Abstract
Because syndecan-4 (SD-4) is expressed by some (but not all) T cells following activation and serves as the exclusive ligand of dendritic cell-associated heparan sulfate proteoglycan-dependent integrin ligand (DC-HIL), we envisioned the DC-HIL/SD-4 pathway to be a therapeutic target for conditions mediated by selectively activated T cells. We conjugated soluble DC-HIL receptor with the toxin saporin (SAP; DC-HIL-SAP) and showed it to bind activated (but not resting) T cells and become internalized by and deplete SD-4+ T cells. In hapten-sensitized mice, DC-HIL-SAP injected i.v. prior to hapten challenge led to markedly suppressed contact hypersensitivity responses that lasted 3 wk and were restricted to the hapten to which the mice were originally sensitized. Such suppression was not observed when DC-HIL-SAP was applied during sensitization. Moreover, the same infusion of DC-HIL-SAP produced almost complete disappearance of SD-4+ cells in haptenated skin and a 40% reduction of such cells within draining lymph nodes. Our results provide a strong rationale for exploring use of toxin-conjugated DC-HIL to treat activated T cell-driven disease in humans.
Tlymphocyte activation is regulated by stimulatory and inhibitory signals transduced by binding of TCRs to corresponding ligands on APCs (1). Stimulatory receptors tend to be present constitutively even on resting T cells, whereas inhibitory receptors require activation for expression (2). Inhibitory receptors include CTLA-4 or CD152 (3–5), programmed cell death-1 (PD-1) (6, 7), B and T lymphocyte attenuator (8–10), and Tim-3 (11–13). T cell expression profiles of these receptors overlap but are disparate; thus, CTLA-4 is expressed by almost all recently activated T cells, whereas PD-1 is restricted to effector T cells, and B and T lymphocyte attenuator and Tim-3 are expressed preferentially by Th1 cells. Moreover, because PD-1 is expressed at a sustained high-level by T cells undergoing exhaustion in chronic viral infections and in melanoma (14–17), inhibitory receptors may serve as a marker for the functional state of T cells.
We discovered and have characterized a novel inhibitory pathway composed of the highly glycosylated APC receptor dendritic cell-associated heparan sulfate proteoglycan-dependent integrin ligand (DC-HIL) and its exclusive T cell ligand syndecan-4 (SD-4) (18–21). DC-HIL is also known as glycoprotein nmb (22), osteoactivin (23), and hematopoietic growth factor-inducible neurokinin-1 type (24). DC-HIL specifically recognizes a particular structure of heparan sulfate on SD-4 peculiar to T cells because it does not bind B cells, which constitutively express SD-4 at a high level (21). SD-4 shares with PD-1 a requirement for T cell activation for its expression (19). In the mouse contact hypersensitivity (CH) model for T cell-mediated immunity, SD-4 is expressed by CD4+ and CD8+ T cells within draining lymph nodes (DLNs) after hapten challenge (and not during hapten sensitization) (20). Inhibiting the DC-HIL/SD-4 pathway in this model by infusing soluble DC-HIL results in enhanced CH responses when DC-HIL is given during challenge (but not during sensitization), consistent with expression of SD-4 on activated (but not resting) T cells.
In this study, we again use the CH model to show that SD-4 is expressed by some (but not all) activated T cells and preferentially by effector memory T cells within DLNs and especially hapten-challenged skin. Moreover, we prove that toxin-conjugated DC-HIL can suppress an activated T cell-driven response by depleting SD-4+ T lymphocytes. Our studies provide a foundation for manipulating the DC-HIL/SD-4 pathway to therapeutically deplete effector memory T cells.
Materials and Methods
Mice
Female BALB/c (6–9 wk old) mice were purchased from Harlan Breeders (Indianapolis, IN). Following National Institutes of Health guidelines, these animals were housed and cared for in the pathogen-free facility of the Institutional Animal Care Use Center of University of Texas Southwestern Medical Center, Dallas, TX. All animal protocols were approved by the institutional review board.
Production of Fc-fused recombinant proteins
A plasmid vector (pSTB-DC-HIL-Fc) encoding the extracellular domain of DC-HIL fused to the Fc portion of human IgG1 was constructed as reported previously (19). The Fc-fusion protein (DC-HIL-Fc) was produced in COS-1 cells and purified as described previously (19). Purity of final preparations was high (19), as judged by a single band (120 kDa) in SDS-PAGE/Coomassie blue staining or in immunoblotting with goat anti-human IgG Ab or 1E4 rat anti–DC-HIL mAb (19).
Conjugation of saporin with Fc protein
DC-HIL-Fc or human IgG1 was biotinylated using EZ-link NHS-Biotin (Pierce, Rockford, IL) following manufacturer's recommendations. Normally, one Fc protein molecule has one to two biotin molecules. Biotinylated Fc protein was then conjugated with streptavidin-saporin (SAP) (Advanced Targeting System, San Diego, CA) by mixing them together at a molecular ratio (Fc-protein:SAP) of 5:1. Resulting SAP conjugates are referred to as DC-HIL-SAP and Ig-SAP, respectively.
Abs
mAbs against CD4 (L3T4), CD8 (53-6.7), CD19 (eBio 1D3), and CD69 (H1.2F3) were purchased from eBioscience (San Diego, CA), anti–SD-4 mAb (KY/8.2) from BD Pharmingen (San Diego, CA), anti-SAP Ab from Advanced Targeting System, and anti-human IgG Ab and secondary Ab from Jackson ImmunoResearch Laboratories (West Grove, PA).
T cell isolation and in vitro assays
CD4+ and CD8+ T cells were purified from spleen or lymph node (LN) cells of naive or immunized BALB/c mice using CD4+ and CD8+ T cell isolation kits (Miltenyi Biotec, Auburn, CA). For experiments examining binding of SAP conjugates to T cells, splenic CD4+ and CD8+ T cells (1 × 106/ml) were activated by immobilized anti-CD3 Ab (2 μg/ml) for 3 d in complete RPMI 1640 media. Freshly isolated (resting) or activated T cells were incubated with 10 μg/ml DC-HIL-SAP or control Ig-SAP plus 2.5 μg/ml PE–anti-human IgG Ab. After washing, cell-bound fluorescence was analyzed by FACSCalibur (BD Biosciences, San Jose, CA).
To assay the effect of DC-HIL-SAP on T cell proliferation, CD4+ or CD8+ T cells (2 × 105/96-well plate) were cultured with immobilized anti-CD3 Ab (2 μg/ml) for 2 d, after which DC-HIL-SAP or Ig-SAP was added to culture at indicated concentrations (calculated as SAP concentration) and incubated for another 1 d. After [3H]thymidine (1 μCi/well) was pulsed for 20–22 h, cells were harvested and measured for [3H] radioactivity. In parallel, T cells were also examined for surface expression of SD-4 by flow cytometry. To confirm specificity of DC-HIL-SAP killing for SD-4+ cells, we used the DO11.10 T cell line (provided by J. Kappler and P. Marrack, National Jewish Medical and Research Center, Denver, CO) that was transfected with SD-4 gene or empty vector (20). These cells (1 × 105) were incubated for 2 h with a SAP conjugate at indicated concentrations. After washing, treated cells were cultured in media containing human rIL-2 (50 U/ml). The next day, [3H] thymidine was pulsed for 20 h and then harvested.
To examine SD-4 expression on memory T cells, H-2Kb–associated OVA peptide (SIINFEKL, 30 μg, synthesized by Protein Chemistry Technology Center, University of Texas Southwestern Medical Center) in TiterMax Gold Adjuvant (Sigma-Aldrich, St. Louis, MO) was injected into the footpad of OT-I transgenic mice (three mice). Seventeen days postimmunization, CD8+ T cells were purified from LN of immunized mice (pool of three mice). These T cells were then stained with FITC–anti-CD44, APC–anti-CD62L, and PE– anti-SD-4 Ab and then examined by flow cytometry.
Immunofluorescence staining
For experiments examining internalization of DC-HIL-SAP, activated CD4+ or CD8+ Tcells(2 × 105) were incubated with 10 nM DC-HIL-SAP for 1 h on ice. After washing, cells were split into two batches: one batch was kept on ice, and the other was incubated at 37°C for 30 min. Cells were then fixed with Fixation/Permealization buffer (eBioscience) for 30 min on ice and incubated with goat anti-SAP Ab, followed by fluorescent labeling with Alexa 594-secondary Ab (0.5 μg/ml; Invitrogen, Carlsbad, CA). After washing, cells were incubated further with rabbit anti-human IgG-Fc mAb (5 μg/ml) and with Alexa 488-secondary Ab (0.5 μg/ml, Invitrogen). Fluorescent images were taken using a TCS-SP1 laser scanning confocal microscope (Leica Microsystems, Bannockburn, IL) at ×100 magnification. Images were analyzed by the ImageJ program (National Institutes of Health, Bethesda, MD).
To examine skin-homing T cells, ear skin biopsies were procured from mice sensitized/challenged with 2-phenyl-4-ethoxymethylene-5-oxazolone (oxazolone or Ox) (day 2 postchallenge), doubly stained with rat anti-CD4 (or anti-CD8) Ab and biotinylated anti–SD-4 mAb (5 μg/ml each), and fluorescently labeled with Alexa 488 goat anti-rat IgG (2 μg/ml) and Alexa 594 streptavidin (2 μg/ml). Fluorescence images were taken using a confocal microscope and merged to analyze coexpression of SD-4 and a T cell marker. T cells doubly stained with anti-CD4 or CD8 Ab and anti–SD-4 Ab were counted using three different views.
CH assays
BALB/c mice (n = 4) were sensitized for CH on day 0 by painting 2% of Ox (Sigma-Aldrich) in acetone-olive oil (4:1 in volume) on shaved abdominal skin (sensitization) (25). Mice were challenged on day 6 by painting 1% Ox and solvent control to right and left ears, respectively (elicitation). In some experiments, mice were sensitized by topical application (20 μl) of 50 μg/ml PMA (dissolved in 1% DMSO/99% methanol) and challenged by 10 μl of 25 μg/ml PMA or the solvent alone (as control) (26). Thereafter, CH was assessed daily from days 7–9 by an unbiased technician who measured ear thickness and calculating changes in ear swelling (thickness of right ear minus that of control left ear) (27). Different panels of mice were injected via the tail vein with 20, 40, or 80 nM DC-HIL-SAP, Ig-SAP (as concentration of SAP), or PBS (200 μl/mice) at indicated time points.
For experiments examining the duration of DC-HIL-SAP effects, sensitized mice were injected i.v. with DC-HIL-SAP (or Ig-SAP) 3 h pre-challenge. A week postinjection, mice were challenged weekly with Ox or solvent control (on right and left ears, respectively) up to four times, after which ear thickness was measured daily.
CH responses of DC-HIL-SAP–treated mice to a different contact allergen, 2,4,6-trinitrochlorobenzene (TNCB; Sigma-Aldrich), were also examined following sensitization, injection of SAP, and challenge as above. On day 6, the same mice were sensitized by epicutaneous application of 7% TNCB (solved in acetone-olive oil) to a different area of shaved abdominal skin and on day 12 challenged with 1% Ox and 1% TNCB on left and right ears, respectively, after measuring thickness of both ears (control).
Histological examination of skin and phenotyping of LN cells
Two days after painting Ox on ears of Ox-sensitized mice treated with SAP conjugates or PBS, ear skin and DLNs were procured. Ear skin was frozen, thin sectioned, and stained with H&E according to Ehrlich (Sigma-Aldrich). Histological examination was carried out under light microscopy at a magnification of ×10.
Cervical DLNs were excised and cells per LN counted and examined for frequency of CD4+ T cells, CD8+ T cells, and CD19+ B cells using respective antimaker Abs. To assay CD69 expression, LN cells (5 × 105) were stained with T cell or B cell surface markers (CD4, CD8, and CD19) (2.5 μg/ml each) in the presence or absence of PE–anti-CD69 mAb or PE-isotypic control hamster IgG (BD Pharmingen) (2.5 μg/ml each) and examined by flow cytometry for surface expression of CD69 in each leukocyte subpopulation.
For SD-4 expression on T cells in DLNs, sensitized mice (n = 3) were treated similarly and their DLNs excised 2 d postchallenge and pooled. CD4+ and CD8+ T cells were purified from the pooled DLN cells and stained with PE–anti-SD-4 mAb or PE-isotypic control rat IgG (2.5 μg/ml each) and examined by flow cytometry for surface expression of SD-4 on T cells.
Cytokine assay
Mice (n = 3) were treated similarly with Ox and injected i.v. with SAP conjugate (40 nM). A day postchallenge, DLN cells were isolated and cultured (2 × 105/well) with immobilized anti-CD3 Ab (2 μg/ml) and soluble anti-CD28 Ab (2 μg/ml) for 2 d. Culture supernatant was harvested and assayed for production of IL-4, IFN-γ, IL-10, and IL-17 using mouse ELISA Ready-SET-Go kits (eBioscience).
Statistical analysis
Data are presented as means ± SD. The significance of differences between experimental variables was determined using the paired Student t test.
Results
SD-4 is expressed by effector memory T cells
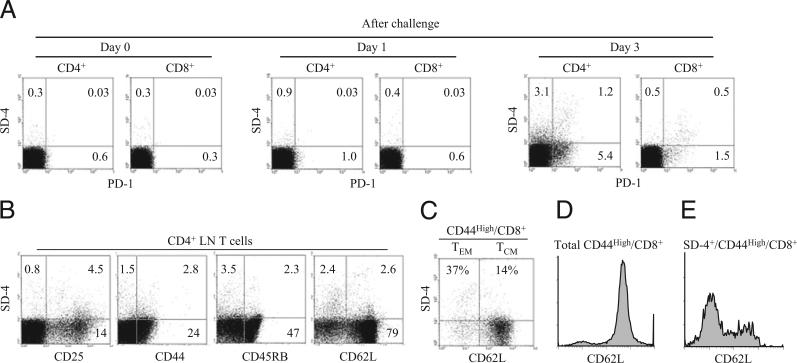
Having shown previously that SD-4 (like PD-1) is expressed by T cells within DLNs of recently challenged hapten-sensitized mice, we analyzed more rigorously the spectrum of SD-4 expression in this experimental model (Fig. 1). CD4+ or CD8+ T cells isolated from DLNs of sensitized mice challenged with Ox were examined for coexpression of SD-4 and T cell activation markers (Fig. 1). Although neither SD-4– nor PD-1–expressing T cells were present in the DLN of mice sensitized alone (day 0, just prior to challenge) and day 1 following challenge, positive cells were found in DLNs 3 d postchallenge (Fig. 1A). Only some of PD-1+ T cells expressed SD-4, indicating overlapping but not identical T cell subpopulations for the two markers. Among CD4+ T cells, 4.3% were SD-4+, and these cells coexpressed the activation markers CD25, CD44, CD45RB, and CD62L at varying levels (Fig. 1B). Conversely, 25% of CD25+ T cells expressed SD-4. To sort SD-4+ expression by memory T cells (Fig. 1C), we purified CD8+ T cells from OT-I transgenic mice immunized with H-2Kb–associated OVA peptide labeled fluorescently with Abs directed against CD44, CD62L, and SD-4. The CD44high/CD8+fraction was gated and fractionated for effector memory (CD62Llow/CD44high/CD8+) and central memory (CD62Lhigh/CD44high/CD8+) T cells. Whereas the majority of CD44high/CD8+ cells were of the central memory subset (Fig. 1D), most SD-4+ cells were of the effector memory subpopulation. SD-4 expression was noted equally between Th1 and Th2 cells harvested from cultures polarized to either fraction by treatment with relevant Abs and cytokines (data not shown). These results indicate that, among DLN cells of hapten-sensitized/challenged mice, SD-4 is expressed by a small population of activated T cells belonging to the effector memory subset.
FIGURE 1.
Characterization of SD-4+ T cells generated in immunized mice. A, CD4+ or CD8+ T cells purified from DLNs of mice sensitized/challenged with Ox day 0 (just prior to challenge), day 1, or day 3 postchallenge were examined for coexpression of SD-4 and PD-1. B, These CD4+ T cells also were analyzed for coexpression of SD-4 and a T cell marker. C–E, Seventeen days postimmunization of OT-I transgenic mice with OVA peptide, CD8+ T cells were purified from DLNs of immunized mice and then stained with FITC–anti-CD44, APC–anti-CD62L, and PE–anti-SD-4 Ab, followed by flow cytometry. C, Dot-blot analysis of SD-4 versus CD62L is shown for CD44high CD8+ cells. CD62Llow and CD62Lhigh fractions represent effector/memory (TEM) and central memory T cells (TCM), respectively. Frequency of SD-4+ cells for each T cell subset is expressed as %. CD62L expression by total CD44high/CD8+ (D) or by SD-4+/CD44high/CD8+ (E) is shown in histograms. All data are representative of at least two experiments.
Toxin-conjugated DC-HIL depletes SD-4+ T cells in vitro
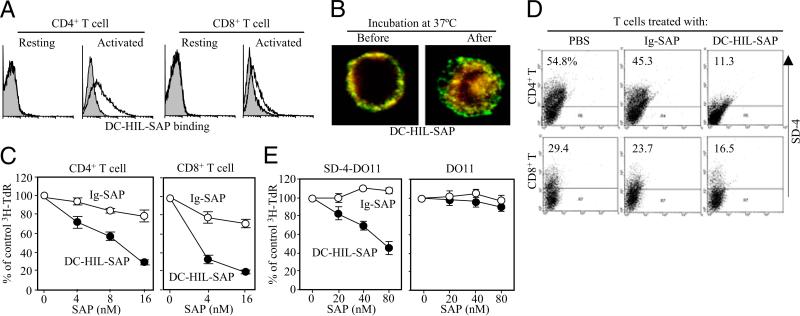
Having shown that soluble DC-HIL receptor (DC-HIL-Fc) binds specifically to SD-4 on T cells (21), we created DC-HIL-SAP by conjugating DC-HIL-Fc to SAP, a potent type I ribosome-inactivating toxin (28). The immunotoxin bound to activated (but not resting) CD4+ or CD8+ T cells (Fig. 2A). Using immunofluorescence staining, we observed DC-HIL-SAP to be internalized by T cells (Fig. 2B): DC-HIL-SAP conjugates (labeled in yellow) were noted on the cell surfaces prior to incubation, followed by their entry into cells postincubation, with DC-HIL clinging to the internal side of the cell membrane, whereas SAP moved more deeply within cells.
FIGURE 2.
Effects of DC-HIL-SAP on T cells in vitro. A, Binding of DC-HIL-SAP to T cells. Splenic CD4+ or CD8+ T cells from BALB/c mice were left untreated (resting) or activated by anti-CD3 Ab (2 μg/ml) for 3 d, and then incubated with 5 μg/ml DC-HIL-SAP (open histograms) or Ig-SAP (shaded), followed by fluorescent labeling with PE–anti-human IgG Ab. Binding was measured by flow cytometry. B, Internalization. Activated CD4+ T cells were incubated with 10 nM DC-HIL-SAP for 1 h on ice. After washing, cells were either left untreated (Before) or incubated (After) at 37°C for 30 min. Cells were then fixed and fluorescently stained with anti-DC-HIL (green fluorescence) or anti-SAP Ab (red), followed by confocal microscopy. Original magnification ×40. C, Effects on T cell proliferation. Splenic CD4+ or CD8+ Tcells(2 × 105 per well) were activated with immobilized anti-CD3 Ab (2 μg/ml) for 2 d, and then different doses of DC-HIL-SAP or Ig-SAP (shown as SAP concentration) were added to the culture. The next day, [3H]thymidine (TdR) incorporation was measured. Relative cpm to the control (0 nM or PBS) are expressed as percentages (mean ± SD; n =3). D, Depletion of SD-4+ T cells. Similarly treated T cells (just prior to harvest for counting [3H] cpm) were examined by flow cytometry for expression of SD-4; percentage of positive cells is shown. E, Specificity to SD-4. DO11.10 T cell lines transfected with SD-4 gene (SD-4-DO11) or empty vector (DO11) were treated with indicated doses of SAP conjugates and cultured for 2 d. Proliferation was measured (mean ± SD; n = 3). Data shown are representative of at least two separate experiments.
We next assessed effects of DC-HIL-SAP on T cell activation (Fig. 2C). CD4+ or CD8+ T cells were activated by incubation with immobilized anti-CD3 Ab for 2 d and then with DC-HIL-SAP (or control Ig-SAP) for 1 d. DC-HIL-SAP blocked proliferation of CD4+ and CD8+ T cells in a dose-dependent manner, achieving 70–80% reduction at the highest dose tested (16 nM SAP). Reduced T cell proliferation was due to selective depletion of SD-4+ Tcells because DC-HIL-SAP treatment markedly reduced the number of SD-4+ T cells (Fig. 2D) and because DC-HIL-SAP potently inhibited proliferation of a SD-4–transfected DO11.10 T cell line (but not vector-transfected control cells) in a dose-dependent manner (Fig. 2E). These results document the ability of toxin-conjugated DC-HIL to selectively kill SD-4+ (activated) T cells.
A single infusion of DC-HIL-SAP blocks elicitation of CH
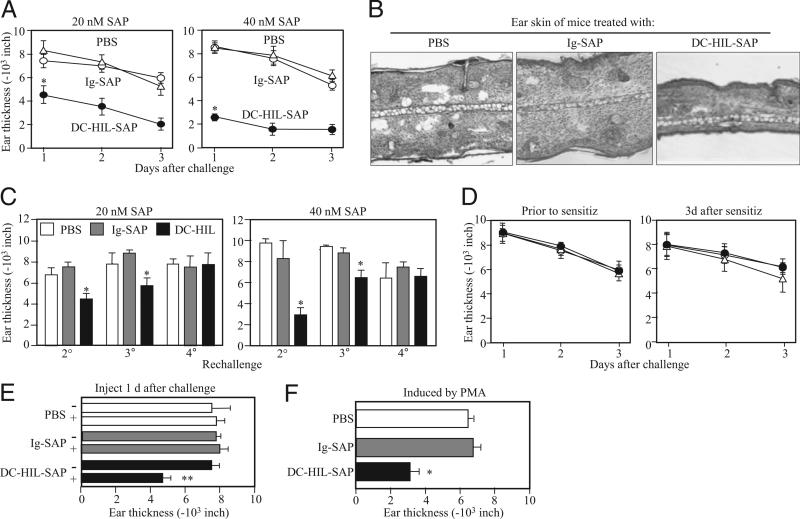
CH is an established model for T cell-mediated, delayed-type hypersensitivity, in which the effect of depleting SD-4+ T cells by DC-HIL-SAP could be tested readily in vivo (Fig. 3). BALB/c mice were sensitized to Ox on shaved abdominal skin (day 0), then challenged with Ox on ear skin (day 6). Mice were injected i.v. with DC-HIL-SAP (20 or 40 nM SAP concentration), Ig-SAP, or PBS 3 h prechallenge (Fig. 3A). Whereas PBS-injected mice developed strong ear swelling, DC-HIL-SAP–injected mice exhibited markedly reduced ear swelling (by 50% and 80% corresponding to 20 and 40 nM SAP, respectively). A dose of 80 nM of DC-HIL-SAP produced similar 80% reduction, with a similar dose of Ig-SAP causing increased toxicity (data not shown), thereby indicating 40 nM to be optimal for suppressing CH. Ig-SAP had minimal effect (<10% reduction for either SAP concentration). Histologic examination of Ox-painted ear skin in DC-HIL-SAP–injected mice versus controls documented less ear thickness and fewer infiltrating leukocytes (Fig. 3B).
FIGURE 3.
Effects of DC-HIL-SAP on CH. On day 0, BALB/c mice (n = 4) were sensitized by painting 2% Ox on shaved abdominal skin. On day 6, CH was elicited in sensitized mice by painting 1% Ox or solvent control to right or left ears, respectively (Challenge). Ear thickness was measured daily from days 1–3 following challenge. A, Mice also were injected i.v. with PBS, Ig-SAP, or DC-HIL-SAP (20 or 40 nM) 3 h prechallenge. Daily change in ear thickness (10−3 inches) was plotted for each panel. B, On day 2, ear skin specimens of mouse representative in each group were stained with H&E and histologically examined under magnification ×10. C, These mice were kept for 1 wk and then rechallenged with Ox weekly for second (2°), third (3°) and fourth challenges (4°). Ear thickness was measured the day following challenge. D, In separate experiments, mice (n = 4) were also injected i.v. with the same SAP conjugates (40 nM) 3 h presensitization or 3 d postsensitization. E, One day postchallenge, mice (n = 4) were injected with PBS, Ig-SAP, or DC-HIL-SAP (40 nM). Ear thickness was measured just before (−) or 1 d after (+) injection. F, Similarly, mice (n = 4) were also sensitized and challenged but with a different chemical, PMA. Ear thickness was measured 1 d following challenge. Statistical significance is denoted by asterisks (*p < 0.001; **p = 0.003) as compared with ear thickness treated with control Ig-SAP. Data shown are representative of four (A) and two (B–F) independent experiments.
The duration of unresponsiveness was assessed by rechallenging mice infused with DC-HIL-SAP (Fig. 3C) with Ox each week for 4 wk. Such mice showed persistently reduced ear swelling after second and third challenges, but regained responsiveness after a fourth challenge.
Having shown previously that infusion of DC-HIL-Fc (unconjugated with toxin) into mice impacted elicitation but not sensitization (19), we revisited this issue (Fig. 3D) and showed consistent results: infusion of DC-HIL-SAP during sensitization (3 h prior or 3 d after) had no impact on CH response.
We also examined whether DC-HIL-SAP injection reduces established ear swelling (Fig. 3E). Sensitized/challenged mice developed similar levels of ear thickness (measured day 1 postchallenge), and they were injected with 40 nM DC-HIL-SAP or controls (PBS or Ig-SAP). DC-HIL-SAP (but not Ig-SAP) reduced ear thickness by 40%.
Finally, we questioned whether injection of DC-HIL-SAP also inhibits CH response to other chemicals. Mice were sensitized and challenged with PMA using a similar time schedule and injected with 40 nM SAP conjugates 3 h prechallenge (Fig. 3F). Infusion of DC-HIL-SAP reduced CH response by 55%. Altogether, these results indicate that a single injection of DC-HIL-SAP markedly suppresses elicitation of CH in sensitized mice and that this immunosuppression lasts 3 wk. Moreover, DC-HIL-SAP effectively inhibits an established CH response.
CH inhibition by DC-HIL-SAP is restricted to Ag at time of infusion
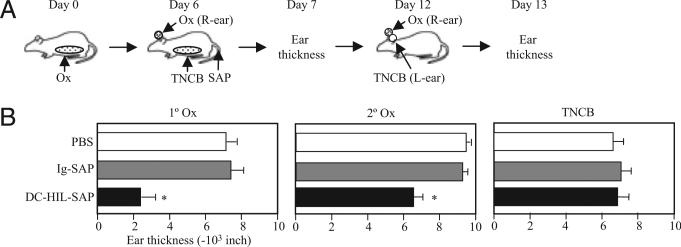
We next examined the immune reactivity of mice treated with DC-HIL-SAP to other haptens during their unresponsive state to the original hapten (Fig. 4). BALB/c mice were sensitized and challenged with Ox and treated i.v. with PBS, Ig-SAP, or DC-HIL-SAP. On the same day (day 6), mice were sensitized to TNCB and then challenged with Ox (right ear) and TNCB (left ear) on day 12 (Fig. 4). Mice treated with DC-HIL-SAP suppressed CH responses to Ox (70% and 30% reduction for first and second challenge) but did not show suppression to TNCB. These results indicate that unresponsiveness caused by DC-HIL-SAP is restricted to the Ag administered at the time of infusion.
FIGURE 4.
Suppression of CH by DC-HIL-SAP is restricted to hapten administrated at the time of infusion. A, Immunization protocol is depicted schematically: BALB/c mice (n = 4) were sensitized with Ox on day 0, and i.v. injected with PBS or SAP conjugate 3 h prechallenge (day 6). On the same day, mice were challenged with Ox and solvent alone on right (R-ear) and left ears (L-ear), respectively, and also sensitized to TNCB. On day 7, ear thickness was measured (1° Ox). Day 12, all mice were challenged with Ox (2° Ox) and TNCB on right and left ears, respectively. Ear thickness shown is measured on day 1 every postchallenge (B). *p < 0.05 as compared with ear thickness of mice treated with Ig-SAP. A second experiment showed similar results.
DC-HIL-SAP reduces the number of activated T cells within DLN and alters cytokine expression patterns
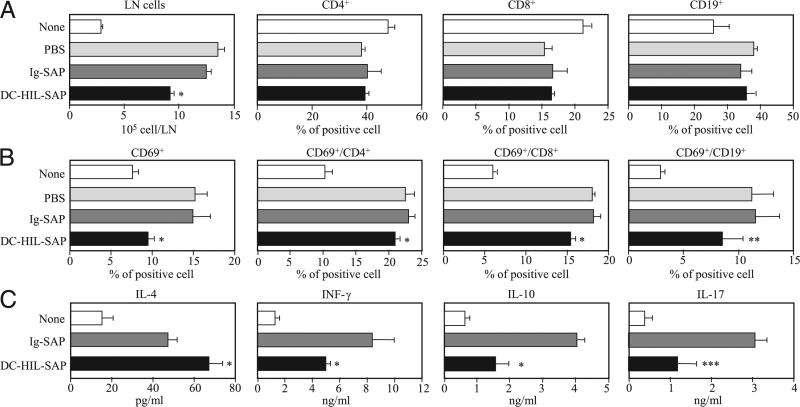
Because significant accumulation of lymphocytes was observed in DLNs 2 d (not 1 d) postchallenge, we procured DLN at this time point and examined effects of DC-HIL-SAP on T cell phenotypes (Fig. 5). DLNs of mice treated with DC-HIL-SAP were smaller compared with controls (mice treated with PBS or Ig-SAP) (Fig. 5A). There were no significant changes in the relative proportions of CD4+, CD8+, and CD19+ cells (Fig. 5A). However, DLNs from mice treated with DC-HIL-SAP contained less numbers of CD69+ (activated) cells in all three subpopulations compared with those for Ig-SAP–treated mice (Fig. 5B). We also examined cytokine production by DLN cells (Fig. 5C). A day after hapten challenge, DLN cells were procured and then cultured for 2 d in the presence of anti-CD3 and anti-CD28 Ab. In mice treated with DC-HIL-SAP, DLN cells produced 40% more IL-4, but secreted 40% less IFN-γ and 60% less IL-10 and IL-17 compared with controls. Thus, not only did DC-HIL-SAP reduce the number of activated T cells in DLN, but it also altered the balance of Th cytokine expression.
FIGURE 5.
Immunological phenotypes of DLN cells from mice treated with SAP conjugates. A and B, Sensitized mice (n = 3) were i.v. injected with PBS, Ig-SAP, or DC-HIL-SAP (each 40 nM) 3 h prechallenge. Two days postchallenge with Ox, DLNs procured from treated mice were counted and expressed as cell number per DLN. Frequency (%) of CD4+, CD8+, and CD19+ B cells was measured by flow cytometry (A). DLN cells also were examined for expression of CD69 on all LN cells, CD4+, CD8+, or CD19+ cells, and frequency (%) was calculated (B). C, DLN cells from similarly treated mice (n = 3) were cultured for 2 d in the presence of anti-CD3 and anti-CD28 Ab and assayed for production of IL-4, IFN-γ, IL-10, and IL-17. As control, DLN cells from unsensitized mice (None) were also analyzed in the same manner. SD was derived from experimental values for three mice. Statistical significance is denoted by asterisks (*p < 0.001; ** p = 0.14; *** p = 0.03) as compared with frequency in LNs of mice treated with control Ig-SAP. Data shown are representative of three separate experiments.
DC-HIL-SAP depletes SD-4+ T cells in hapten-painted skin and DLNs
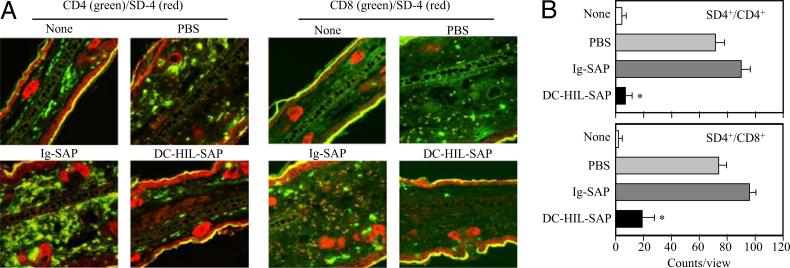
We also examined effects of DC-HIL-SAP on SD-4+ T cells in Ox-painted ear skin. Two days after challenging sensitized mice treated with SAP conjugates (40 nM) or controls, Ox-painted ear skin biopsies were procured and stained fluorescently with anti-CD4 (or anti-CD8) Ab and anti–SD-4 Ab (Fig. 6A). Doubly positive cells were counted and averaged from three separate microscopic views (Fig. 6B). There were none to very few T cells in untreated skin (None) but many CD4+ and CD8+ T cells in Ox-painted skin injected with PBS, almost all of which were SD-4+ (Fig. 6A). Numbers of CD4+ and CD8+ T cells in skin of mice injected with Ig-SAP were similar to those of mice treated with PBS; both were reduced markedly following DC-HIL-SAP infusion (90% reduction for CD4+ and 80% for CD8+ T cells). These results indicate that a single infusion of DC-HIL-SAP depletes almost all SD-4+ T cells in Ox-challenged skin.
FIGURE 6.
Depletion of SD-4+ T cells in Ox-painted skin. A, BALB/c mice (n = 3) were sensitized, injected with PBS or SAP conjugate, and challenged. Two days postchallenge, ear skin biopsies were procured and doubly stained with anti-CD4 or CD8 Ab (shown in green fluorescence) and anti–SD-4 Ab (red). Original magnification ×10. Merged confocal images are shown. B, Using these images, numbers of SD-4+/CD4+ or SD-4+/CD8+ T cells were counted in three separate views, and the average with SD is shown graphically. *p < 0.01 as compared with percentage in skin of mice infused with Ig-SAP. Data shown are representative of three separate experiments.
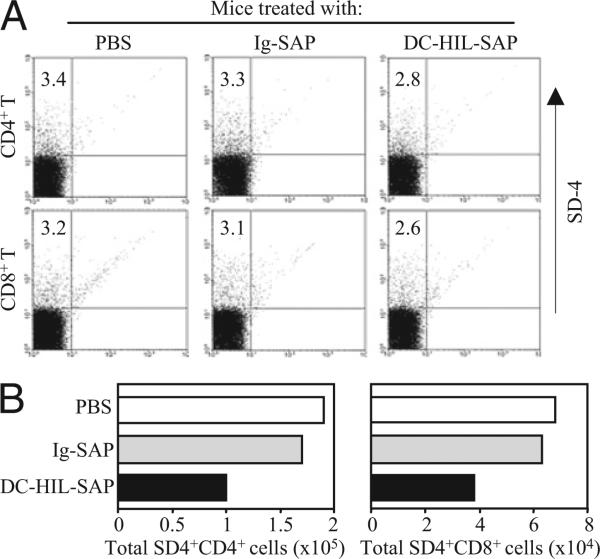
We also examined effects of DC-HIL-SAP on DLN cells (Fig. 7). Two days after challenging sensitized mice infused with SAP conjugates, CD4+ and CD8+ T cells were purified from DLNs and assayed for SD-4+ by flow cytometry. SD-4+ T cells comprised 3.4% of CD4+ and 3.2% of CD8+ T cells in LNs of mice treated with PBS (Fig. 7A). Mice treated with DC-HIL-SAP showed reduced frequency of SD-4+ cells: 2.8% for CD4+ and 2.6% for CD8+ cells. By contrast, there were no changes in mice treated with PBS or Ig-SAP control. Because the total number of CD4+ T cells per LN varied (5.6 × 106 cells/LN of mice treated with PBS, 5.2 × 106 cells/LN with Ig-SAP, and 3.6 × 106 cells/LN with DC-HIL-SAP), the total number of SD-4+ CD4+ T cells was 1.9 × 105 cells/LN for mice with PBS, 1.7 × 105 cells/LN with Ig-SAP, and 1.0 × 105 cells/LN with DC-HIL-SAP (Fig. 7B). For CD8+ T cells, LNs of mice treated with DC-HIL-SAP contained 3.8 × 104 SD-4+ CD8+ T cells (versus 6.8 × 104 cells for PBS and 6.3 × 104 cells for Ig-SAP). These results document the ability of a single infusion of DC-HIL-SAP to deplete by 40% CD4+ and CD8+ T cells from DLNs.
FIGURE 7.
DC-HIL-SAP deletes SD-4+ T cells within DLNs. Mice (n = 3) were sensitized, injected, and challenged similarly. Two days post-challenge, DLN cells were isolated from treated mice and pooled, from which CD4+ or CD8+ T cells were purified and examined for expression of SD-4 by flow cytometry. A, The frequency (%) is shown in dot blots. B, Total number of SD-4+/CD4+ or SD-4+/CD8+ T cells per DLN was calculated and shown graphically. A second experiment showed similar results.
Discussion
Several immunotoxins and cytotoxic mAbs have been used to kill T lymphocytes in diseases in which these cells play critical pathogenic roles. A well-studied example is DAB389IL-2 (Denileukin Diftitox or Ontak), an immunotoxin created by replacing the receptor-binding domain of diphtheria toxin with the entire IL-2 molecule, that targets intermediate (CD122/CD132) and high-affinity (CD25/CD122/CD132) IL-2Rs (29–31) expressed by activated T and B cells and macrophages. Originally used to treat psoriasis, it is now approved by the U.S. Food and Drug Administration to treat cutaneous T cell lymphoma (32), a condition in which T cells overexpress high affinity IL-2R. Although useful for these diseases, the potential of DAB389IL-2 to kill all activated T lymphocytes including those in the memory pool may have debilitating consequences with respect to protective immunity. Thus, in cases in which only a restricted subpopulation of T cells are pathologic, the use of toxin-bearing DC-HIL may hold a special advantage because of its specific binding to SD-4 expressed on effector memory T cells.
CTLA-4 also has been targeted by immunotoxins because of its central role in inhibiting T cell activation (33). Recombinant anti-CTLA single-chain fragment of variable domain conjugated with SAP was shown to effectively deplete activated T cells in MLRs and in infiltrating tumors in mice (34). By contrast, it was not effective for inhibiting established inflammatory conditions because CTLA-4 expression is restricted to early activated T cells that comprise only a small portion of T cells in secondary responses. Like DAB389IL-2, anti–CTLA-4 immunotoxin was more efficacious for treating CTLA-4–expressing neoplastic cells of acute myeloid leukemia (35). We found SD-4 to be highly expressed by CD4+ T cells of patients with mycosis fungoides and Sezary syndrome (a leukemic variant of cutaneous T cell lymphoma) and showed our DC-HIL immunotoxin to effectively kill these cells (J.-S. Chung, L.H. Shiue, A. Dougherty, X. Ni, M. Duvic, P.D. Cruz, Jr., and K. Ariizumi, manuscript in preparation).
Because SD-4 and PD-1 share several features (20), including overlapping T cell expression profiles and functions, we also examined the ability of SAP-bearing PD-L1-Fc (the extracellular domain fused with the Fc portion) to suppress elicitation of CH responses. Surprisingly, it failed to suppress CH responses regardless of infusion during sensitization or challenge (data not shown). These negative outcomes were due to the inability of PDL1-Fc to bind stably activated T cells. The biological activity of our PD-L1-Fc preparation was ascertained by the ability of its immobilized form to strongly inhibit the anti-CD3 response (36). Thus, among coinhibitory receptors, our effort to target SD-4 is the first successful attempt at inhibiting previously primed T cell-mediated inflammation. Future applications targeting SD-4 may prove highly beneficial because a single infusion of DC-HIL-SAP strongly suppressed elicitation of CH response, lasting 3 wk.
Because infusion of DC-HIL-SAP markedly reduces the number of SD-4+ T cells in hapten-challenged skin (but less efficiently depletes these T cells in DLNs), we speculate that SD-4 may be involved in homing of T cells to skin, especially because it facilitates binding of the chemokine RANTES/CCL5 to the specific receptor CCR5 responsible for recruiting leukocytes into inflammatory sites (37). Moreover, SD-4 was reported to bind the chemokine, albeit at a much lower affinity (37). However, it is not known whether SD-4 is involved in binding of the CCL27 chemokine to the receptor CCR10 that directs T cell homing specifically to skin (38). Alternatively, infused DC-HIL-SAP may be differentially accessible to SD-4+ T cells in DLNs versus in blood. Because T cells migrate to skin as quickly as 1 h following hapten challenge, peaking at 3 h (39, 40), blood-circulating DC-HIL-SAP may more readily find SD-4+ T cells migrating from DLNs or spleen to the skin. By contrast, infused DC-HIL-SAP may not reach high levels in DLNs.
In sum, our studies document the effectiveness of DC-HIL–mediated targeting of SD-4 to alleviate a cutaneous inflammatory response in mice. This immunotherapy may benefit management of graft-versus-host disease and protection of transplanted organs from rejection because SD-4 also is expressed by cytotoxic T lymphocytes directed to allo-Ag (M. Tomihari and K. Ariizumi, unpublished observations).
Acknowledgments
We thank Irene Dougherty and Julie Yang for technical and secretary assistance.
This work was supported by National Institutes of Health Grant AI064927-05A2 and a Pilot and Feasibility Study grant from Galderma.
Abbreviations used in this paper
- CH
contact hypersensitivity
- DC-HIL
dendritic cell-associated heparan sulfate proteoglycan-dependent integrin ligand
- DLN
draining lymph node
- LN
lymph node
- Ox
2-phenyl-4-ethoxymethylene-5-oxazolone
- PD-1
programmed cell death-1
- SAP
saporin
- SD-4
syndecan-4
- TNCB
2,4,6-trinitrochlorobenzene
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Chambers CA, Allison JP. Co-stimulation in T cell responses. Curr. Opin. Immunol. 1997;9:396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 2.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu. Rev. Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 3.Carreno BM, Bennett F, Chau TA, Ling V, Luxenberg D, Jussif J, Baroja ML, Madrenas J. CTLA-4 (CD152) can inhibit T cell activation by two different mechanisms depending on its level of cell surface expression. J. Immunol. 2000;165:1352–1356. doi: 10.4049/jimmunol.165.3.1352. [DOI] [PubMed] [Google Scholar]
- 4.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 6.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int. Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 7.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 9.Carreno BM, Collins M. BTLA: a new inhibitory receptor with a B7-like ligand. Trends Immunol. 2003;24:524–527. doi: 10.1016/j.it.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Han P, Goularte OD, Rufner K, Wilkinson B, Kaye J. An inhibitory Ig superfamily protein expressed by lymphocytes and APCs is also an early marker of thymocyte positive selection. J. Immunol. 2004;172:5931–5939. doi: 10.4049/jimmunol.172.10.5931. [DOI] [PubMed] [Google Scholar]
- 11.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 13.Sabatos CA, Chakravarti S, Cha E, Schubart A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat. Immunol. 2003;4:1102–1110. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 14.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol. Immunother. 2007;56:739–745. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 16.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr. Opin. Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shikano S, Bonkobara M, Zukas PK, Ariizumi K. Molecular cloning of a dendritic cell-associated transmembrane protein, DC-HIL, that promotes RGD-dependent adhesion of endothelial cells through recognition of heparan sulfate proteoglycans. J. Biol. Chem. 2001;276:8125–8134. doi: 10.1074/jbc.M008539200. [DOI] [PubMed] [Google Scholar]
- 19.Chung JS, Sato K, Dougherty II, Cruz PD, Jr., Ariizumi K. DC-HIL is a negative regulator of T lymphocyte activation. Blood. 2007;109:4320–4327. doi: 10.1182/blood-2006-11-053769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung JS, Dougherty I, Cruz PD, Jr., Ariizumi K. Syndecan-4 mediates the coinhibitory function of DC-HIL on T cell activation. J. Immunol. 2007;179:5778–5784. doi: 10.4049/jimmunol.179.9.5778. [DOI] [PubMed] [Google Scholar]
- 21.Chung JS, Bonkobara M, Tomihari M, Cruz PD, Jr., Ariizumi K. The DC-HIL/syndecan-4 pathway inhibits human allogeneic T-cell responses. Eur. J. Immunol. 2009;39:965–974. doi: 10.1002/eji.200838990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weterman MA, Ajubi N, van Dinter IM, Degen WG, van Muijen GN, Ruitter DJ, Bloemers HP. nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int. J. Cancer. 1995;60:73–81. doi: 10.1002/ijc.2910600111. [DOI] [PubMed] [Google Scholar]
- 23.Safadi FF, Xu J, Smock SL, Rico MC, Owen TA, Popoff SN. Cloning and characterization of osteoactivin, a novel cDNA expressed in osteoblasts. J. Cell Biochem. 2001;84:12–26. doi: 10.1002/jcb.1259. [DOI] [PubMed] [Google Scholar]
- 24.Metz RL, Patel PS, Hameed M, Bryan M, Rameshwar P. Role of human HGFIN/nmb in breast cancer. Breast Cancer Res. 2007;9:R58. doi: 10.1186/bcr1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz PD, Jr., Bergstresser PR. The low-dose model of UVB-induced immunosuppression. Photodermatol. 1988;5:151–161. [PubMed] [Google Scholar]
- 26.Kadoshima-Yamaoka K, Goto M, Murakawa M, Yoshioka R, Tanaka Y, Inoue H, Murafuji H, Kanki S, Hayashi Y, Nagahira K, et al. ASB16165, a phosphodiesterase 7A inhibitor, reduces cutaneous TNF-alpha level and ameliorates skin edema in phorbol ester 12-O-tetradecanoylphorbol-13-acetate-induced skin inflammation model in mice. Eur. J. Pharmacol. 2009;613:163–166. doi: 10.1016/j.ejphar.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Cruz PD, Jr., Nixon-Fulton J, Tigelaar RE, Bergstresser PR. Local effects of UV radiation on immunization with contact sensitizers. I. Down-regulation of contact hypersensitivity by application of TNCB to UV-irradiated skin. Photodermatol. 1988;5:126–132. [PubMed] [Google Scholar]
- 28.Flavell DJ. Saporin immunotoxins. Curr. Top. Microbiol. Immunol. 1998;234:57–61. doi: 10.1007/978-3-642-72153-3_4. [DOI] [PubMed] [Google Scholar]
- 29.Kelley VE, Bacha P, Pankewycz O, Nichols JC, Murphy JR, Strom TB. Interleukin 2-diphtheria toxin fusion protein can abolish cell-mediated immunity in vivo. Proc. Natl. Acad. Sci. U.S.A. 1988;85:3980–3984. doi: 10.1073/pnas.85.11.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pastan I, Chaudhary V, FitzGerald DJ. Recombinant toxins as novel therapeutic agents. Annu. Rev. Biochem. 1992;61:331–354. doi: 10.1146/annurev.bi.61.070192.001555. [DOI] [PubMed] [Google Scholar]
- 31.Phillips SM, Bhopale MK, Constantinescu CS, Ciric B, Hilliard B, Ventura E, Lavi E, Rostami A. Effect of DAB(389)IL-2 immunotoxin on the course of experimental autoimmune encephalomyelitis in Lewis rats. J. Neurol. Sci. 2007;263:59–69. doi: 10.1016/j.jns.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 32.Foss FM. DAB(389)IL-2 (ONTAK): a novel fusion toxin therapy for lymphoma. Clin. Lymphoma. 2000;1:110–116. doi: 10.3816/clm.2000.n.009. discussion 117. [DOI] [PubMed] [Google Scholar]
- 33.Pistillo MP, Tazzari PL, Ellis JH, Ferrara GB. Molecular characterization and applications of recombinant scFv antibodies to CD152 co-stimulatory molecule. Tissue Antigens. 2000;55:229–238. doi: 10.1034/j.1399-0039.2000.550306.x. [DOI] [PubMed] [Google Scholar]
- 34.Tazzari PL, Polito L, Bolognesi A, Pistillo MP, Capanni P, Palmisano GL, Lemoli RM, Curti A, Biancone L, Camussi G, et al. Immunotoxins containing recombinant anti-CTLA-4 single-chain fragment variable antibodies and saporin: in vitro results and in vivo effects in an acute rejection model. J. Immunol. 2001;167:4222–4229. doi: 10.4049/jimmunol.167.8.4222. [DOI] [PubMed] [Google Scholar]
- 35.Pistillo MP, Tazzari PL, Palmisano GL, Pierri I, Bolognesi A, Ferlito F, Capanni P, Polito L, Ratta M, Pileri S, et al. CTLA-4 is not restricted to the lymphoid cell lineage and can function as a target molecule for apoptosis induction of leukemic cells. Blood. 2003;101:202–209. doi: 10.1182/blood-2002-06-1668. [DOI] [PubMed] [Google Scholar]
- 36.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slimani H, Charnaux N, Mbemba E, Saffar L, Vassy R, Vita C, Gattegno L. Binding of the CC-chemokine RANTES to syndecan-1 and syndecan-4 expressed on HeLa cells. Glycobiology. 2003;13:623–634. doi: 10.1093/glycob/cwg083. [DOI] [PubMed] [Google Scholar]
- 38.Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006;27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Marelli-Berg FM, Cannella L, Dazzi F, Mirenda V. The highway code of T cell trafficking. J. Pathol. 2008;214:179–189. doi: 10.1002/path.2269. [DOI] [PubMed] [Google Scholar]
- 40.Huang V, Lonsdorf AS, Fang L, Kakinuma T, Lee VC, Cha E, Zhang H, Nagao K, Zaleska M, Olszewski WL, Hwang ST. Cutting edge: rapid accumulation of epidermal CCL27 in skin-draining lymph nodes following topical application of a contact sensitizer recruits CCR10-expressing T cells. J. Immunol. 2008;180:6462–6466. doi: 10.4049/jimmunol.180.10.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]