basis of phosphodiesterase 6 inhibition by the C-terminal region of the gamma-subunit. Embo J. 2009;28:3613–22. doi: 10.1038/emboj.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen NF, Yu JZ, Skiba NP, Hamm HE, Rasenick MM. A specific domain of Gialpha required for the transactivation of Gialpha by tubulin is implicated in the organization of cellular microtubules. J Biol Chem. 2003;278:15285–90. doi: 10.1074/jbc.M300841200. [DOI] [PubMed] [Google Scholar]
- 4.Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005;6:204. doi: 10.1186/gb-2004-6-1-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Etienne-Manneville S. From signaling pathways to microtubule dynamics: the key players. Curr Opin Cell Biol. 2009 doi: 10.1016/j.ceb.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira PA. Characterization of RanBP2-associated molecular components in neuroretina. Methods Enzymol. 2000;315:455–68. doi: 10.1016/s0076-6879(00)15861-6. [DOI] [PubMed] [Google Scholar]
- 7.Granovsky AE, Artemyev NO. A conformational switch in the inhibitory gamma-subunit of PDE6 upon enzyme activation by transducin. Biochemistry. 2001;40:13209–15. doi: 10.1021/bi011127j. [DOI] [PubMed] [Google Scholar]
- 8.Guo LW, Ruoho AE. The retinal cGMP phosphodiesterase gamma-subunit - a chameleon. Curr Protein Pept Sci. 2008;9:611–25. doi: 10.2174/138920308786733930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanson SM, Francis DJ, Vishnivetskiy SA, Klug CS, Gurevich VV. Visual arrestin binding to microtubules involves a distinct conformational change. J Biol Chem. 2006;281:9765–72. doi: 10.1074/jbc.M510738200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houdart F, Girard-Nau N, Morin F, Voisin P, Vannier B. The regulatory subunit of PDE6 interacts with PACSIN in photoreceptors. Mol Vis. 2005;11:1061–70. [PubMed] [Google Scholar]
- 11.Karan S, Zhang H, Li S, Frederick JM, Baehr W. A model for transport of membrane-associated phototransduction polypeptides in rod and cone photoreceptor inner segments. Vision Res. 2008;48:442–52. doi: 10.1016/j.visres.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MH, Cierpicki T, Derewenda U, Krowarsch D, Feng Y, Devedjiev Y, Dauter Z, Walsh CA, Otlewski J, Bushweller JH, Derewenda ZS. The DCX-domain tandems of doublecortin and doublecortin-like kinase. Nat Struct Biol. 2003;10:324–33. doi: 10.1038/nsb918. [DOI] [PubMed] [Google Scholar]
- 13.Mou H, Cote RH. The catalytic and GAF domains of the rod cGMP phosphodiesterase (PDE6) heterodimer are regulated by distinct regions of its inhibitory gamma subunit. J Biol Chem. 2001;276:27527–34. doi: 10.1074/jbc.M103316200. [DOI] [PubMed] [Google Scholar]
- 14.Ovadi J, Orosz F. An unstructured protein with destructive potential: TPPP/p25 in neurodegeneration. Bioessays. 2009;31:676–86. doi: 10.1002/bies.200900008. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–25. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 16.Slep KC, Kercher MA, He W, Cowan CW, Wensel TG, Sigler PB. Structural determinants for regulation of phosphodiesterase by a G protein at 2.0 A. Nature. 2001;409:1071–7. doi: 10.1038/35059138. [DOI] [PubMed] [Google Scholar]
- 17.Slepak VZ, Artemyev NO, Zhu Y, Dumke CL, Sabacan L, Sondek J, Hamm HE, Bownds MD, Arshavsky VY. An effector site that stimulates G-protein GTPase in photoreceptors. J Biol Chem. 1995;270:14319–24. doi: 10.1074/jbc.270.24.14319. [DOI] [PubMed] [Google Scholar]
- 18.Song J, Guo LW, Muradov H, Artemyev NO, Ruoho AE, Markley JL. Intrinsically disordered gamma-subunit of cGMP phosphodiesterase encodes functionally relevant transient secondary and tertiary structure. Proc Natl Acad Sci U S A. 2008;105:1505–10. doi: 10.1073/pnas.0709558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinmetz MO. Structure and thermodynamics of the tubulin-stathmin interaction. J Struct Biol. 2007;158:137–47. doi: 10.1016/j.jsb.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Tsang SH, Gouras P, Yamashita CK, Kjeldbye H, Fisher J, Farber DB, Goff SP. Retinal degeneration in mice lacking the gamma subunit of the rod cGMP phosphodiesterase. Science. 1996;272:1026–9. doi: 10.1126/science.272.5264.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wersinger C, Sidhu A. Disruption of the interaction of alpha-synuclein with microtubules enhances cell surface recruitment of the dopamine transporter. Biochemistry. 2005;44:13612–24. doi: 10.1021/bi050402p. [DOI] [PubMed] [Google Scholar]
- 22.Xuan C, Qiao W, Gao J, Liu M, Zhang X, Cao Y, Chen Q, Geng Y, Zhou J. Regulation of microtubule assembly and stability by the transactivator of transcription protein of Jembrana disease virus. J Biol Chem. 2007;282:28800–6. doi: 10.1074/jbc.M702823200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.